二氰基亚甲基-四氢吡喃-苯并噻二唑的多功能逻辑门应用
2014-07-19喻艳华付成文丹贺贤然
喻艳华,付成,文丹,贺贤然
(江汉大学交叉学科研究院,湖北武汉430056)
二氰基亚甲基-四氢吡喃-苯并噻二唑的多功能逻辑门应用
喻艳华,付成,文丹,贺贤然*
(江汉大学交叉学科研究院,湖北武汉430056)
合成了基于二氰基亚甲基-四氢吡喃和苯并噻二唑的新型荧光化合物,并研究了该化合物通过改变二价铜离子和氟离子/溴离子滴加顺序对应的分子荧光现象。研究发现先加入二价铜离子导致该化合物的最大发射波长“蓝移”,继续滴加氟离子导致荧光淬灭,若继续滴加的是溴离子则荧光不变;反之先加入氟离子,该化合物荧光强度微弱降低,继续滴加铜离子对荧光强度影响不大,若滴加溴离子,该化合物荧光不变,继续滴加铜离子,最大发射波长强度降低,并且在485nm处出现新的荧光;这些荧光变化的机制也得到研究与证实。分子逻辑门是在分子水平上的逻辑操作来描述逻辑门,输入和输出信号。通过改变离子性质(加入氟离子或溴离子)、滴加顺序、激发波长获得不同的荧光发射波长和荧光强度,模拟了EnYES、EnNOT、EnIMP、EnINH、EnNAND逻辑门,1∶2信号分离器和键盘锁。
分子荧光;络合作用;离子识别;逻辑门;键盘锁
Biographies:YU Yanhua(1985—),female,assistant research fellow,majors in organic chemistry.
0 Introduction
The current"Information Age"requires an everincreasing amount of data storage and processing,whereas miniaturization of silicon-based electronic components is about to reach its limit[1].The con⁃cepts related to digital information technology have been developed on the basis of Boolean binary log⁃ic,meaning that information is stored and processed by a combination of two different values:0 or 1,cor⁃responding to the presence or absence of an electric current[2].The pioneering work of de Silva on molec⁃ular logic have demonstrated that information could be treated at the molecular level using chemical in⁃puts and fluorescence signals as outputs to mimic the function of an AND logic gate[3].Since then,sev⁃eral research groups have focused their efforts on the realization of molecular devices that could act as components for molecular computers;and the field of molecular logic developed from an academic curi⁃osity to a mature interdisciplinary research area with applications in a myriad of fields ranging from chemi⁃cal sensing,biological diagnosis,targeted therapy,information and security technologies[4-5].
Unlike classical electronic components that can be easily connected together to create devices with higher complexity,the"wiring"of molecular logic gates in a serial manner is challenged by a number of bottlenecks such as homogenization of inputs and outputs,physical integration of logic gates,decrease in propagation delay and increase of the fan-out[4]. Consequently,the approach consisting in the devel⁃opment of molecular logic gate arrays,that is,mole⁃cules not only mimicking a single logic gate but an entire electronic circuit,and able to perform multi⁃ple logic operations through reconfiguration of the in⁃puts and/or outputs,which is now emerging as a way to overcome these limitations[4].
Thanks to their excellent optical-electronic properties,dicyanomethylene-4H-pyran(DCM)devivatives have been investigated as OLED emit⁃ters,logic gates and optical chemosensors[4].Benzo⁃thiadiazole devivatives have also been used in the de⁃velopment of organic light-emitting materials or fluo⁃roionophores[6].As a continuing interest on the de⁃velopment of fluorescent molecules[7],we reported here in the synthesis,ion-sensing properties and functionnal integration into various arrays of logic gates of a DCM-benzothiadiazole conjugate mole⁃cule 7(Scheme 1).
1 Results and Discussion
In the context of developing new molecular de⁃vices for ion sensing and data processing,we were interested in the synthesis of fluorescent compound 7,designed by a combination of the azido-DCM 4 and triazolyl-BZT 6 dyes(Scheme 1).Our choice was motivated by the spectral overlap between BZT fluorescence emission(λem≈490 nm)and DCM ab⁃sorption(λmax≈460 nm),which should allow ob⁃servation of fluorescence around 605 nm upon either DCM excitation at 457 nm or BZT excitation at 370 nm.Such a cross-talk between the fluorophores wasexpectedtobeofinterestinthecontextofmolecular information handling,since various chemical stimu⁃li might affect either fluorophore to different extent.
1.1 Synthesis of fluorophores 4,6 and 7
Scheme 1 Synthesis of fluorophore 7 and model compounds 4 and 6
The target compound 7(Scheme 1)was effi⁃ciently synthesized from DCM azide 4 and known BZT derivative 5[8].Mesylation of hydroxy-aldehyde 1 followed by substitution with sodium azide in DMF at 90℃led to azide 2,which was reacted with 3 in the presence of piperidine to yield 75%of DCM 4 as the(E)-isomer exclusively[9].Mono-deprotection of 5 was achieved by careful treatment with a catalytic amount of KOH in MeOH,followed by copper-cata⁃lyzed Huisgen[3+2]cycloaddition of the crude product with azide 4,to afford the desired fluores⁃cent triazole 7 in 46%overall yield.Triazolyl-BZT 6 was obtained from 5 and ethyl 3-azidopropanoate[10]in 62%yield for the two steps using a similar proce⁃dure.
1.2 Ion sensing properties
The ability of 4,6 and 7 to act as chemical sen⁃sors for various anions and cations was investigated by following the evolution of absorbance and fluores⁃cence of 10 μM solutions in MeCN after addition of 8 equiv.ions.Upon excitation at 457 nm,4 exhibit⁃ed fluorescence emission centered around 610 nm,which remained unchanged after addition of various anions(Fig.1 ).In contrast,the presence of Cu2+re⁃sulted in a complete quenching of fluorescence,while Fe2+and Hg2+induced a decrease of fluores⁃cence intensity to ca.75%and 60%of their initial value,respectively.The fluorescence emission spec⁃tra of triazolyl-BZT 6 upon excitation at 370 nm are reported in Fig.2 .With a notable exception of F-,which induces TMS-alkyne deprotection[10]and re⁃sults in a slight fluorescence intensity decrease asso⁃ciated with a blue shift of the maximum from 496 to 489 nm,none of the tested anions affected fluores⁃cence emission of 6.Among the metal cations test⁃ed,Cu2+,Ni2+,and to a lower extent Hg2+,Co2+and Fe2+induced a significant decrease in fluorescence intensity.
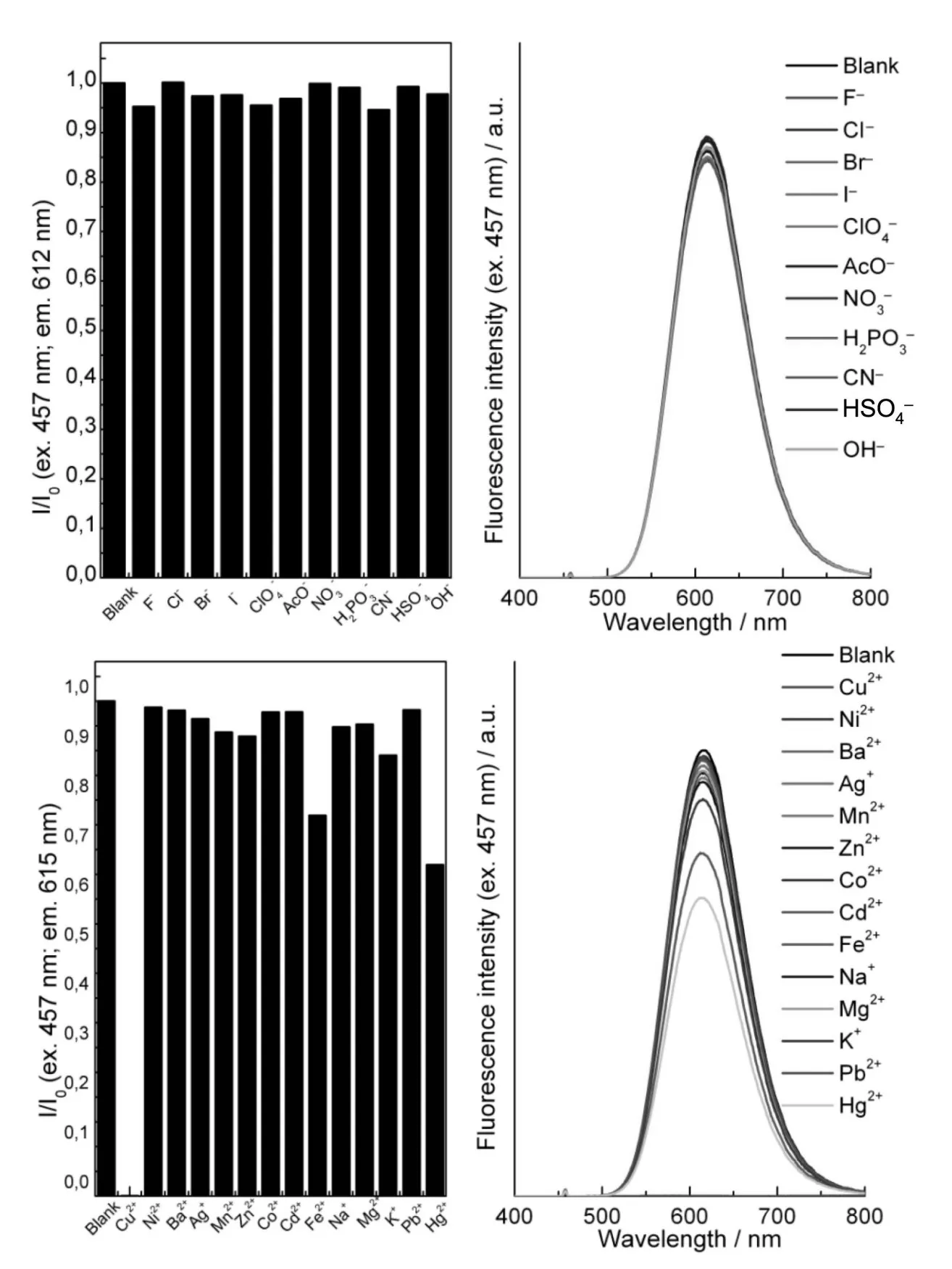
Fig.1 Fluorescence intensity ratio(left)and emission spectra(right)of 4(10μMin MeCN)after addition of8 equiv.of ions(λex=457 nm)
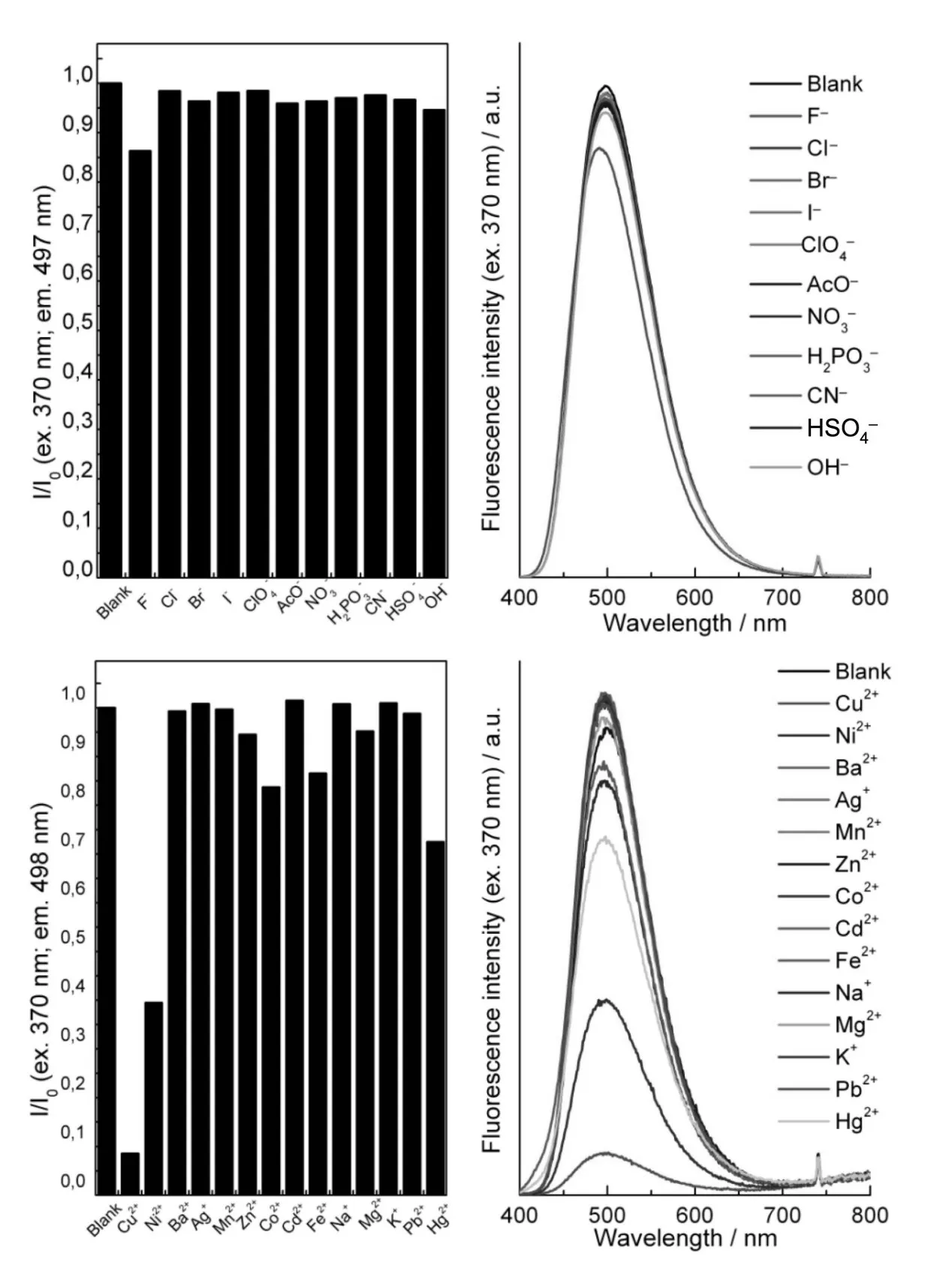
Fig.2 Fluorescence intensity ratio(left)and emission spectra(right)of 6(10μMin MeCN)after addition of8 equiv.ions(λex=370 nm)
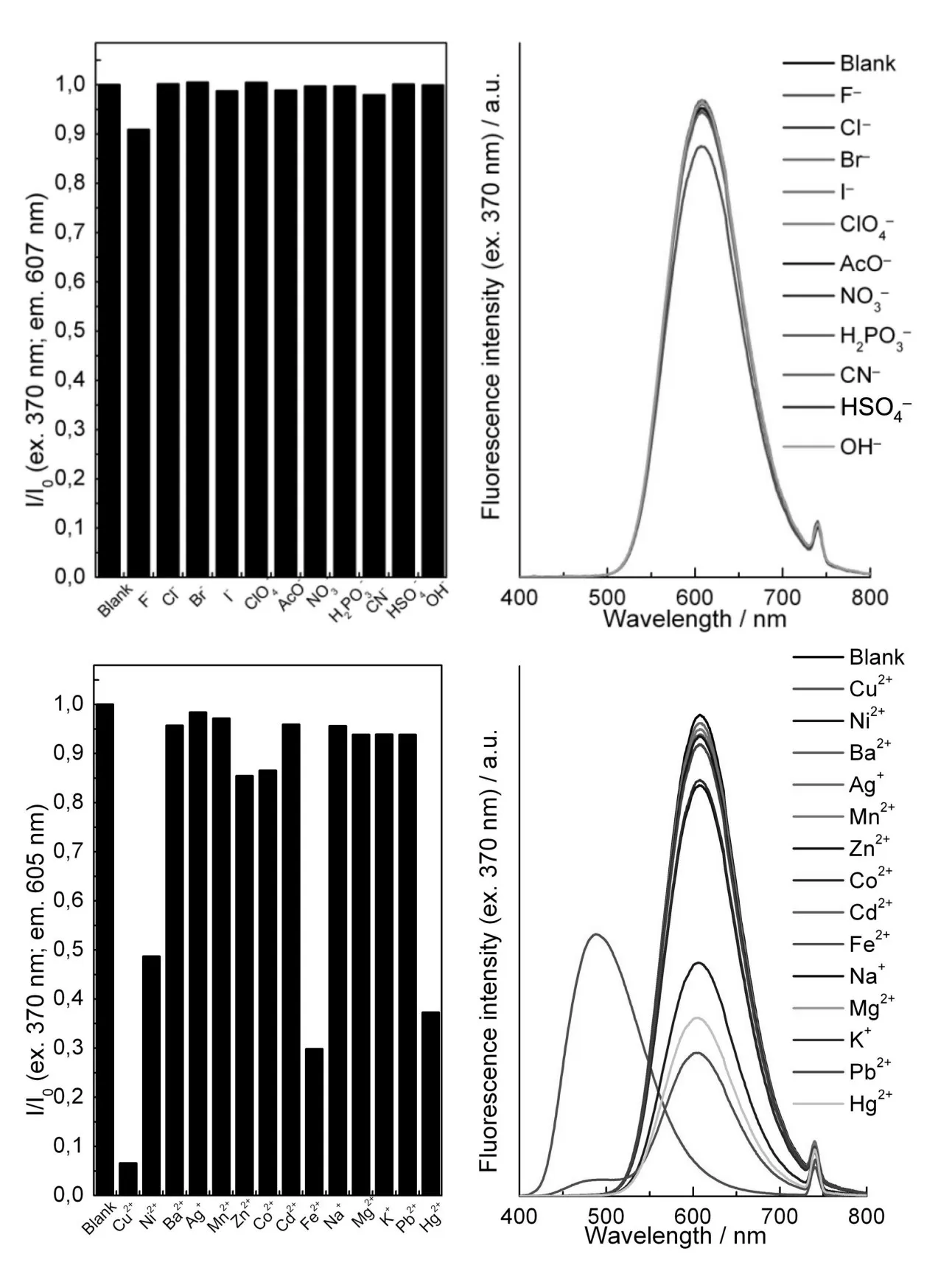
Fig.3 Fluorescence intensity ratio(left)and emission spectra(right)of 7(10μMin MeCN)after addition of8 equiv.of ions(λex=370 nm)
Our attention then turned to compound 7,re⁃garded as a combination of the previously studied flu⁃orophores 4 and 6(Fig.3 ).As expected,excitation of BZT(at 370 nm)resulted in fluorescence emis⁃sion at ca.605 nm,as a consequence of intramolecu⁃lar energy transfer.Addition of anions did not influ⁃ence the fluorescence intensity,except F–through deprotection of the TMS-alkyne moiety[11].In con⁃trast,addition of Cu2+immediately resulted in a dis⁃coloration of the solution,associated with the appari⁃tion of the characteristic blue fluorescence of BZT around 490 nm.This result clearly indicates that Cu2+affects the DCM moiety of 7 and quenches its fluorescence,which allows BZT fluorescence emis⁃sion to be observed.Among other metal cations test⁃ed,the most notable effects were exhibited by Ni2+,Fe2+and Hg2+,resulting in a ca.50%-70%quench⁃ing of fluorescence as compared to the blank sample.
1.3 Sequential ion addition
Having established the selectivity of ion recog⁃nition by fluorophores 4,6 and 7,our interest then focused on studying the effect of combining cations and anions in a sequence-dependent manner.Our choice turned to Cu2+as a cation,since it affects both DCM and BZT moieties;F-(which induces TMS-alkyne deprotection)and Br-(inert)were se⁃lected as anions,on the basis of their ability to form stable complexes of the type[Cu(X)n](2-n)+in MeCN,characterized by association constants in the range 107-1015[12].
The behavior of compound 4 was thus investi⁃gated in the presence of various combinations of Cu2+,F-and Br-(1 equiv.each,Fig.4 ).Addition of Cu2+resulted in an instantaneous and complete ex⁃tinction of fluorescence,which was only very weakly recovered after halide ions addition(Fig.4 a).This behaviour is in keeping with the transformation of 4 through Cu2+-mediated air oxidation of DCM as de⁃picted in Scheme 2.However,the oxidation mecha⁃nism remains to be clarified.Cu2+-promoted oxida⁃tion reaction is well known in living system and has been applied to fluorescence turn-on detection of Cu2+[13].In our case,pyran 8 was isolated as a com⁃plex mixture of regio and/or stereoisomers.This hy⁃pothesis was further confirmed by the observation that when Cu2+was added to a degassed solution of 4 in MeCN under inert atmosphere,no reaction oc⁃curred during at least 30 min.,as revealed by theabsence of discoloration of the red solution.In con⁃trast,the presence of F–or Br–in solution prior to Cu2+maintained a significantly high fluorescence in⁃tensity(ca.70%-90%of the blank value;Fig.4 b),by preventing 4 from air oxidation,presumably through formation of the complex[CuX]-.It to be no⁃ticed that oxidation of DCM devrivatives by Cu2+has not been reported before[5].
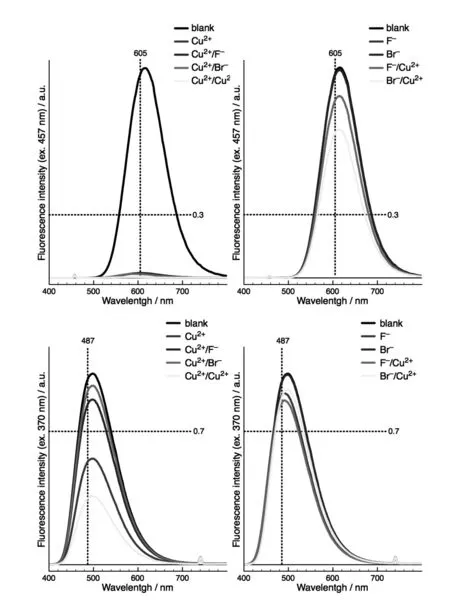
Fig.4 Emission spectra of 4(top:10μMin CH3CN,λex457 nm)and 6(bottom:10μMin CH3CN,λex370 nm)after addition of 1 equiv.Cu(ClO4)2,Bu4NF and/or Bu4NBr
Addition of 1 equiv.Cu2+to 6 induced a ca. 2-fold decrease in BZT fluorescence emission inten⁃sity,which further diminished when another equiva⁃lent Cu2+was added to the solution(Fig.4 c).In con⁃trast,when 1 equiv.F-or Br-was introduced after 1 equiv.Cu2+,fluorescence intensity was restored to reach ca.80%-90%of its initial level.Interestingly,when halide ions addition preceded Cu2+,the fluores⁃cence intensity of BZT remained almost unchanged(Fig.4 d).

Scheme 2Cu2+-mediated aerobic oxidative cyclization of 4 into 8
Such a behavior presumably originates from the Cu2+complexation between a nitrogen atom of benzo⁃thiadiazole and N(3)of triazole leading to complex[6.Cu][14-15],in which photoinduced electron trans⁃fer or charge transfer is likely to occur and affect the intensity of the fluorescence(Scheme 3)[8].Subse⁃quent halide addition results in the formation of com⁃plexes of the type[6.CuX],with de-coordination of one of the N-atoms.Further halide addition results in the regeneration of 6 and the formation of species of the type[Cu(X)n](2-n)+[11].Conversely,the pres⁃ence of halide ions prior to Cu2+addition presumably prevents formation of[6.Cu](through formation of[Cu(X)n](2-n)+),as revealed by the conservation of optical properties.
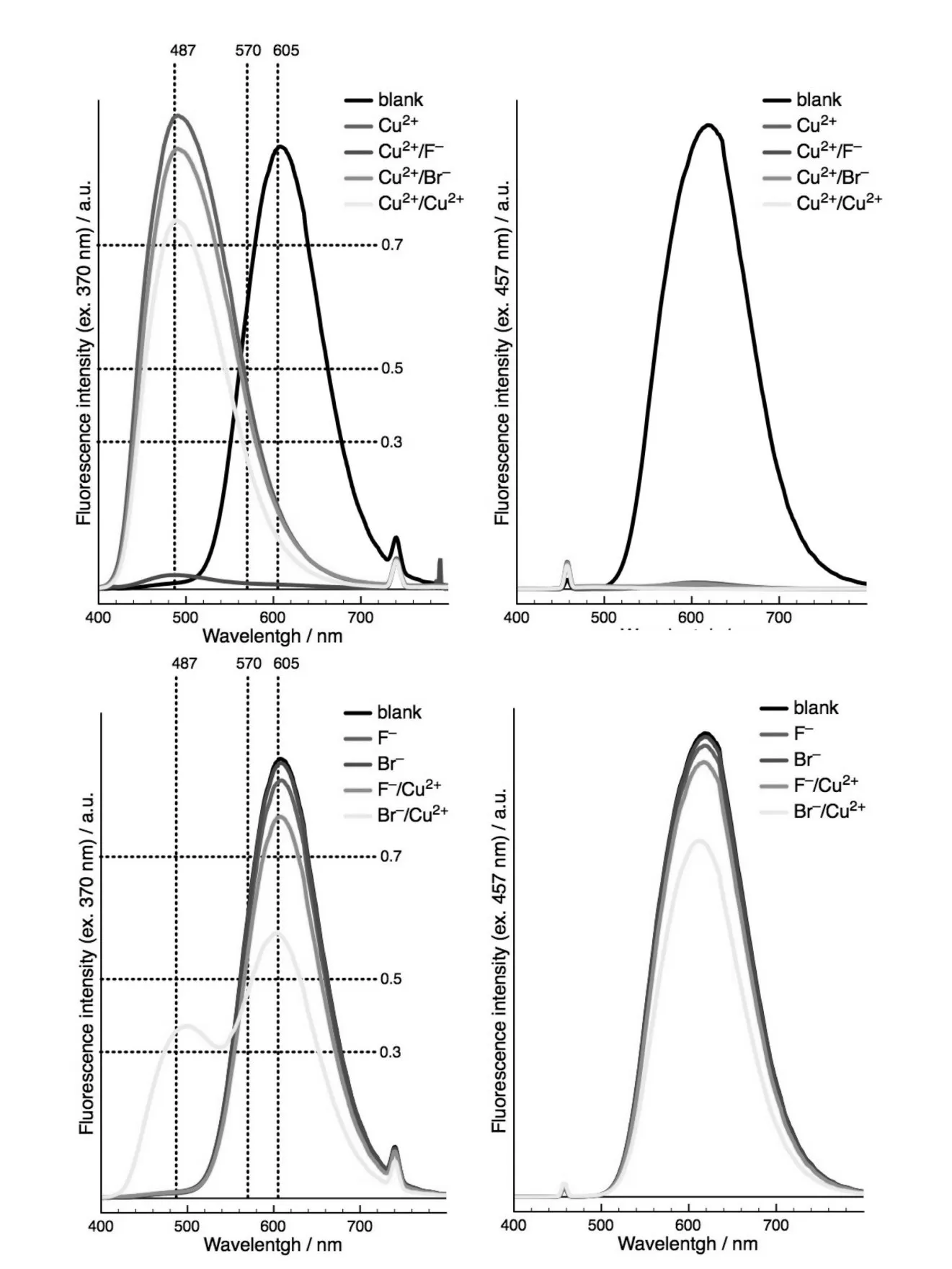
The spectroscopic and visual outcome of 7 in the presence of combinations of two sequential in⁃puts chosen from F-,Br-and Cu2+,at two excitation wavelengths(370 and 457 nm)are reported in Fig.5 and Fig.6 .The most dramatic impact was observed when Cu2+was associated with halide ions since such a combination proved to be dependent not only on the sequence of addition but also on the nature of the anion.Indeed,addition of Cu2+followed by F-result⁃ed in a complete extinction of fluorescence,while using Br-led to a conservation of BZT emission around 490 nm.The inversed sequence F-/Cu2+only induced a slight reduction of fluorescence intensity at ca.600 nm,whereas the couple Br-/Cu2+resulted in the apparition of an additional band around 490 nm. In the light of these studies,the behavior of 7 upon sequential addition of ions could be rationalized as follows:as a first input,F-affects BZT(TMS-al⁃kyne deprotection)without significantly modifying the fluorescence intensity of 7;Br-has no effect while Cu2+induces quenching of DCM fluorescence through oxidative dimerization(vide supra)thus re⁃vealing BZT fluorescence.

Fig.5 Solution of fluorophore 7 in MeCN(A),after sequential addition of F–/Cu2+(B),Br–/Cu2+(C),Cu2+/ F-(D)or Cu2+/Br–(E),upon excitation at 365 nm
When F-was present in solution prior to Cu2+ad⁃dition,no striking modification of fluorescence in⁃tensity of 7 could be observed;in contrast,addition of Cu2+following Br-leads to partial quenching of DCM fluorescence with concomitant partial appear⁃ance of BZT fluorescence.Such a behavior is pre⁃sumably the consequence of a competition between formation of complexes of the type[Cu(X)n](2-n)+and DCM oxidative cyclization.Utilization of Br-as a second input following Cu2+has no detectable ef⁃fect,whereas F–leads to complete quenching of fluo⁃rescence,revealing a modification of the BZT moi⁃etypossiblyinvolvingtheformationof acop⁃per-acetylide complex[16].
1.4 Keypad lock
The sequence-dependent character of 7 was ex⁃ploited for the realization of a molecular keypad lock able to deliver various secret codes,depending on the sequence of inputs(Fig.7 ).Indeed,attributing the letters"E","M"and"N"respectively to Cu2+,F-and Br-inputs,and"W","D"and"U"respec⁃ tively to red,blue and absence of fluorescence(up⁃on excitation at 365 nm,see Fig.5 )as outputs,the four words"END","MEW","EMU"or"NEW" could be read.
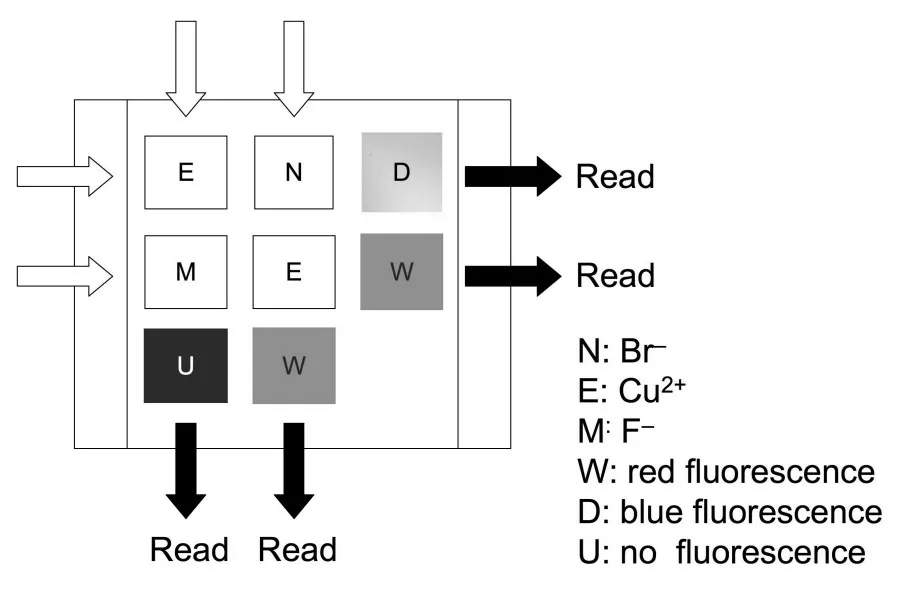
Fig.7 Compound 7 as a fluorescence-based molecular keypad lock
1.5 Logic gate arrays
The ion-responsive properties of fluorophores 4,6 and 7 were also employed to perform logic oper⁃ations.In the case of BZT 6,using both chemical and optical inputs(Cu2+,Br-and excitation at 370 nm),associated with the reading of fluorescence at 487 nm(relative threshold value of 0.7 as compared to the blank sample)as an output,the truth table depicted in Tab.1 was constructed(see Fig.4 c/d).Interest⁃ingly,6 performs enabled IMPLICATION(EnIMP)logic operation,that is,output will be 0 only in the presence of In1(Cu2+)alone.In3(λex370 nm)acts as the enabling channel,since no logic functionality could be observed when its value is 0;therefore,BZT 6 is a molecular mimic of the electronic logic circuit represented in Fig.8 a.

Fig.8 Logic circuits corresponding to the functions of(a)6 and(b)4
As a consequence of the sequence-dependent character of DCM 4(compare Cu2+/Br-and Br-/Cu2+in Fig.4 a/b),this compound can act as two logic cir⁃cuits performing either the functions enabled IN⁃VERTER(EnINV,resulting in output 0 when In1 is 1)when the order of addition is Cu2+/Br-,or EnIMP(resulting in output 0 only if In2 is present alone) when the order is Br–/Cu2+(Tab.1 ).The enabling optical input In3 is excitation at 457 nm,and a rep⁃resentation of the electronic circuits corresponding to the functions of 4 can be found in Fig.8 b.Note⁃worthy is the fact that same results were obtained for both 6 and 4 when F-was used instead of Br-.
In compound 7 however,the nature of the ha⁃
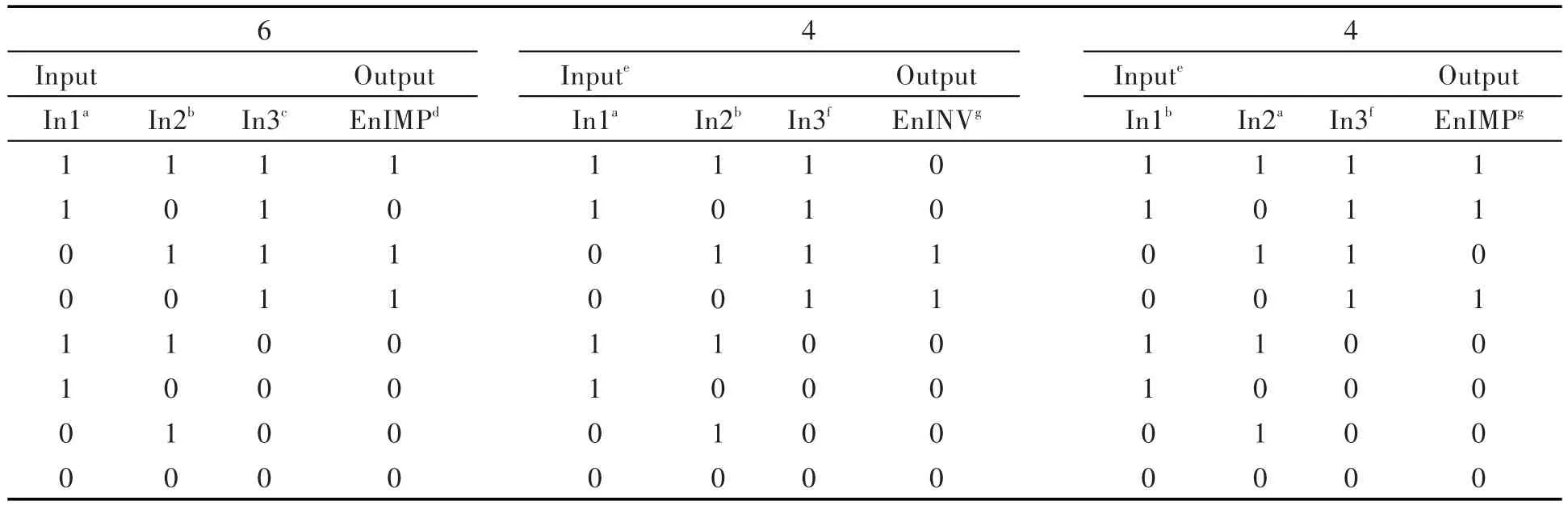
Tab.1 Truth table for compounds 6 and 4
lide(F-or Br-)also influences fluorescence emis⁃sion resulting in four molecular molecular logic gate arrays able to perform enabled YES(EnYES),en⁃abled NOT(EnNOT),EnIMP,enabled INHIBIT(EnINH)and enabled NOT AND(EnNAND)func⁃tions,through simple modification of the order of ion addition,the nature of the halide ion(F-or Br-)and the fluorescence reading wavelength and/or rela⁃tive intensity threshold values.For instance,using Cu2+and Br-as sequence-controlled In1 and In2,re⁃spectively,and BZT excitation at 370 nm as the en⁃abling In3,EnYES and EnNOT logic operations were revealed upon reading fluorescence at 487 nm(If,rel=0.3)and 605 nm(If,rel=0.3),respectively(Tab.2 a,Fig.6 and Fig.9 a).Practically,irrespec⁃tive of the value of In2,EnYES returns the value of In1,while EnNOT returns the opposite of In1.Re⁃versing the order of addition(Br–as In1 and Cu2+as In2)conversely lead to EnYES and EnNOT func⁃tions with respect to In2,whatever the value of In1(Tab.2 b,Fig.6 and Fig.9 b).In addition,EnIMP and EnINH functions were obtained by following flu⁃orescence emission at 605 nm(If,rel=0.3)and 487 nm(If,rel=0.5),respectively.When the logic device is enabled(i.e.In3 is 1),EnIMP returns 0 only when In2 is present and In1 absent,while En⁃INH returns 1 in the same situation.
Furthermore,compound 7 acts as a 1∶2 demul⁃tiplexer,an electronic device allowing the individu⁃al recovery of multiple signals transmitted on the same data line[4,17].
Depending on the value of the address input Ad(Cu2+),7 is able to deliver the value of input In(excitation at 370 nm)to either outputs O1 or O2(Tab.3 ),thus mimicking the circuit depicted in Fig.10 .
As mentioned above,the nature of the halide ion also influences the behavior of 7.Indeed,using F-as the first input followed by Cu2+,with excitation at 370 nm as the enabling input,resulted in EnNOT(λobs605 nm,thresholdIf,rel=0.3),EnINH(λobs487 nm,thresholdIf,rel=0.3)and En⁃NAND(λobs570 nm,thresholdIf,rel=0.3)logic functions(Tab.2 c,Fig.6 and Fig.9 c).The latter returns 0 when both In1 and In2 are 1.Noteworthy is the fact that NAND gate(together with NOR gate)is considered to be universal since it could be used to generate any logic function[2].Finally,when Cu2+is followed by F-,compound 7 exhibits the behavior of EnIMP(λobs605 nm,thresholdIf,rel=0.3)and EnINH(λobs605 nm,thresholdIf,rel=0.3)functions(Tab.2 d,Fig.6 and Fig.9 d).
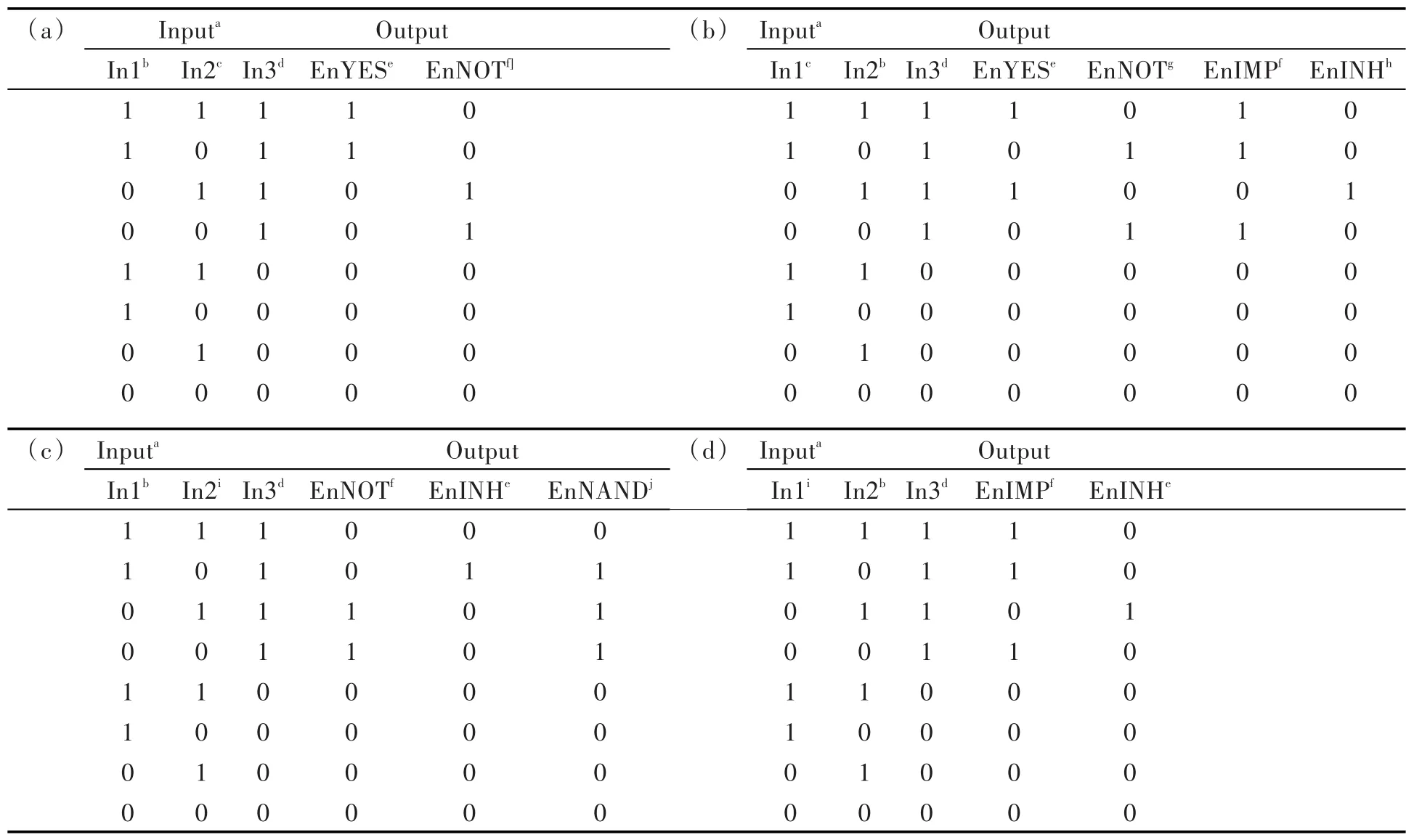
Tab.2 Truth table for compound 7
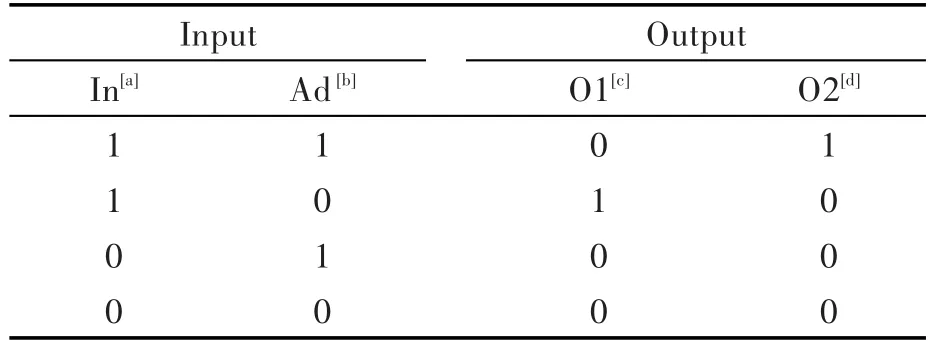
Tab.3 Truth table of a 1∶2 demultiplexer
2 Conclusion
Three novel fluorescent molecules based on DCM,triazolyl-BZT and a combination thereof were synthesized and exhibited a propensity to selectively modify their fluorescence behavior in the presence of some anions and cations.The Cu2+-promoted oxida⁃ tive dimerization of DCM moiety has been observed for the first time.Combinations of Cu2+,F-and/or Br-proved to be highly sequence-and halide-de⁃pendent for fluorescence emission in the case of DCM-based compounds 4 and 7.This property has been used to construct a keypad lock for compound 7.By appropriate selection of inputs(nature of ions,sequence of additions and excitation wavelength)and outputs(fluorescence reading wavelength and threshold),these fluorophores were able to mimick the functions of complex logic gate arrays performing up to the six logic operations EnYES,EnNOT,En⁃IMP,EnINH,EnNAND and 1∶2 demultiplexer. Although our system remains irreversible,this study might open the way for the design of new chromo⁃phores as molecular logic gates.
3 Experimental Section
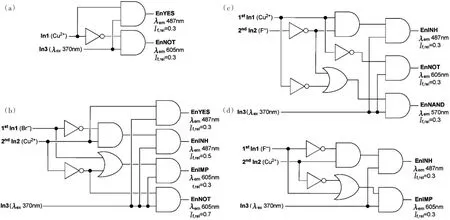
Fig.9 Logic circuits corresponding to the functions of compound 7
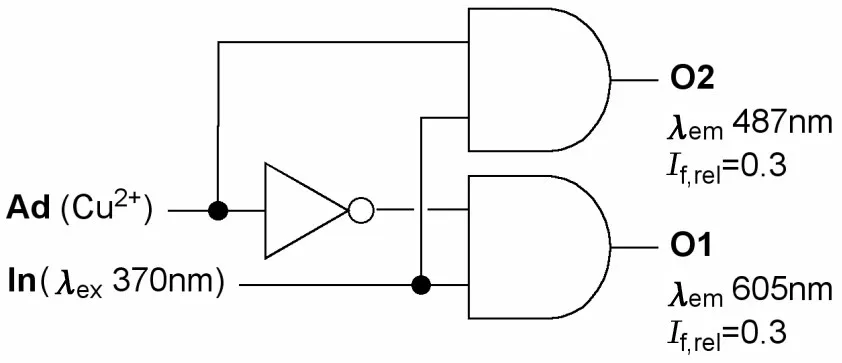
Fig.10 Molecular 1∶2 demultiplexer corresponding to the function of 7
General:Commercially available solvents and reagents were used without further purification ex⁃cept MeCN which was distilled over CaH2.Melting points were measured on a Kofler bench.Column chromatography was performed on Carlo Erba Silica Gel 60A(40-63 μm).Analytical thin layer chroma⁃tography was performed on E.Merck aluminum per⁃colated plates of Silica Gel 60F-254 with detection by UV.1H and13C-NMR spectra were recorded on a Jeol ECS-400 spectrometer.ESI-HRMS spectra were recorded on a Bruker microTOF-Q II spectrom⁃eter or Bruker maxis using standard conditions.IR spectra were recorded on Shimadzu FTIR-8400S spectrometer(Shimadzu Corporation,Kyoto,Ja⁃pan).Absorptionspectrawererecordedona Uvikon-940KON-TRONspectrophotometerand corrected emission spectra were performed on a Jo⁃bin-Yvon Fluoromax 3 spectrofluorometer(1 cm quartz cell was used).The fluorescence quantum yield(ΦF)was determined by the standard method using quinine sulphate in 0.5 M H2SO4as a refer⁃ence.The refractive index of the solvent was taken into account in the measurement[18].All anions were of the form(Bu4N+,X-),all cations of the form[Mn(+HClO4-)n],except AgNO3.
4-[N-(2-Azidoethyl)-N-methyl]amino⁃benzaldehyde 2:To a stirred solution of 4-[N-(2-hydroxyethyl)-N-methyl]aminobenzaldehyde(9.03 g,0.050 mol)in CH2Cl2(125 mL)at 0℃,were added respectively Et3N(21 mL,0.150 mol)and MsCl(5.8 mL,0.075 mol).The mixture was stirred for 20 to 30 min.until the brown color changedtoyellow.Themixturewastreatedwith150mL of water,the aqueous layer was extracted with CH2Cl2(2×50 mL).The organic layers were com⁃bined,washed with brine,dried over MgSO4and evaporated under vacuum to give 13.84 g of crude mesylate which was used without purification for the next step.To a solution of this crude product in DMF(90 mL)was added sodium azide(4.89 g,0.750 mol). The mixture was stirred at 90℃for 90 min.(moni⁃toring by TLC),cooled to RT and the solvent was evaporated under vacuum.The residue was parti⁃tioned in CH2Cl2/H2O(150/150 mL),the aqueous layer was extracted with CH2Cl2(2×50 mL),then or⁃ganic layers were combined,washed with saturated NaHCO3,dried over MgSO4and evaporated under vacuum.Column chromatography(gradient EtOAc/ petroleum ether 9/1,8/2,7/3,6/4)afforded 2(10.15 g,99%)as a brown oil.TLC:Rf=0.31(petroleum ether/EtOAc:2/1).1H NMR(400 MHz,CDCl3):δ=9.74(s,1H,CHO),7.74(d,J=8.7 Hz,2H,Ph),6.74(d,J=8.7 Hz,2H,Ph),3.63(t,J=5.9 Hz,2H,CH2),3.51(t,J= 6.0 Hz,2H,CH2),3.11(s,3H,N-Me).13C NMR(100 MHz,CDCl3):δ=190.4,153.0,132.5,126.1,111.2,77.3,51.6,48.9;39.2. HRMS(ESI)forC10H13N4O[M+H]+calcd 205.1084,found 205.1 088;for C10H12N4ONa[M+ Na]+calcd 227.090 3,found 227.090 4.
(E)-2-(2-(4-((2-Azidoethyl)(methyl)amino)styryl)-6-tert-butyl-4H-pyran-4-yli⁃dene)malononitrile 4:To a stirred solution of 4-[N-(2-azidoethyl)-N-methyl]aminobenzaldehyde(6.02 g,0.029 mol)and 2-(2-tert-butyl-6-meth⁃yl-4H-pyran-4-ylidene)malononitrile[19](6.32 g,0.029 mol)in distilled MeCN(60 mL)under ar⁃gon,was added piperidine(3.9 mL,0.039 mol). The mixture was refluxed for 3 to 4 h(monitoring by TLC)and cooled to RT.The filtered precipitate was washed with cold MeCN(10-20 mL)and cyclohex⁃ane(200-400 mL).The filtrate was evaporated un⁃der vacuum and the residue triturated with a mini⁃mum of cold MeCN and filtered(sonication could be used during trituration).Combination of the two sol⁃ids afforded 4(8.79 g,75%)as an orange-red sol⁃id.A portion of 4(6.2 g)was recrystallized from MeCN(110 mL).TLC:Rf=0.33(petroleum ether/ EtOAc:7/3).m.p.156°C.1H NMR(400 MHz,CD⁃Cl3):δ=7.45(d,J=8.7 Hz,2H,Ph),7.34(d,J=15.6 Hz,1H,CH=),6.73(d,J=8.7 Hz,2H,Ph),6.61(d,J=1.8 Hz,1H,CH=),6.53(d,J=1.8 Hz,1H,CH=),6.53(d,J= 1.8 Hz,1H,CH=),6.51(d,J=16.0 Hz,1H,CH=),3.63(t,J=6.0 Hz,2H,CH2),3.51(t,J=5.7 Hz,2H,CH2),3.11(s,3H,N-Me),1.38(s,9H,tBu).13C NMR(100 MHz,CDCl3):δ=172.0,160.1,156.9,150.3,138.2,129.9,123.2,115.84,115.77,113.6,112.1,105.8,102.5,77.2,58.2,51.6,49.0,39.0,36.8,28.2.HRMS(ESI)for C23H25N6O[M+H]+calcd 400.2007,found 401.208 8;for C23H24N6ONa[M+ Na]+423.190 4,found 423.190 3.
(E)-2-(2-Tert-butyl-6-(4-(methyl(2-(4-(7-((trimethylsilyl)ethynyl)benzo[c][1,2,5]thiadiazol-4-yl)-1H-1,2,3-triazol-1-yl)eth⁃yl)amino)styryl)-4H-pyran-4-ylidene)malo⁃nonitrile 7:To a solution of 4,7-bis((trimethylsi⁃lyl)ethynyl)benzo[c][1,2,5]thiadiazole 58(200 mg,0.61 mmol)in MeOH(15 mL)was add⁃ed 1M aqueous KOH(30 mL),and the mixture was stirred at RT under argon over night.After evapora⁃tion of the solvent under vacuum,the residue was dissolved in EtOAc(30 mL),washed with water(30 mL)and brine(30 mL),dried over MgSO4,fil⁃tered,and concentrated.To the obtained solid dis⁃solved in CH2Cl2(10 mL)and H2O(1 mL),were added 3(243 mg,0.61 mmol),CuSO4·5H2O(25 mg,0.1 mmol)and sodium ascorbate(40 mg,0.2 mmol). The reaction mixture was vigorously stirred at RT for 12 h,then CH2Cl2(20 mL)was added.The solution was washed with water(30 mL),dried over MgSO4,filtered,and concentrated to a solid which was puri⁃fied by column chromatography(petroleum ether/ EtOAc/CH2Cl2:3/3/1)to afford compound 7(300 mg,46%).TLC:Rf=0.34(petroleum ether/EtOAc:2/1).m.p.132℃.1H NMR(400 MHz,CDCl3):δ=8.65(s,1H,CH),8.50(d,J=7.4 Hz,1H,CH),7.88(d,J=7.4 Hz,1H,CH),7.42(d,J=8.7 Hz,2H,Ph),7.30(d,J=16.0 Hz,1H,CH),6.71(d,J=8.7 Hz,2H,Ph),6.60(d,J=2.3 Hz,1H,CH=),6.53(d,J=2.3 Hz,1H,CH=),6.47(d,J=16.0 Hz,1H,CH=),4.71(m,2H,CH2),4.05(m,2H,CH2),2.94(s,3H,CH3),1.37(s,9H,3×CH3),0.34(s,9H,Si(CH3)3).13C NMR(100 MHz,CDCl3):δ=172.0,159.9,156.8,155.0,151.4,149.8,143.1,137.9,134.2,129.9,125.2,125.1,123.6,123.5,116.0,115.7,115.6,113.9,112.2,105.9,102.5,100.2,58.3,52.7,47.8,39.0,36.7,28.1,0.0.IR(KBr):2 207,1 642,1 597,1 548,1 498,1 421,1 377,1 304,1 252,1 175,1 119,1 053,928,848,750 cm-1.UV/Vis(MeCN):λmax(ε)=457 nm(40 700 L·mol–1·cm–1). ΦF(λex372 nm)=(12±1.2)%.HRMS(ESI)for C36H37N8OSSi[M+H]+:calcd 657.250 2,found 657.257 1.
Ethyl 3-(4-(7-((trimethylsilyl)ethynyl)benzo[c][1,2,5]thiadiazol-4-yl)-1H-1,2,3-triazol-1-yl)propanoate 6:To a solution of 4,7-bis((trimethylsilyl)ethynyl)benzo[c][1,2,5]thi⁃adiazole 5[5](200 mg,0.61 mmol)in MeOH(15 mL)was added 1M aqueous KOH(30 mL),and the mix⁃ture was stirred at rt under argon over night.After evaporation of the solvent under vacuum,the resi⁃due was dissolved in EtOAc(30 mL),washed with water(30 mL)and brine(30 mL),dried over Mg⁃SO4,filtered,and concentrated.To the solid dis⁃solved in CH2Cl2(10 mL)and H2O(1 mL),were added ethyl 3-azidopropanoate(87 mg,0.61 mmol),CuSO4·5H2O(25 mg,0.1 mmol)and sodium ascor⁃bate(40 mg,0.2 mmol).The reaction mixture was vigorously stirred at RT for 12 h,then CH2Cl2(20 mL)was added.The solution was washed with water(30 mL),dried over MgSO4,filtered,and concen⁃trated to a solid which was purified by column chro⁃matography(petroleumether/EtOAc:10/1)to afford compound 6(250 mg,62%).TLC:Rf=0.36(petroleum ether/EtOAc:15/1).m.p.124℃.1H NMR(400 MHz,CDCl3):δ=8.84(s,1H,CH),8.50(d,J=7.4 Hz,1H,CH),7.88(d,J=7.4 Hz,1H,CH),4.79(t,J=6.4 Hz,2H,CH2),4.17(q,J=7.2 Hz,2H,CH2),3.06(t,J=6.4 Hz,2H,CH2),1.25(t,J=7.2 Hz,3H,CH3)0.34(s,9H,Si(CH3)3),13C NMR(100 MHz,CDCl3):δ=170.4,154.9,151.4,142.8,134.2,125.1,123.9,115.8,102.2,100.3,61.3,45.8,34.8,14.1,0.0;IR(KBr):2 146,1 729,1 591,1 464,1 442,1 404,1 378,1 249,1 197,1 163,1 096,838,850,757 cm-1.UV/Vis(MeCN):λmax(ε)=390 nm(11 800 L·mol–1·cm–1). ΦF(λex368 nm)=(79±7.9)%.HRMS(ESI)for C18H22N5O2SSi[M+H]+:calcd 400.1185,found 400.126 3.
Copper(II)-catalyzed oxidative cyclisation of 4:To a solution of 4(50 mg,0.125 mmol)in 3 mL MeCN was added Cu(ClO4)2·6H2O(46 mg,0.125 mmol).After stirring at RT for 5 min,the sol⁃vent was removed under vacuum to give a green solid which was exclusively soluble in MeCN.The green solid was dissolved in 5 mL MeCN,washed with 1M EDTA,then extracted with EtOAc(2×20 mL).The combined organic layers were dried over MgSO4,evaporated to give a yellow solid which was purified by column chromatography(petroleum ether/EtO⁃Ac:2/1)to afford 10 mg of compound 8 as a light yellow solid.TLC:Rf=0.34(petroleum ether/EtO⁃Ac:1/1).HRMS(ESI)for C46H49N12O3[M+H]+:calcd 817.404 5,found 817.404 3.
Procedure for ion addition
Stock solutions of compounds 4,6 or 7 were prepared in MeCN at a concentration of 10-3M.Solu⁃tions of Cu(ClO4)2,Bu4NF,Bu4NBr were prepared in MeCN at a concentration of 10-2M.In a quartz cu⁃vette,25 μL stock solutions of compounds 4,6 or 7 were diluted with 2 475 μL MeCN(final concen⁃tration of 10 mM),then 2.5 μL of a solution of Cu2+,F-or Br-was added.The mixture was shacked for 1 min(followed by a second addition and shack⁃ing for another minute if necessary)before recording absorption and fluorescence spectra.
4 Acknowledgments
Thanks to RUAN Yibin for helpful discussions.
[1]MATHARU A S,JEEVA S,RAMANUJAM P S.Liq⁃uid crystals for holographic optical data storage[J]. Chem Soc Rev,2007,36:1868-1880.
[2]HAYES J P.Introduction to Digital Logic Design[M]. Boston:Longman Publishing Co.,Inc.,1993.
[3]DE SILVA A P,GUNARATNE H Q N,MCCOY C P. A molecular photoionic AND gate based on fluorescent signalling[J].Nature,1993,364:42-44.
[4]DE SILVA A P.Molecular logic gate arrays[J].Chem Asian J,2011,6:750-766.
[5]MARGULIES D,FELDER C E,MELMAN G,et al.A molecular keypad lock:a photochemical device capa⁃ble of authorizing password entries[J].J Am Chem Soc,2007,129:347-354.
[6]FANG Q,XU B,JIANG B,et al.Bisindoles contain⁃ing a 2,1,3-benzothiadiazole unit:novel non-doping red organic light-emitting diodes with excellent color purity[J].Chem Commun,2005,11:1468-1470.
[7]DAVID O,MAISONNEUVE S,XIE J.Generation of new fluorophore by click chemistry:synthesis and prop⁃erties of β-cyclodextrin substituted by 2-pyridyl tri⁃azole[J].Tetrahedron Lett,2007,48:6527-6530.
[8]DASILVEIRA NETO B A,SANT′ANA LOPES A,EB⁃ELING G,et al.Photophysical and electrochemicalproperties of π-extended molecular 2,1,3-benzothiadi⁃azoles[J].Tetrahedron Lett,2005,61:10975-10982.
[9]ANDREU R,CARRASQUER L,GARIN J,et al.New one-andtwo-dimensional4H-pyranylideneNLO-phores[J].Tetrahedron Lett,2009,50:2920-2924.
[10]REUX B,WEBER V,GALMIER M J,et al.Synthesis and cytotoxic properties of new fluorodeoxyglucose-cou⁃pled chlorambucil derivatives[J].Bioorg Med Chem,2008,16:5004-5020.
[11]LU H,WANG Q,LI Z,et al.A specific chemodosime⁃ter for fluoride ion based on a pyrene derivative with tri⁃methylsilylethynylgroups[J].OrgBiomolChem,2011,9:4558-4562.
[12]SESTILI L,FURLANI C,CIANA A,et al.Polarogra⁃phy of copper(II)-halide complexes in non-aqueous solvents[J].Electrochim Acta,1970,15:225-235.
[13]WANG D,SHIRAISHI Y,HIRAI T.A BODIPY-based fluorescent chemodosimeter for Cu(II)driven by an oxi⁃dative dehydrogenation mechanism[J].Chem Com⁃mun,2011,47:2673-2675.
[14]PAPAEFSTATHIOU G S,TSOHOS A,RAPTOPOU⁃LOU C P,et al.Cystal engineering:stacking interac⁃ tions control the crystal structures of benzothiadiazole(btd)and its complexes with copper(II)and copper(I)chlorides[J].Cryst Growth Des,2001(1):191-194.
[15]TAMANINI E,RIGBY S E J,MOTEVALLI M,et al. Responsive metal complexes:a click-based"allosteric scorpionate"complex permits the detection of a biologi⁃cal recognition event by EPR/ENDOR spectroscopy[J]. Chem Eur J,2009,15:3720-3728.
[16]CHOW Y L,BUONO-CORE G E.Triplet-state benzo⁃phenone-sensitized photoreduction of bis(acetylaceto⁃nato)copper(II):the generation and stability of copper(I)complexes[J].Can J Chem,1983,61:795-800.
[17]ANDRÉASSON J,PISCHEL U,STRAIGHT S D,et al. All-photonic multifunctional molecular logic device[J].J Am Chem Soc,2011,133:11641-11648.
[18]LAKOWICZ J R.Principles of fluorescence spectrosco⁃py[M].3rd ed.New York:Springer Science,2006.
[19]YAO Y S,XIAO J,WANG X S,et al.Starburst DCM-type red-light-emitting materials for electrolumi⁃nescence applications[J].Adv Funct Mater,2006,16:706-718.
(责任编辑:陈旷)
2013-12-15
The National Natural Science Foundation of China(No.21302065)
O644;O69
A
1673-0143(2014)02-0049-12
*Corresponding Author:HE Xianran(1983—),male,associate research fellow,doctor,majors in sugar chemistry and pharmacochemistry.E-mail:hexianran@163.com
