Effects of Colloidal Silica Binder on Catalytic Activity and Adhesion of HZSM-5 Coatings for Structured Reactors☆
2014-07-12GuozhuLiuJinhuaGuoFanxuMengXiangwenZhangLiWang
Guozhu Liu**,Jinhua GuoFanxu Meng**,Xiangwen ZhangLiWang*
1Key Laboratory of Green Chemical Technology of Ministry of Education,School of chemical engineering and Technology,Tianjin University,Tianjin 300072,China
2Department of chemical engineering,Texas A&MUniversity,College Station,TX 77843,USA
Effects of Colloidal Silica Binder on Catalytic Activity and Adhesion of HZSM-5 Coatings for Structured Reactors☆
Guozhu Liu1,**,Jinhua Guo1,Fanxu Meng2,**,Xiangwen Zhang1,LiWang1,*
1Key Laboratory of Green Chemical Technology of Ministry of Education,School of chemical engineering and Technology,Tianjin University,Tianjin 300072,China
2Department of chemical engineering,Texas A&MUniversity,College Station,TX 77843,USA
A R T I C L E I N F o
Article history:
Received 24 December 2013
Received in revised form 6 January 2014
Accepted 17 February 2014
Available online 18 June 2014
HZSM-5 coating
colloidal silica binder
Catalytic activity
Structured reactor
HZSM-5 coating using three colloidal silica binders,acidic colloidal silica(ACS),neutralcolloidal silica(NCS)and basic colloidal silica(BCS),was prepared to study the effect of binders on their adhesion and catalytic activity. Scanning electron microscopy characterization indicated that the zeolite coating using BCS shows the smoothest surface with higher homogeneity and adherence strength.The specific surface area,relative crystallization and acid site strength of zeolites are also dependent on the binder used.Catalytic cracking of supercritical ndodecane over the series of zeolite coating with various binders indicated that HZSM-5 coating with BCS exhibits the highest and the most stable catalytic activity compared with other kinds of binders,and also exhibits a stable catalytic activity ascribed to its proper acid property and microstructure.
©2014 The Chemical Industry and Engineering Society of China,and ChemicalIndustry Press.Allrights reserved.
1.Introduction
As speed of aircraft reaching supersonic or hypersonic regimes, aerodynamic heat will raise vehicle heat load beyond the scope that structure materials could bear.Under such situations,hydrocarbon fuel,as an ideal coolant,can offer suf fi cient cooling capacity (heatsink)for supersonic aircrafts,through both significant physical sensible heat and heat-adsorbing chemical reactions[1-3].In the cooling system of advanced aircraft,the hydrocarbon fuelis under high pressure(3.4-6.9 MPa)and high temperature(above 400°C), i.e.,the supercritical conditions.Supercritical catalytic cracking of hydrocarbon fuels over structured catalyst(zeolite coatings in cooling microchannels)has been highlighted in previous works as an attractive method of improving cooling capacity of hydrocarbon fuels for the advanced aircrafts[4-6].The catalyst loading method offers a low pressure drop,efficient mass and heattransfer,high geometric surface area,enhanced thermal stability,high mechanical strength,and better control of product selectivity[3].
Washcoating(or dip-coating)is an easy and efficient method to prepare ready-made zeolite coatings on the fine channels of cooling structure[7].Generally,the preparation of the zeolite coatings always involves washing the substrates with slurry,blowing air to remove the excess liquid,drying,and calcination.From a technologicalpoint of view,there were also some essentialfactors to prepare desirable coatings including particle size of zeolites,viscosity and rheological properties of the slurries,the number of washcoating,the blowing conditions,as well as the binder used[7-9].The binders added in the slurries are very necessary to help zeolite coating produce desired morphology and mechanical strength for practical applications and to avoid their poor self-binding property.Although they may not have catalytic property,binders could influence the physical and acidic properties of zeolites as a result of changes in the proton-exchange efficiency,trapping by the binder of coke precursors and/or blocking of zeolite channels when they are introduced[10-12].This change can have a strong influence on the catalytic performance in chemical reaction process. Also,binders will significantly affect the microstructure of coating when being used in washcoat slurry.The viscosity of slurry,the adhesion,thickness,loading and morphology of coating are alltightly related to the binder introduced.All these influences lead to the difference in microstructure when different binders were added,consequently resulting in various performances in catalytic reaction and adhesion. Some researchers also noted that binders help to increase the washcoat adhesion,butlittle work has been done on the effect of binders on the catalytic cracking activity of zeolite coating for the hydrocarbon fuels, which is one of the most importantissues in the developing and designing structured catalyst(wall-coated zeolite catalyst).
Colloidal silica is a common binder used in preparing zeolite washcoating.Peters and coworkers deposited composite catalytic zeolite H-USY layers on silica membranes by dip-coating using acidic TEOS suspension and Ludox AS-40 as binder material[13].Boix and coworkers used silica binders to washcoat PtCo/Ferrierite on a ceramic monolith to improve the adherence and to obtain a selective catalyst [14].Mitra and Kunzru prepared several zeolites(ZSM-5,β-zeolite,mordenite,and zeolite Y)washcoatings on cordierite monoliths with colloidal silica binders to establish the relationship between washcoat characteristics and the powder and slurry properties[7].Meng et al. and Qu etal.also applied neutralcolloidal silica to prepare HZSM-5 zeolite coatings on 304 SS tube reactorforcatalytic cracking of n-dodecane [4-6,15,16].Therefore,in this work colloidal silica binder was selected to study the effect ofbinder on the physicaland catalytic activities of HZSM-5 coatings.
The objective of this work is to study the effect of colloidal silica binders on the microstructure and catalytic performance of HZSM-5 coating.HZSM-5 coating was prepared using three colloidal silica binders with different pH values:acidic colloidal silica(ACS),neutral colloidal silica(NCS)and basic colloidal silica(BCS).The effects of binders on the surface,crystal and acidity properties were characterized using SEM,XRD,and NH3-TPD.The adhesive strength of the prepared coatings on the substance was also studied.The catalytic activity and stability of the prepared coatings with different binders were studied using catalytic cracking of supercritical n-dodecane.It is recommended that BCS leads to the smoothest surface and best strength and helps HZSM-5 coating to keep higher conversion in a wider range of zeolite loading and reduce coke accumulation due to its microstructure and activity.
2.Experimental
2.1.Materials
HZSM-5 zeolites(SiO2/Al2O3mole ratio is 140)with the particle size of2-5μm were purchased by Nankai University Catalyst Plant(Tianjin, China).Before experiment,fresh zeolites were calcined at450°C for2 h. n-Dodecane(purity 99%,Sinopharm Chemical Reagent Co.,Shanghai, China)was used as received.Three binders are used:acidic colloidal silica(ACS)was prepared by mixing TEOS,ethanol,water and 65%nitric acid(composition by mass percent:38.4:32.2:27.1:1.0)together and stirring for 2 h under 60°C;neutral colloidal silica(NCS,ZA-25)and basic colloidal silica(BCS,JX-25)were commercially obtained from Qingdao Haiyang Chemical Co.,China.Table 1 shows the properties of binders used in this work.
Dynamic light scattering(DLS)measurements of colloidal silica were performed at25°C and 514 nmusing BI200SMdynamic light scattering apparatus(Brookhaven).The hydrodynamic radius distribution was determined from the Laplace inversion of the measured intensityintensity time correlation function using the CONTIN program on the basis of the Stokes-Einstein equation.
2.2.Preparation of zeolite coatings
Before the washcoating process,the stainless steel tube was pretreated with organic solvent to remove adsorbed impurities and then with inorganic acid erosion.A typical slurry(zeolite: SiO2:ethanol:water=10:10:40:40)containing 10%(by mass) HZSM-5,10%(by mass)SiO2,40%(by mass)ethanol and 40%(by mass)water was homogenized by ball-milling to make it a stable suspension.Then,the slurry was used to coat the inner surface of stainless steel tubes(Φ3 mm×0.5 mm×300 mm)by the washcoating method,leading to the formation of uniform wall-coated layer.Finally, the prepared zeolite coatings were dried overnight at room temperature and then calcined at 600°C for 2 h.A series of HZSM-5 zeolite coating were prepared by washcoating method by adding different single binders respectively.ZC(BCS)is assigned to the HZSM-5 coating with BCS binder,and so forth.
Viscosity of the zeolite slurries was determined by a stress-controlled AR-1000 rheometer(TA Instruments,UK)equipped with a 40 mm parallelplate geometry in the shear rate range of 0.03-1088 s-1.Zeta potential values for the microscale and nanoscale HZSM-5 zeolite crystals dispersing in distilled water at solid concentration of 1%(by mass)were determined by a Zeta Probe Analyzer(Zetasirer nano ZS,Malvern,UK) atthe room temperature.
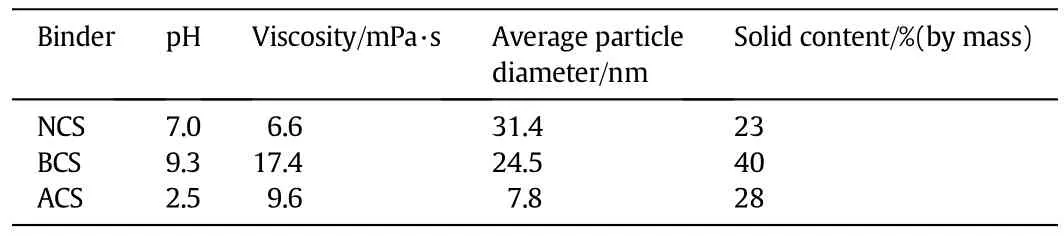
Table 1 Properties of binders used in this work
2.3.Characterizations of zeolite coatings
The solid loading amounts on stainless steel tubes were measured by gravimetry.X-ray diffractions(XRD,Philips X'PERT MPD diffractometer, Cu Kαradiation)were used to investigate the influences of the binder introduction and coating treatment procedure on crystalstructures of zeolites.The prepared coatings were characterized by an emission gun scanning electron microscope(SEM,Nano-Sem 430,FEICorporation, USA)to observe the coating morphologies.The samples were glued to the sample holder with silver paint and covered with a thin gold layer to improve the images.To evaluate the adherence of the zeolite coating, ultrasonic test was carried out as in the previous work[16].
The acid properties of the catalysts were determined by ammonia temperature-programmed desorption(TPD)in Micromeritics 2910 (TPD/TPR)instrument(Micromeritics Instrument Co.,USA).Previously, the samples were outgassed under a He fl ow(60 N ml·min-1),heating with a rate of 20°C·min-1.After cooling to 100°C,an ammonia flow of 30 N·ml·min-1was passed through the sample for 30 min.The physisorbed ammonia was removed by fl owing He at 100°C for 60 min.The chemically adsorbed ammonia was determined by increasing the temperature up to 600°C with a heating rate of 10°C·min-1.The ammonia concentration in the ef fl uent He steam was monitored by a thermal conductivity detector.
2.4.Catalytic activity experiments

Fig.1.Particle size distributions of different colloidal silica binders.(a)NCS;(b)BCS; (c)ACS.
Catalytic cracking of hydrocarbon fuel was carried out using asprepared zeolite coated tube as the reactor.The experimental apparatus used to evaluate the catalytic activity of zeolite coatings was described in our previous paper[4-6].n-Dodecane was pumped with a HPLC pump and the flow rate was also calibrated by electronic balance online.The zeolite-coated tube(stainless steel304 tubes,Φ3 mm×0.5 mm×300 mm)was heated directly by directcurrent(DC)power,and a backpressure valve was utilized to keep the system pressure constant.Fuel outlet and wall temperatures were measured by K-type thermocouples.At the end of experiments,the cracking product was firstly cooled with the condenser,and then fl owed into a gas-liquid separator.Liquid samples were collected for an interval of 5 min to reduce the experimental errors in the material balance.The error of mass balance was less than 2.5%between feeds and products involving gas,and liquid products.

Fig.2.Morphologies of various binder particles.(a)NCS;(b)BCS;(c)ACS.
2.5.Analysis methods
The gas products were analyzed by SP3420 gas chromatograph (Beijing Beifen-Ruili Analytical Instrument Co,China),using a flame ionization detector(FID)and an Al2O3/S capillary column (50 m×0.53 mm).The liquid products were identified by Agilent 7890A GC(Agilent Technologies,USA)with a FID and a PONA column (50 m×0.20 mm).Conversion of n-dodecane,as an index of catalyst coating activity,was defined as the ratio of n-dodecane consumed in the reactor to n-dodecane fed.
3.Results and Discussion
3.1.Characterizations of binders and slurries
To obtain further information on the binders used in this work,the particle diameter distributions of the binders were further measured by DLS.The distribution of binder particle was depicted in Fig.1.It can be seen that NCS has the largest average particle diameter(APD)of 31.4 nm,followed by BCS of 24.5 nm,while the ACS has a much smaller APD of only 7.8 nm(see Table 1).Furthermore,we used SEMimages(Fig.2)to observe particle morphology of each binder. Obviously,the binder particle diameters in SEMcan be finely corresponding to the DLS results.The solid content was measured by weighting the solid after calcination,indicating that both the viscosity and solid content of colloidal silica increase by the following order:ACS<NCS<BCS.Therefore,the main difference between different binders lies in acidic property and particle size distribution, which could influence the catalytic activity and adherence strength of zeolite coating.
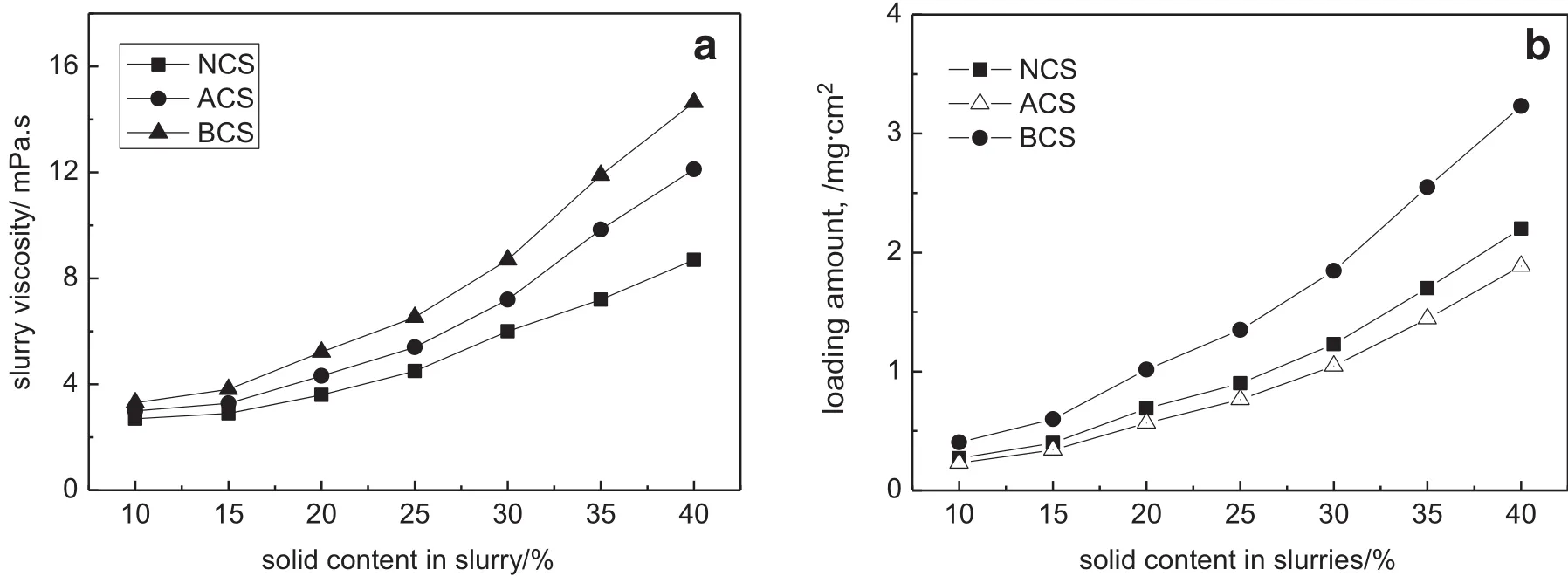
Fig.3.Effect of solid content on the slurry viscosity and solid loading(zeolite:SiO2=1.50:1;ethanol:water=1:1,by mass).
The relationship between binder content in slurry and the properties of loaded HZSM-5 coating was also studied to further explore the effect of binders on the slurry properties(see Fig.3),which is helpful to reveal the roles of binders on catalytic activity and adhesion.It is found that the slurry viscosity increases with the increasing solid content and with the binder type as following:BCS>ACS>NCS,which leads to the increasing solid loading amount on substrate tube.The zeta potential,as agood indication of the DLVO potential barrier,was generally used as an indicator for dispersion stability.The surface charge of zeolite slurry results from the protonation or dissociation of the hydroxyl groups,so that the pH controls the particle surface charge,and hence the zeta potential.With the introduction of various binders with different pH values,the zeta potential of the slurry also changes remarkably.The zeta potential of ACS slurry is-12.4 mV(see Table 2),while for NCS and BCS decrease to-25.2 and-38.4 mV,indicating that the stabilities of slurries may be responsible for the high solid loading when using BCS binder.
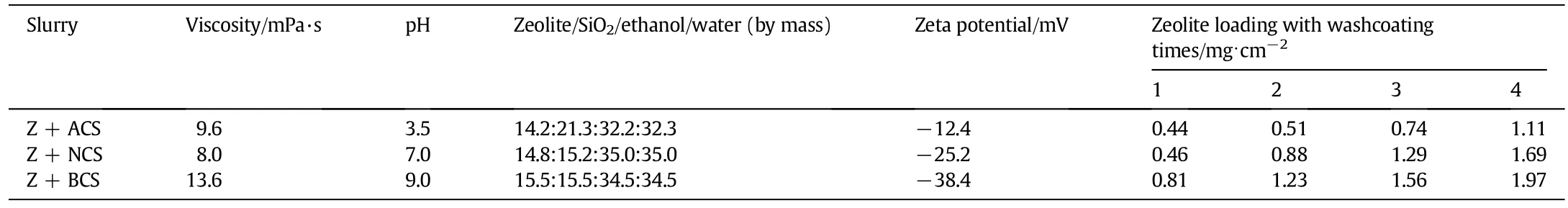
Table 2 Properties of slurries and HZSM-5 coatings with various binders
3.2.Characterizations ofHZSM-5 coatings
As the first step of preparing coatings with different binders,attempts were made to prepare HZSM-5 washcoating with different amounts of NCS binders(10%-40%)at given total solid content of 25%.Fig.4 presents the coating morphologies of those coatings. When the binder amount is less than 20%,the zeolite crystal and surface defects could be observed clearly.Further adhesion analysis also shows that the adhesion increases with the binder amounts up to 30%.Therefore,the percentage of binders in the solid was greater than 30%in the following work.For the ACS,the binder amount even exceeds the zeolite amount to obtain a similar loading amount as the NCS and BCS.
Table 2 lists the properties of slurries and HZSM-5 coatings with various binders,showing that repeated washcoating increases the loading amount.Under the same washcoating number,loading amount increases in the order of BCS>NCS>ACS.This may infer that BCS seems to be a better adhesive in maintaining more zeolite in the microchannel surface.ACS may not be a suitable binder for HZSM-5 coating prepared in SS 304 microchannels,and too big and heterogeneous size particle should be responsible.

Fig.4.Coating morphologies using NCS SiO2binder with different solid contents in slurry.(a)10%;(b)20%;(c)30%;(d)40%;zeolite:SiO2=1:1;ethanol:water=1:1;by mass percent.
Fig.5 shows SEMtop view morphology of zeolite coatings with various binders deposited onto the 304 SS tube surface by washcoating.It isapparent that many hollows and cracks are shown in coating a1which is prepared with NCS by two washcoating.As discussed in previous work [4],repeated washcoating helps to obtain a smooth,compact film without significant holes and cracks(coating a2),whereas a smooth and compactcoating is finely obtained with BCS even with one washcoating (coating b1).Actually,the dominating factor is the degree of dispersion of the particles of zeolite in the washcoating suspension,which are deposited on the surface.This is evident in the agglomeration of the coating obtained with ACS.In addition,more homogeneous distribution of BCS colloidal particles may help to form more uniform coating.Interestingly,repeated washcoating will not modify the coating more smooth butcause very slightly coarse surface(b2).This is notsurprising because little cavities exist in former coating and penetration of adequately smallparticles may notoccur,which is considered as the reason for the improved performance during layer-by-layer washcoating.Contrarily,repeated washcoating willamplify the tinny unsmoothness.The change from coating c1to c2can testify the improved performance of repeated washcoating that usually occurs when the former coating is not so smooth.The c2coating doesn't show very homogeneous surface, which proves that too tiny colloidal particle and extreme pHwould not be in favor of the compact coating formation.
For the zeolite coatings with ACSand NCS,the surface charge density is too low to maintain stability,and the particles coalesce to form aggregates in an early stage of the dip coating process,which leads to the formation of large agglomerates,and thus poor particle packing and poor adhesion[Fig.5(a)].However,for the coating with BCS binder the lower zeta potential results in a significant improvement of the quality of the zeolite Y layer[Fig.5(c)].The coating surface is very uniform and compacteven after one time washcoating[Fig.5(b)],and only slight agglomeration of zeolite particles can be seen together with virtually complete support coverage.
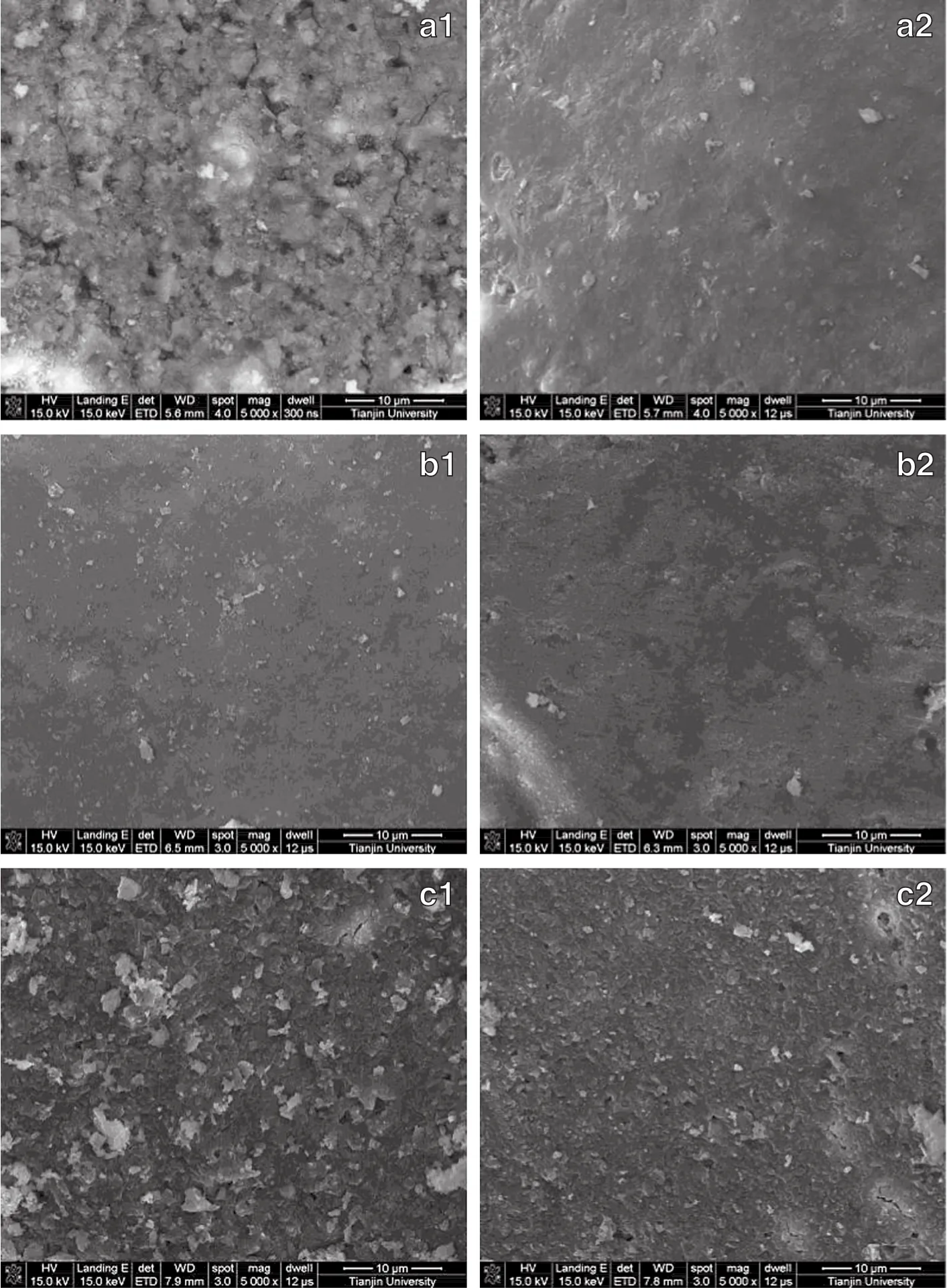
Fig.5.SEMtop view of HZSM-5 coatings with binders.(a)NCS;(b)BCS;(c)ACS;1—two washcoating;2—four washcoating.
3.3.Adherence strength
The strength of the HZSM-5 coatings increased as repeated washcoating with NCS and ACS binders(see Fig.6).The loss of stabilityfrom the first to the second washcoating was due to the weak interlocking strength of the zeolite particles with the substrate and washcoat particles among themselves[4,17].This resultis well matched with previous reports that penetration of adequately small particles into cavities in former layerleads to improved performance and can increase the adherence of HZSM-5 coating.This increase in coating strength is well related to the surface morphology.For the coating with BCS in which no modified performance occurs due to favorable uniform coating prepared by one washcoating,the strength of the coating slightly decreased as repeated washcoating.It matches well with the surface morphology of the favorable uniform coating by one washcoating(b1) and that of the slightly unsmooth surface coating by four washcoating (b2).

Fig.6.Mass loss of HZSM-5 coatings with various binders after ultrasonic test.
3.4.Physical and acidic properties
The XRD patterns of the catalysts added with various binders are shown in Fig.7,all of which are similar to that of the typical HZSM-5 catalyst.It is confirmed that the HZSM-5 crystal structure was maintained with various binders introduced.
Table 3 summarized that binders affected differently the specific surface area and crystallinity of HZSM-5 coating catalysts according to the decreasing order of ACS>NCS>BCS for both properties.Interestingly,the catalyst prepared with ACS has a larger surface area but smaller crystallinity.Generally,the solid contents may be more important factor which can in fl uence the crystallization ofdifferent samples.The decreased crystallinity may also be a result of structure damage of HZSM-5 in the powders,while the increased specific surface may be ascribed to SiO2nanoparticles introduced into slurries,which have a particle size 4 times smaller than the other binders.
The number and strength of acid sites present in the zeolite catalyst are important parameters that affect the activity.There are two adsorption peaks on the NH3-TPD profiles of zeolite samples with different binders,one centered at 190-220°C and the other at 375-445°C,corresponding to the weak and strong acid sites,respectively.The amount of total acid sites also exhibits signi fi cant decay trend(see Table 3),wherein the total strong acid decreases about 38%(from 0.44 to 0.27 mmol NH3·g-1)and weak acid about 45%.It can be explained that the decrease in the acidity of the catalyst with BCS binder was induced by neutralization with basic silica binder added.The increasing amount of those acidic sites is conductive to enhance the initial cracking activity of catalyst.However,increasing these acid sites,particularly the strong acid sites,will inevitably result in the formation of cokes,which will cause catalyst deactivation by hindering the access of reactant molecules to active sites.
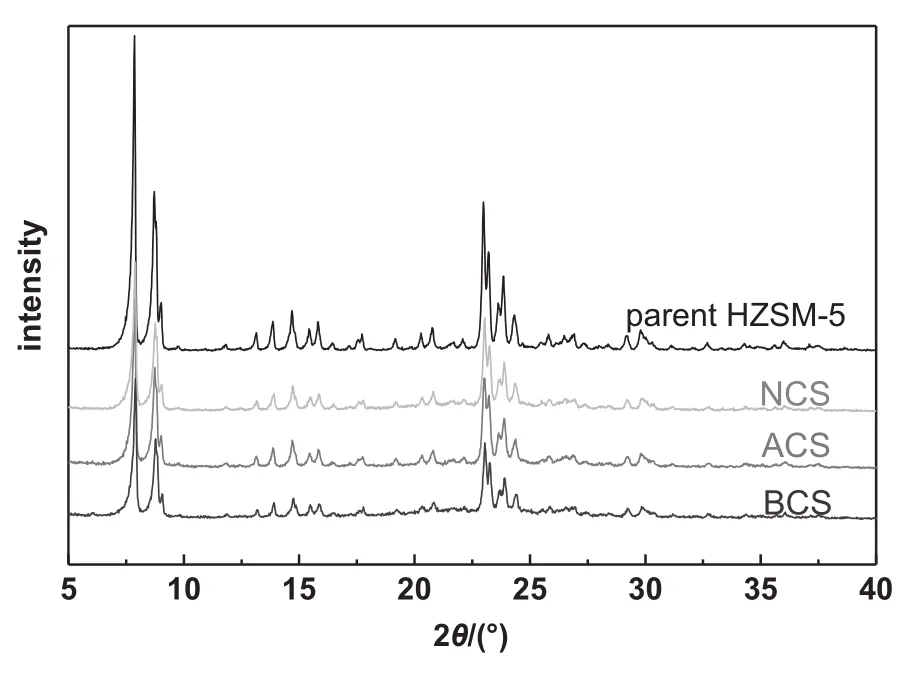
Fig.7.XRD patterns of HZSM-5 coating catalysts with various binders.
3.5.Catalytic activities of HZSM-5 coatings with different binders
HZSM-5 coating series with various binders(with 4 consecutive coatings,the solid loading can be found in Table 2)were tested in the supercritical cracking of n-dodecane.Fig.8 presents the catalytic cracking conversion of supercritical n-dodecane as a function of timeon-stream(TOS)for each catalytic tube in a reaction time of 30 min (550°C,4 MPa).In the bare tube the cracking conversion of ndodecane is ca.12%,which keeps stable in the TOS range of 30 min. The conversion of n-dodecane raises to 15%over the ZC(NCS)coating and only slightly deactivates after TOS=20 min.For the ZC(ACS)coating the initial conversion reaches ca.25%and then rapidly losses 40%activity in 25 min,while the initial activity of ZC(BCS)coating is 24.5% (slightly lower 25%)but only 8%activity loss is observed after 30 min. In spite of the highestacid amount,ZC(ACS)exhibits the rapid deactivation attributed to the coke formed over the zeolite catalyst,which may block the acid sites and channels resulting in acid sites not available and affecting activity.However,ZC(NCS)has similar deactivation with the ZC(BCS),but with lower conversion than that of ZC(BCS).The high and stable catalytic activity may be a result of high loading amount up to 1.97 mg·cm-2compared with 1.69 mg·cm-2for ZC(NCS).Of course,the changes in the mesopores structures during the alkalitreatment in the ACS may also be responsible for the excellent performance. Therefore,the ZC(BCS),with mild catalyst activity and smooth coating microstructure,displays less deactivation performance during the supercritical cracking of dodecane.

Table 3 Specific surface area and crystallinity of HZSM-5 coating catalysts with various binders

Fig.8.Catalytic activities ofHZSM-5 coatings with various binders as a function of stream time.Conditions:4 MPa,10 ml·min-1,550°C,30 min.
4.Conclusions
HZSM-5 coating with three colloidal silica binders,such as acidic colloidal silica(ACS),neutral colloidal silica(NCS)and basic colloidal silica (BCS),was prepared inΦ2 mm stainless tubular reactors.The effect of binders on the catalyst activity and microstructure of HZSM-5 coating in catalytic cracking of supercritical n-dodecane process was observed.
The HZSM-5 coating with BCS showed smooth and compacted surface morphology due to special characteristics of BCS and lower zeta potential of the slurry.Such microstructure leads to appreciable binding strength and higher loading amount.Binders also influence the acidic properties and catalytic cracking activities of zeolite coatings.The total acid amount of zeolite coatings decreases in the following order: ZC(ACS)>ZC(NCS)>ZC(BCS),while the catalytic cracking activities of coatings increase by the same order possibly due to rapid pore mouth plugging of coating induced by higher acidic amount.
[1]T.Edwards,Cracking and deposition behavior of supercritical hydrocarbon aviation fuels,Combust.Sci.Technol.178(2006)307-334.
[2]D.R.Sobel,L.J.Spadaccini,Hydrocarbon fuel cooling technologies for advanced propulsion,J.Eng.Gas Turbines Power 119(1997)344-351.
[3]H.Huang,L.J.Spadaccini,D.R.Sobel,Fuel-cooled thermal management for advanced aero-engines,J.Eng.Gas Turbines Power 126(2004)284-293.
[4]F.X.Meng,G.Z.Liu,L.Wang,S.D.Qu,X.W.Zhang,Z.T.Mi,Effect of HZSM-5 coating thickness upon catalytic cracking of n-dodecane under supercritical condition, Energy Fuels 24(2010)2848-2856.
[5]F.X.Meng,G.Z.Liu,S.D.Qu,L.Wang,X.W.Zhang,Z.T.Mi,Catalytic cracking and coking of supercritical n-dodecane in microchannelcoated with HZSM-5 zeolites, Ind.Eng.Chem.Res.49(2010)8977-8983.
[6]S.D.Qu,G.Z.Liu,F.X.Meng,L.Wang,X.W.Zhang,Catalytic cracking of supercritical n-dodecane over wall-coated HZSM-5 with different Si/Al ratios,Energy Fuels 25 (2011)2808-2814.
[7]B.Mitra,D.Kunzru,Washcoating of different zeolites on cordierite monoliths,J.Am. Ceram.Soc.91(2008)64-70.
[8]J.M.Zamaro,M.A.Ulla,E.E.Miro,Zeolite washcoating onto cordierite honeycomb reactors for environmental applications,Chem.Eng.J.106(2005)25-33.
[9]J.M.Zamaro,M.A.Ulla,E.E.Miro,The effect of different slurry compositions and solvents upon the properties of ZSM5-washcoated cordierite honeycombs for the SCR of NOxwith methane,Catal.Today 107-108(2005)86-93.
[10]B.T.Holland,V.Subramani,S.K.Gangwal,Utilizing colloidal silica and aluminumdoped colloidal silica as a binder in FCC catalysts:effects on porosity,acidity,and microactivity,Ind.Eng.Chem.Res.46(2007)4486-4496.
[11]H.Sun,B.Shen,J.Liu,N-paraf fi ns adsorption with 5A zeolites:the effect ofbinder on adsorption equilibria,Sep.Purif.Technol.64(2008)135-139.
[12]R.V.Jasra,B.Tyagi,Y.M.Badheka,V.N.Choudary,T.S.G.Bhat,Effectofclay binder on sorption and catalytic properties of zeolite pellets,Ind.Eng.Chem.Res.42(2003) 3263-3272.
[13]T.A.Peters,J.van der Tuin,C.Houssin,M.A.G.Vorstman,N.E.Benes,Z.A.E.Vroon,P.A. Holmen,J.T.F.Keurentjes,Preparation of zeolite-coated pervaporation membranes for the integration of reaction and separation,Catal.Today 104(2005) 288-295.
[14]A.V.Boix,J.M.Zamaro,E.A.Lombardo,E.E.Miro,The beneficial effect of silica on the activity and thermalstability of PtCoFerrierite-washcoated cordierite monoliths for the SCR of NOxwith CH4,Appl.Catal.B Environ.46(2003)121-132.
[15]Z.Q.You,G.Z.Liu,L.Wang,X.W.Zhang,Binderless nano-HZSM-5 zeolite coatings prepared through combining washcoating and dry-gelconversion(DGC)methods, Microporous Mesoporous Mater.170(2013)235-242.
[16]G.Z.Liu,G.L.Zhao,F.X.Meng,S.D.Qu,L.Wang,X.W.Zhang,Catalytic cracking of supercritical n-dodecane over wall-coated HZSM-5 zeolites with micro-and nanocrystal sizes,Energy Fuels 26(2012)1220-1229.
[17]J.Zhu,Y.Q.Fan,N.P.Xu,Preparation and characterization of alumina membranes on capillary supports:effect of film-coating on crack-free membrane preparation,Chin. J.Chem.Eng.18(2010)377-383.
☆Supported by the National Natural Science Foundation of China(91116001).
*Corresponding author.
E-mailaddress:wlytj@tju.edu.cn(L.Wang).
**Both authors contributed equally.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Separation Science and Engineering Performance Prediction of Structured Packing Column for Cryogenic Air Separation with Hybrid Model☆
- Enhanced Photocatalytic Properties of Silver Oxide Loaded Bismuth Vanadate☆
- Recovery and Recycling of Ti Supported Bimodal Mesoporous Catalysts Prepared via Ship-in-a-bottle Method in the Epoxidation of Cyclohexene☆
- Promoting Xylene Production in Benzene Methylation using Hierarchically Porous ZSM-5 Derived from a Modified Dry-gel Route☆
- Energy,Resources and Environmental Technology Experimental and Modeling Study on de-NOxCharacteristics of Selective Non-catalytic Reduction in O2/CO2Atmosphere☆
- Energy,Resources and Environmental Technology CO2Removal from Biogas by Water Washing System☆