Kinetics Analysis on Mixing Calcination Process of Fly Ash and Ammonium Sulfate☆
2014-07-25PengWangLaishiLiDezhouWei
Peng Wang*,Laishi Li,Dezhou Wei
Energy,Resources and Environmental Technology
Kinetics Analysis on Mixing Calcination Process of Fly Ash and Ammonium Sulfate☆
Peng Wang*,1,2,Laishi Li2,Dezhou Wei1
1College of Resources and Civil Engineering,Northeastern University,Shenyang 110819,China2Shenyang Aluminum and Magnesium Engineering and Research Institute,Shenyang 110001,China
A R T I C L EI N F O
Article history:
Mixing calcinations
Kinetics
Activation energy
Reaction mechanism
The further development of the extraction of alumina that is produced in the calcination process of ammonium sulfate mixed with f l y ash was limited because of the lack of systematic theoretical study.In order to aggrandize theresearchofthecalcinationprocess,thekineticsandreactionmechanismofthecalcinationswerestudied.The result suggests that there are two stages in the calcination process,and the alumina extraction rate increases swiftly in the initial stage,but slows down increasing in the later stage.The apparent activation energy of the initial and later stages equals to 13.31 and 35.65 kJ·mol−1,respectively.In the initial stage,ammonium sulfate reacts directly with mullite in the f l y ash to form ammonium aluminum sulfate,while in the later stage,aluminum sulfate is formed by the reaction between ammonium aluminum sulfate and ammonium sulfate.
©2014TheChemicalIndustry andEngineeringSocietyofChina,andChemicalIndustryPress.Allrightsreserved.
1.Introduction
At present,the f l y ash is mainly used in the f i eld of civil engineering as constructing material and agriculture in China,which belongs to utilizationwithlowaddedvalue,especiallyforf l yashwithaluminacontent higher than 30%.Themain components of f l y ashare silicon dioxide and alumina.Since the reserve of bauxite is not rich in China,it will not only promote the utilizing value of f l y ash,but also bring big economic benef i t and solve the problem of aluminum resource strain if the alumina contained in f l y ash can be used[1-3].The ammonia sulfate calcination method is an effective way to activate f l y ash.Fly ash is mixed with ammonia sulfate and calcined to activate the non-reactive substance for extracting the alumina using the non-reactive feature between silicon dioxide and acid[4,5].There are some advantages of treatingf l yashbythismethodsuchaslargeamountoff l yashtreatment, high extracting ratio,simple process,circulating use of ammonia sulfate and simple equipment,and thus it is easy for industrialization[6-10].At present,a fewstudyaboutthef l yashactivatedbythemethodof ammoniasulfatecalcinationsisreported.Park[11]usedtheammoniasulfateto extractthehighconcentratedaluminafromf l yashwiththelowtemperature sintering method and the recovery percentage of aluminum can reach 94.36%.Li[12]studied the process of extracting alumina from f l y ash via ammonia sulfate with the recovery percentage of 95.6%.Jin[13] studied the phase transformation of calcination products of f l y ash and ammonium sulfate.However,all these studies were focused on the alumina extraction technique and there was less care about the related theory.It lacked the systematic thermodynamics and kinetics analysis for the whole calcination process as well as the systematic analysis of factorsaffectingtheprocess.Allthesehindered the furtherdevelopment of alumina extraction from f l y ash via the ammonia method.
In this study,the mixing calcinations of ammonia sulfate and f l y ash process are used to treat the high alumina f l y ash from a power plant in Liaoning,China.The thermodynamics and kinetics,and associated factors in calcination process are examined.This fundamental study shall be benef i cial to further understand the calcinations of ammonia sulfate and f l y ash,promoting the alumina extraction rate,improving the calcination condition,and optimizing the calcination mechanism. Italsoprovidessomeguidanceandreferenceforcomprehensiveutilization of high alumina f l y ash.
2.Experimental
2.1.Materials
The f l y ash was taken from a power plant in Liaoning,China, anditschemical analysisislistedinTable1.Themajorchemicalcomponents of the f l y ash are Al2O3,SiO2and Fe2O3.Moreover,the sample is the kind of high aluminum f l y ash containing 40.7%Al2O3in the mass fraction.
The X-ray diffraction(XRD)pattern of the f l y ash is shown in Fig.1. The main composition in f l y ash is mullite,crystal silica and vitrif i ed slag,while iron mainly exists in the form of hematite.The utilization of f l yashis relatively diff i cultbecausethe f l y ashis cooleddown rapidly from a high temperature and the chemical activity of aluminum and silicon is undermined.
Ammonia sulfate used in the test is industrial grade,the water used is de-ionized water and the other reagents are analytical grade.
2.2.Procedures
The ammonium sulfate and f l y ash were mixed with a mass ratio of 7:1,and the mixture was placed in a ceramic crucible after thoroughly grinded in the ball mill.Then,the ceramic crucible was placed in a box oven to get the calcinations started.After that,the calcinate is placed in a beaker with a magnetic rotator,and an adequate amount of water was added to make the ratio of liquid to solid reach 10:1.The beaker was placed in the water-bath at the constant temperature of 90°C and the solution was stirred for 2 h.Finally,the leach liquor was f i ltered, and the residue was washed for 4 times usinghot water with a temperature of 90°C.The extraction rate of alumina is determined by the following equation:

Table 1Chemical composition of f l y ash

where A and B are themass ratios of aluminatosilicon dioxide in thef l y ash and residue,respectively.
X-ray Fluorescence(XRF,Bruker S8-TIGER)spectrometry is used for chemical composition analysis.A Ricoh D/max-RB XRD is used for examining the phase composition of solid matter.The JEOL JSM-6360LV SEM is used for observing SEM images of microstructure.
3.Results and Discussion
3.1.Effect of calcination temperature and time on the extraction rate of alumina
In order to discover the calcination kinetics of ammonium sulfate and f l y ash,the law of the change in alumina extraction rate of f l y ash in different calcining times and temperatures is studied.The result is shown in Fig.2.
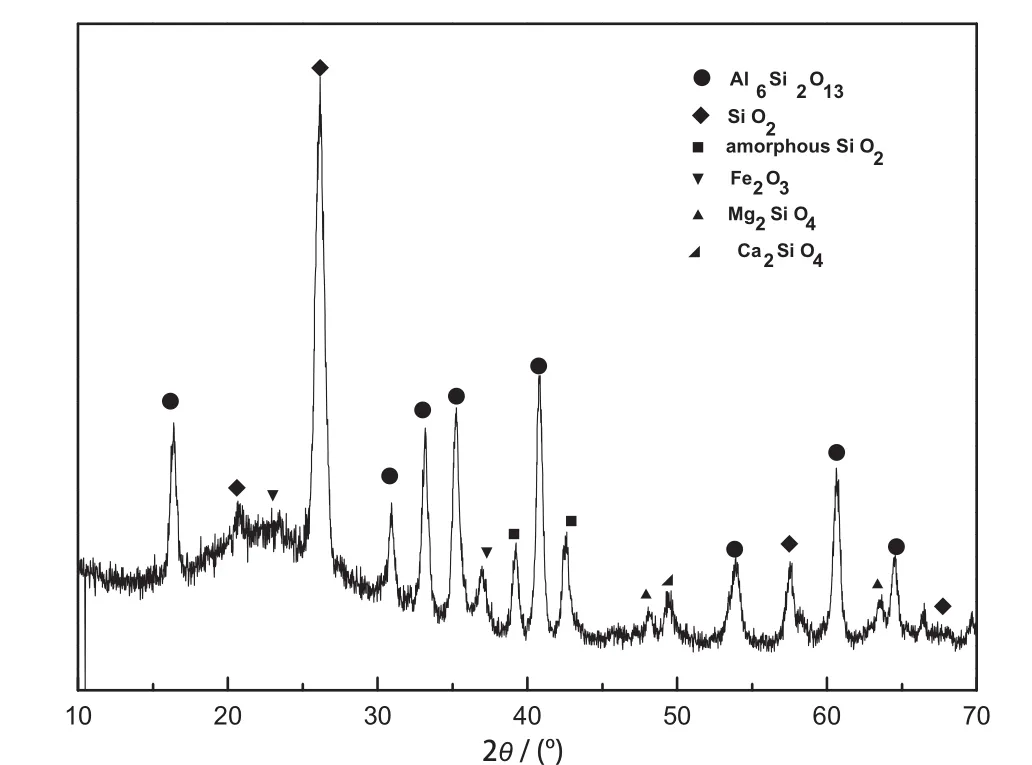
Fig.1.XRD pattern of f l y ash.
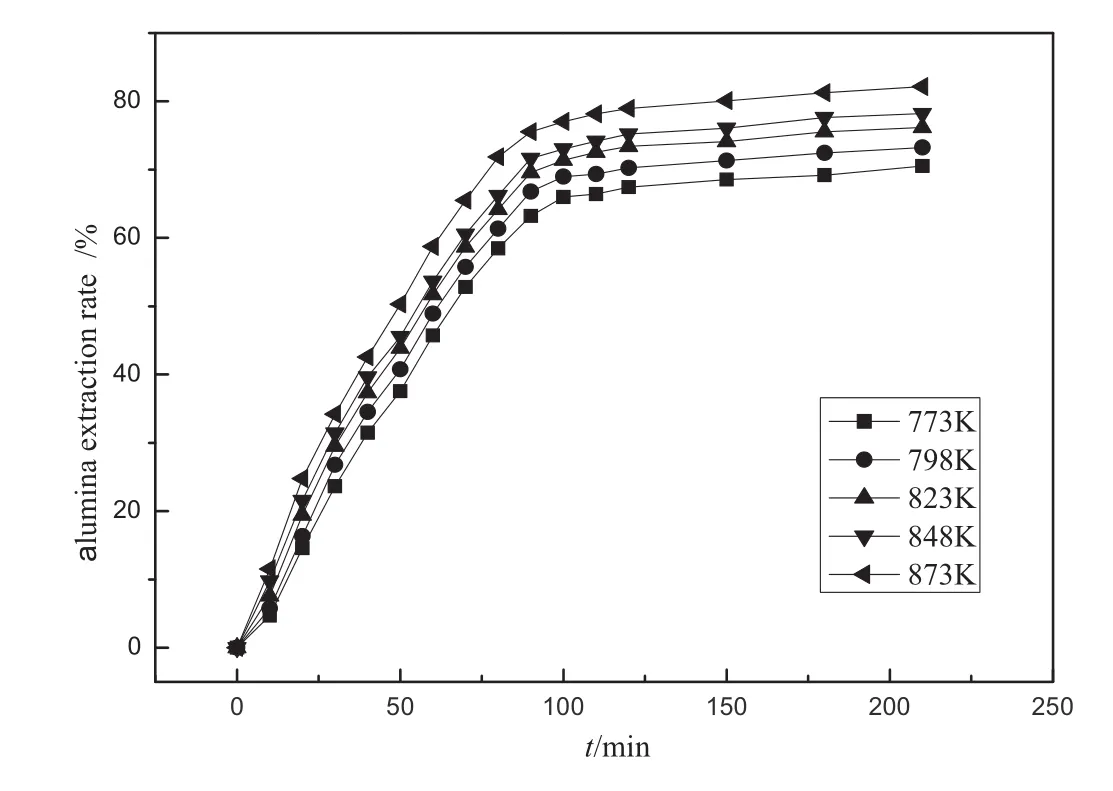
Fig.2.Effect on alumina extraction rate in different temperatures and durations.
Accordingto Fig.2,the factor of temperature is more affective on the alumina extraction rate,leading to a higher alumina extraction at a higher temperature.In the range of the studied temperature(773-873 K),the f l y-ash-alumina extraction rate grows rapidly in 0-90 min, but slows down between 90 and 210 min.At 873 K,the rate is up to 75.56%,however,it increases by only 6.62%at 210 min.In conclusion, the process of mixing calcinations can be divided into two stages: rapid reaction and slow reaction.
3.2.Calcination reaction kinetics analysis
Shrinking core model has some features as following:(1)There is a solid interface between unreacted solid phase and solid product; (2)the reaction takes place at the interface;(3)the reaction surface shrinks continuously from the outside to the center of the particle; (4)unreacted solid particles continuously shrink inward;and(5)the solid product layer will be constantly expanded inward which increases the thickness.The calcination system is a typical gas-liquid-solid heterogeneous reaction system and any part of the reaction could be the control step.However,in general,the reaction process is completely in the category of solid-solid and f l uid-solid reactions.Therefore,the kineticscanbedescribedin themodelofthesetworeactions,asis mentioned above[14-17].Assuming that the f l y ash is a spherical particle that the mesh size and the surface activity are homogeneous and the volume of the solid sample in calcination process changes little,the calcination reaction kinetics can be determined according to the shrinking core model,in which the reaction is controlled by internal and external diffusion aswell asthechemistryreaction.Thus,thealuminaextraction rateXanddurationtshouldf i tintoEqs.(2),(3)and(4),asismentioned below.The control step of the reaction is judged by if the relationship of durations and extraction f i ts into these specialized equations well [15-19].

where X is alumina extraction rate,k is the overall rate constant of reaction,t is reaction time.
To reveal the control step of the calcination process,the alumina extraction at various reaction times at 873 K is plotted,calculated by the left parts of Eqs.(2),(3)and(4).The result is shown below in Fig.3.
AccordingtoFig.3(a),whenprocessingthedataofaluminaextraction rate in the calcinations based on the shrinking core model,all the controlling-model-based characteristic functions and durations are not in the linear relationship.There is a deviation in every function at the time of 90-100 min,dividing the curve into two parts.It means that the rate control step or the reaction mechanism has changed during the calcinationprocess.Fittingthealuminaextractionrateat873Kincalcination reaction initial stage(0-90 min)and later stage(100-210 min)into all the controlling-model-based characteristic functions,and employing the linear regression analysis of the characteristic functions and reaction timebytheleastsquaremethod,theresultisshowninFig.3(b)and3(c). It is clear that in the initial stage characteristic function 1−(1−X)1/3= ktisthemostlinearrelatedwithtimet,whileinthelaterstagecharacteristic function 1−2X/3−(1−X)2/3=kt is the most linear related with time t.Thus,at the temperature of 873 K,the calcination process is controlled f i rst by chemical reaction and f i nally by interface diffusion.
To determine the kinetics parameters in different phases of the calcination process,the alumina extraction at various reaction temperatures is plotted based on the characteristic functions of 1−(1−X)1/3= kt and 1−2X/3−(1−X)2/3=kt in initial stage and later stage, respectively.The result is shown in Fig.4.
According to Fig.4,at the range of 727-827 K,the relationship between the extraction rate in the initial and later stages and the duration isthesamewiththelinearrelationshipshowninEqs.(3)and(4),which indicates that the initial stage f i ts in the chemical-reaction-controlled model,while the later stage suits the interface-diffusion-controlled model.Theapparentreactionrateconstantkintwostagescan bedetermined by the slope of the curves,as is listed in Table 2,in which k is higher in the initial stage than that in the later stage by one magnitude. Therefore,in the range of the temperature studied,the reaction mechanism does change during the calcination process.
Based on the apparent reaction rate constant of the initial and later stages measured in different temperatures,the apparent reaction energy of activation in each stage can be calculated according to Arrhenius formula(5)[20,21]:

where k0is the pre-exponential factor and Eais the apparent activation energy in the unit of J·mol−1.R is the molar gas constant which equals to 8.314 J·mol−1·K−1,while T is the thermodynamic temperature. Formula(6)is the natural logarithm form of formula(5):

The plot of reaction rate constant versus different temperatures is showninFig.5.Basedontheslopeofthecurves,theapparentactivation energy of the initial and later stages can be determined,which equals to 13.31 and 35.65 kJ·mol−1,respectively.The result proves further that the reaction mechanism has changed in the different stages.

Fig.3.Relationships between different models and reaction time at 873 K.Curve 1—F(X)=X;Curve 2—F(X)=1−(1−X)1/3;Curve 3—F(X)=1−2X/3−(1−X)2/3.

Fig.4.Relationship of characteristic functions and durations in different temperatures.

Table 2Apparent reaction rate constants of the initial and later stages of the calcinations in different temperatures
3.3.Reaction mechanism analysis
Thepossiblechemicalreactionsofcalciningthemixtureofammonia sulfate and f l y ash are listed below[22-24]:
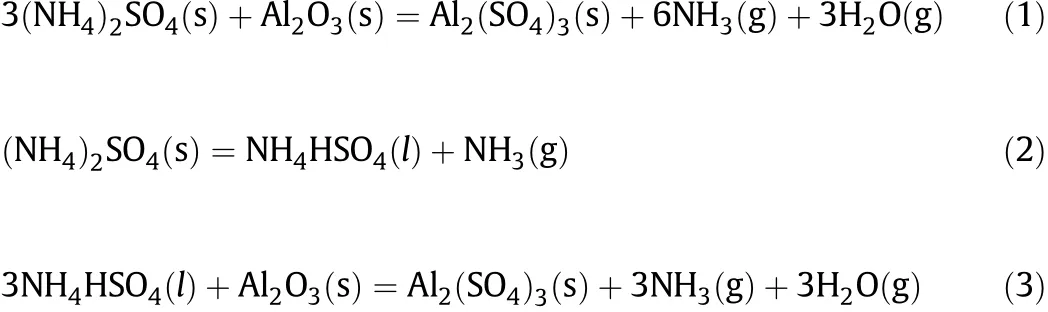
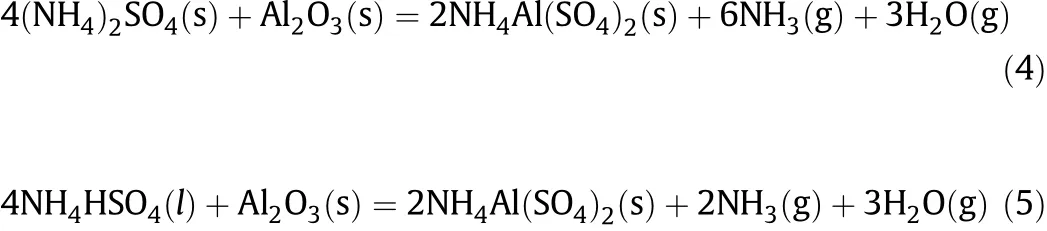
Throughthecalcinations,thealuminainf l yash can betransferredto soluble ammonium aluminum sulfate and aluminum sulfate.
In the condition of the temperatures of 573-723 K and 773-873 K, and duration of 3.5 h,the XRD patterns of the calcination product are shown in Fig.6.
From Fig.6(a),it is clear that at temperature of 573 K,the major product is ammonium sulfate,indicating apparently that no reaction has happened between f l y ash and ammonium sulfate.However, when thetemperature rises to673K,it is ammonium aluminum sulfate that becomes the major product,and it remains at up to 723 K.From Fig.6(b),at temperature of 773 K,the major product is still ammonium aluminum sulfate while a small amount of aluminum sulfate has occurred.As the temperature rises,the amount of ammonium aluminum sulfate has decreased and that of aluminum sulfate has enhanced. Thus,we can only get ammonium aluminum sulfate when the temperatureislowerthan 673K,andonlyifthetemperatureishigherthan873K can aluminum sulfate be formed.The results of thermodynamic calculation of reactions are in close agreement with the test ones[25].
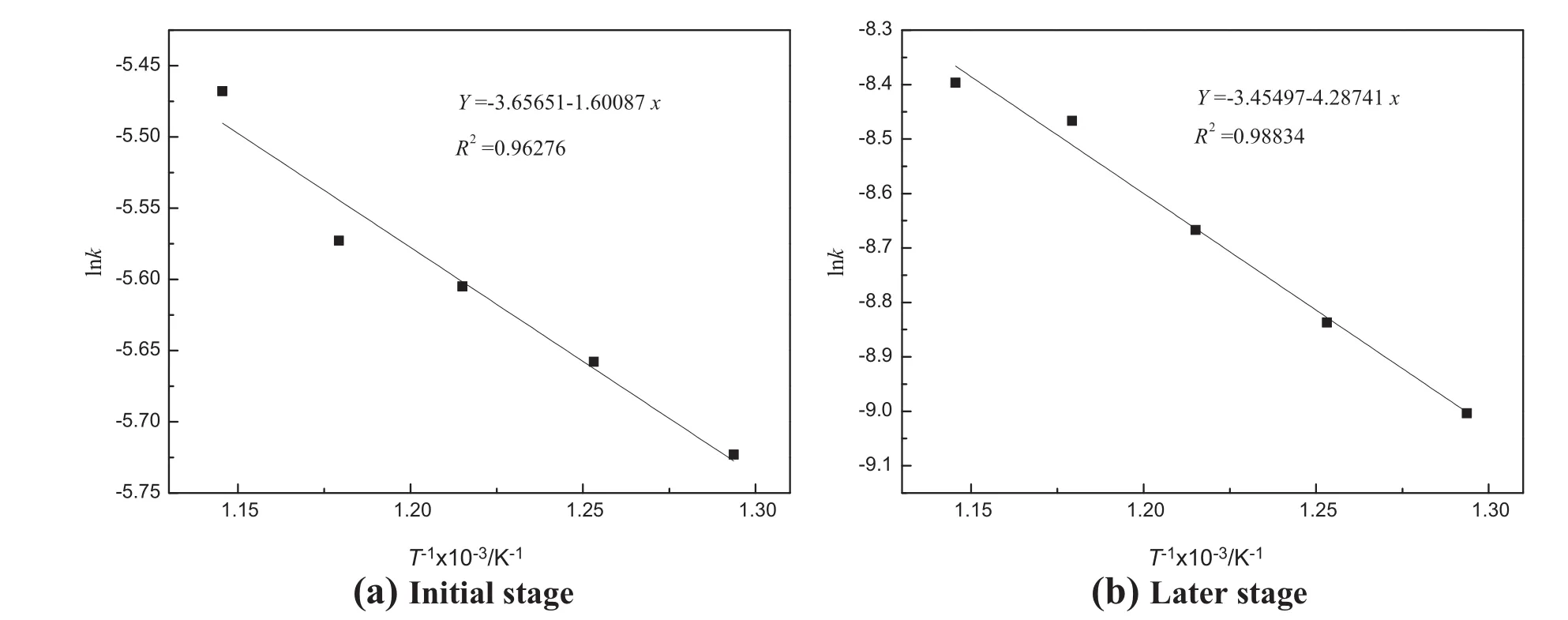
Fig.5.Relationship between K and temperature in the calcinations.

The SEM images of calcination product treated at 873 K with durations of 10,90 and 210 min are shown in Fig.7.
According to Fig.7,vitrif i ed slag mainly exists in small f l y ash particles,while porous vitrif i ed slag is rich in larger particles.At the duration of 10 min ammonium sulfate inf i ltrates at the surface of f l y ash but the structure is not completely changed.However,at 90 min the reaction has occurred between ammonium sulfate and f l y ash,making the superf i cial structure of the calcination product clear and integrated, whichcausesthemineralmorepolyporousandmakesthereactionfaster. The more regular crystal is formed at 210 min.Comparing the phase variationandthekineticsresultofthereaction,itiseasytoseethatintheinitial stage,ammonium sulfate reacts directly with mullite in the f l y ash to form ammonium aluminum sulfate.The apparent reaction energy of activation of the initial stage is lower and the reaction rate is faster.In the later stage,ammonium aluminum sulfate adsorbed on the surface of f l y ash reacts with ammonium sulfate to form aluminum sulfate.Its apparentreactionenergyofactivationishigher,whichmeansthatthereaction rateisslowerandahightemperatureisneededtocompletethereaction. Therefore,in order to make the product to be aluminum sulfate only,the later stage of the calcinations should be adequately aware of.
4.Conclusions

Fig.7.The SEM images of the product in different durations.
(1)In the calcination process of ammonium sulfate and the f l y ash, temperature highly affects the extraction rate of alumina and a higher temperature causes a higher extraction rate.The rate increases swiftly in the initial stage,but slows down increasing in the later stage.(2)There are two stages in the calcination process—initial stage and later stage.The former is controlled by a chemical reaction while the latter by interface diffusion.The apparent reaction energy of activation of initial and later stages equals to 13.31 and 35.65 kJ·mol−1,respectively.
(3)The reaction mechanism of the two stages is different:In the initial stage,ammonium sulfate reacts directly with mullite in the f l y ash to form ammonium aluminum sulfate.The apparent reaction energy of activation of the initial stage is lower and the reaction rate is faster.In the later stage,ammonium aluminum sulfate adsorbed on the surface of f l y ash reacts with ammonium sulfate to form aluminum sulfate.Its apparent reaction energy of activation is higher,which means that the reaction rate is slower andahightemperatureisneededtomakethereactioncomplete.
[1]J.D.Wang,Y.C.Zhai,X.Y.Shen,Alumina extraction from de-silicate f l y ash with lime sintering process,Light Met.6(2009)14-16.
[2]L.S.Li,Y.Y.Liu,Y.C.Zhai,Y.Wu,J.D.Wang,Alumina extraction from f l y ash by sulfuric acid calcinations,J.North.Univ.30(S2)(2009)169-172.
[3]K.Gborphlip,S.J.Hooque,Q.Charles,Dissolution behavior of Fe,Co,and Ni from non-ferrous smelter slag in aqueous sulphurdioxide,Hydrometallurgy 81(2006) 130-141.
[4]G.H.Bai,Y.H.Qiao,B.Shen,S.L.Chen,Thermal decomposition of coal f l y ash by concentrated sulfuric acid and alumina extraction process based on it,Fuel Process. Technol.92(2011)1213-1219.
[5]H.Liu,V.G.Papanglak,Thermodynamic equilibrium of the O2-ZnSO4-H2SO4-H2O system from 25 to 250°C,Fluid Phase Equilib.234(2005)122-130.
[6]R.L.Fang,S.Lu,X.B.Xie,The study of high-purity super-f i ne alumina powder preparation with f l y ash,Environ.Eng.5(2003)40-42.
[7]H.Zhao,S.Lu,The study of high-purity super-f i ne alumina powderpreparation with fl y ash,Compr.Util.Fly Ash 6(2002)8-10.
[8]S.Lu,R.L.Fang,H.Zhao,The study of high-purity super-f i ne alumina powder recovered from f l y ash by lime sintering self-powder method,Fly Ash 1(2003)15-17.
[9]Y.R.Ren,Z.K.Zhu,D.L.Xu,P.X.Fu,The study of high-purity alumina preparation by thermal decomposition of aluminum ammonium sulfate,Inorg.Chem.Ind.6(1991) 21-25.
[10]C.Chen,L.B.Evans,A local composition model for the excess Gibbs energy of aqueous electrolyte systems,AIChE J.32(1986)444-454.
[11]H.C.Park,Y.J.Park,R.Stevens,Synthesis of alumina from purity alum derived from coal f l y ash,Mater.Sci.Eng.367(2004)166-170.
[12]L.S.Li,Y.C.Zhai,J.G.Qin,Y.Wu,Y.Y.Liu,Extracting high-purity alumina from f l y ash, J.Chem.Ind.Eng.57(9)(2006)2189-2193(in Chinese).
[13]X.L.Jin,T.j Peng,H.J.Sun,Mineral composition and variation rule of calcinations products of ammonia sulfate and f l y ash,Acta Mieralogica Sinica 33(2)(2013) 147-151.
[14]C.S.Fu,Non-ferrous Metal Principle,Metallurgical Industry Press,Beijing,1993.
[15]K.Sun,Macro reaction Kinetics and Its Analytic Method,Metallurgical Industry Press,Beijing,1998.
[16]A.M.Ginstling,B.I.Brounshtein,Diffusion kinetics of reaction in spherical particles, Russ.J.Appl.Chem.23(12)(1950)1327-1338.
[17]D.S.Abrams,J.M.Prausnitz,Statistical thermodynamics of liquid mixtures:A new expression for the excess Gibbs energy of partly or completely miscible systems, AIChE J.21(1975)116-128.
[18]H.G.Li,The Principle of Metallurgy,Science Press,Beijing,2005.
[19]Z.Y.Zhang,J.H.Peng,Z.B.Zhang,Leachingzincfromspentcatalyst:Processoptimization using response surface methodology,J.Hazard.Mater.176(2010)1113-1117.
[20]A.Haghtalab,V.G.Papanglakis,X.Zhu,The electrolyte NRTL model and speciation approach as applied to multicomponent aqueous solutions of H2SO4,Fe2(SO4)3, MgSO4and Al2(SO4)3at 230-270°C,Fluid Phase Equilib.220(2004)199-209.
[21]K.Thomsen,P.Rasmussen,R.Gani,Correlation and prediction of thermal properties and phase behavior for a class of aqueous electrolyte systems,Chem.Eng.Sci.51 (1996)3675-3683.
[22]S.J.Wu,X.Yu,Z.H.Hu,L.L.Zhang,Optimizingaerobicbiodegradationof dichloromethane using response surface methodology,J.Environ.Sci.21(9)(2009)1276-1283.
[23]H.Liu,V.G.Papangelakis,Chemical modeling of high temperature aqueous processes,Hydrometallurgy 79(2005)48-61.
[24]K.Ravikumar,K.Pakshirajan,T.Swaminathan,K.Balu,Optimization of batch process parameters using response surface methodology for dye removal by a novel adsorbent,Chem.Eng.105(2005)131-138.
[25]L.S.Li,Y.Y.Liu,Thermodynamics of extracting alumina from f l y ash by ammonium sulfate calcinations process,Light Met.9(2009)12-14.
16 December 2013
☆Supportedbythe National Science andTechnology Projectof China(2012BAB01B00).
*Corresponding author.
E-mail address:wp_zx@sina.com(P.Wang).
http://dx.doi.org/10.1016/j.cjche.2014.06.033
1004-9541/©2014 The Chemical Industry and Engineering Society of China,and Chemical Industry Press.All rights reserved.
Received in revised form 17 March 2014
Accepted 29 April 2014
Available online 30 June 2014
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Preparation of a Novel Coal Gangue-Polyacrylamide Hybrid Flocculant and Its Flocculation Performance☆
- Oxygen Gasif i cation of Municipal Solid Waste in a Fixed-bed Gasif i er☆
- Thermodynamic Analysis of Methane-fueled Solid Oxide Fuel Cells Considering CO Electrochemical Oxidation☆
- In-situ IR Monitoring the Synthesis of Amphiphilic Copolymery P(HEMA-co-tBMA)via ARGET ATRP☆
- ComprehensiveAlcohol-/Ion-ResponsivePropertiesof Poly(N-Isopropylacrylamide-co-Benzo-18-Crown-6-Acrylamide)Copolymers☆
- Preparation and Application of the Sol-Gel Combustion Synthesis-Made CaO/CaZrO3Sorbent for Cyclic CO2Capture Through the Severe Calcination Condition☆
