Acidic Montmorillonite/Cordierite Monolithic Catalysts for Cleavage of Cumene Hydroperoxide☆
2014-07-12LiHanYanjunWangJieZhangZhigangLeiChongpinHuangBiaohuaChenStateKeyLaboratoryofChemicalResourceEngineeringBeijingUniversityofChemicalTechnologyBeijing0009China
LiHan,Yanjun Wang,Jie Zhang,*,Zhigang Lei,Chongpin Huang,Biaohua ChenState Key Laboratory of Chemical Resource Engineering,Beijing University of Chemical Technology,Beijing 0009,China
2No.3 Chemical Work of Beijing Yanshan Petrochemical Co.,SINOPEC,Beijing 102500,China
Acidic Montmorillonite/Cordierite Monolithic Catalysts for Cleavage of Cumene Hydroperoxide☆
LiHan1,Yanjun Wang2,Jie Zhang1,*,Zhigang Lei1,Chongpin Huang1,Biaohua Chen11State Key Laboratory of Chemical Resource Engineering,Beijing University of Chemical Technology,Beijing 100029,China
2No.3 Chemical Work of Beijing Yanshan Petrochemical Co.,SINOPEC,Beijing 102500,China
A R T I C L E I N F o
Article history:
Received 24 December 2013
Received in revised form 12 February 2014
Accepted 17 March 2014
Available online 18 June 2014
Monolithic catalyst
Acidic montmorillonite
Cumene hydroperoxide
Cleavage
In this work,a series of acidic montmorillonite/cordierite monolithic catalysts were prepared by a coating method using silica sol as the binder.The morphology and structure of the acidic montmorillonite/cordierite samples were characterized by means of X-ray diffraction(XRD),N2adsorption/desorption isotherms,and scanning electron microscope(SEM).The cleavage of cumene hydroperoxide(CHP)in a conventional fixed-bed reactor was chosen as a model reaction to evaluate the catalytic activity of the monolithic catalysts.The influences of acidic montmorillonite loading,reaction temperature,CHP concentration,and weight hourly space velocity (WHSV)on the catalytic activity and selectivity of phenol were studied.The results indicated that the obtained acidic montmorillonite/cordierite monolithic catalysts were firm and compact,and the loading of acidic montmorillonite was found to reach 40%(by mass)after three coating operations.The surface area of acidic montmorillonite/cordierite catalysts increases greatly as acidic montmorillonite loading increases due to higher surface area of acidic montmorillonite.Under the optimalreaction conditions(acidic montmorillonite loading of32.5%(by mass),temperature of80°C,a mass ratio of CHP to acetone of1:3,and WHSV of CHP of 90 h-1), the conversion of CHP can reach 100%,and the selectivity of phenolis up to 99.8%.
©2014 The Chemical Industry and Engineering Society ofChina,and Chemical Industry Press.Allrights reserved.
1.Introduction
Both phenol and acetone are important chemicals in the organic chemical industry.The cumene peroxidation method for the preparation of phenoland acetone is the most important technology in the current world[1],which was first developed in the 1950s.The process consists of the following three steps:(1)cumene generation by alkylation of benzene with propylene,(2)cumene oxidization by oxygen to cumene hydroperoxide(CHP),and(3)CHP cleavage over acid catalysts to phenoland acetone[2-4].The following reaction presents this process:
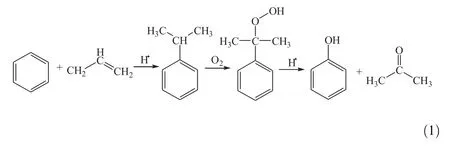
Among these processes,CHP cleavage has an important impact on the yield of the product.The commercialacid catalyst most commonly used for CHP cleavage is concentrated sulfuric acid.However the concentrated sulfuric acid shows intrinsic disadvantages including: (1)less selectivity and more byproducts[5];(2)serious equipment erosion due to addition ofinorganic or organic alkalifor neutralization; (3)the addition of alkalimakes the subsequent operation complicated and the distillation column life cycle shortened;(4)phenol-containing waste water is produced due to added solvent water,leading to environmentalpollution[6].
In the recent two decades,solid acid catalysts were extensively studied due to the possibility to avoid the disadvantages of the traditional process.The solid acid catalysts consist of acid resin,zeolite, acidic montmorillonite,metallic oxide,heteropoly acid,loaded L acid,etc.[7].Among these catalysts,acidic montmorillonite and acid resins exhibit appropriate catalytic performance[8].The ionexchange resin catalyst was proposed in the industry to catalyze CHP cleavage to produce phenol[9,10].However,the fragmentation, instability,easy erosion and nonuniform distribution of acidic center are the main disadvantages for these solid acid catalysts,which restrict their wide industrial application.Therefore,it is necessary to develop new and appropriate acidic catalysts to replace solid acid catalysts.
At present,the monolithic catalyst as a novel kind of catalyst with the internal structure of a regular honeycomb structure exhibitssome advantages compared with the conventional particulate catalyst, e.g.,low pressure drop,high effectiveness factor,minimum axial dispersion stemming from the uniquely structured multichannel configuration,high catalyst utilization efficiency and good heat and mass transfer performances[11-14].Moreover,the monolithic structures are beneficial to improve the surface area per unit of bed volume, which is conducive to timely access of the reactants and timely discharge of the products so as to intensify the chemical reaction process. In addition,it is convenient to install or uninstall the monolithic catalyst into or out of the reactor and also easy for the maintenance,resulting in low operational cost[15-17].CHP cleavage is a strong exothermic reaction and there even exists the danger of explosion.A fast and effective removal of reaction heat is absolutely necessary for the process.The typical method of heat removal is by acetone evaporation.The low bed pressure drop of monolithic catalysts is conducive to heat transfer so as to reduce the formation of byproducts and improve the selectivity. Currently,monolithic catalysts have been applied to plenty of catalytic reactions,e.g.,the catalytic combustion and the catalytic oxidation [18],the methane steam reforming[19],the hydrogenation dehydrogenation reaction[20]and the Fischer-Tropsch synthesis[21],and exhibit excellent catalytic performances.Most monolithic catalysts adopt the cordierite as substrate due to its excellent thermal stability,small thermalexpansion coefficient and impact resistance[22-24].Because the specific surface area of cordierite is very small,the intermediate coating usually is loaded firstly and then the active component is coated, thus the preparation technology is more complicated.Thus,the onestep coating method of preparing monolithic catalysts is simple,easy to operate and in favor ofindustrialproduction.
In this work,the one-step coating method was used to prepare a series of acidic montmorillonite/cordierite monolithic catalysts,and then the samples were characterized by X-ray diffraction(XRD),N2adsorption/desorption and scanning electron microscope(SEM)to characterize the morphology and structure of catalysts.The catalytic performance of the catalysts for the cleavage of cumene hydroperoxide was evaluated in a fixed-bed reactor.The influences of acidic montmorillonite loading,reaction temperature,CHP concentration,and WHSV of CHP on the conversion of CHP and selectivity of phenol were studied. Finally the optimum operation conditions were obtained.
2.Experimental
2.1.Materials
Cordierite monolith support(31 cells per square centimetre,hole size:2 mm×2 mm,surface roughness:~4.0μm)was purchased from Beijing Chuangdaoaofu Fine Ceramics Co.Amberlyst 15(concentration of acid sites≥4.7 mol·kg-1,surface area:40 m2·g-1,pore volume: 0.4 cm3·g-1,pore size:25 nm)was provided by Aoyang Chemical Engineering Co.,Zhangjiagang,Jiangsu.CHP[about 80%(by mass)in cumene]was obtained from Yanshan Petrochemical Co.,Beijing.Other chemicals and reagents such as sulfuric acid,montmorillonite(neutral, average particle size:10.0μm,specific surface area:32.1 m2·g-1),silica sol[aqueous dispersion of30%(by mass)ofnano SiO2],oxalic acid and acetone were of analytical grade and supplied by Tianjin Guangfu Fine Chemical Research Institute without further purification.
2.2.Preparation of catalysts
The montmorillonite powder was activated using 30%(by mass) sulfuric acid,heated at 100°C for 4 h,and then washed with deionized water repeatedly tillpH≥4,and finally the acidic montmorillonite was dried in an oven.The acidic montmorillonite,silica sol[30%(by mass) dispersion],and deionized water were mixed together at a certain proportion(mass ratio of acidic montmorillonite:silica sol:deionized water being 1:1.5:2),then the mixture was stirred on a magnetic stirring apparatus at room temperature for 3 h,and the pH value was kept at 3.0 by addition of dilute nitric acid when needed so as to obtain the active slurry.
The cordierite samples with a diameter of 8 mm and length of 20 mm were cut from a commercial honeycomb cordierite with a cell density of 31 cpsc(cells per square centimetre).The samples were pretreated using 20%(by mass)oxalic acid solution boiling for 2 h and then washed with deionized water.Afterwards,these cordierite samples were calcined in a muffle furnace at the temperature of550°C for 4 h to remove the absorbed impurities.The dried cordierite monolith sample was dipped into the active slurry obtained above for 5 min, and the excess slurry in the monolith channels was removed with compressed air,and then dried horizontally in air at120°C for 30 min.The coating procedure was repeated 1-5 times to achieve the expected loading of13.0%-38.5%(by mass).Afterward,the monolithic catalysts were calcined in air at 550°C for 4 h.Some properties of the prepared monolithic catalysts were listed in Table 1.
2.3.Characterization ofcatalysts
The adhesion degree of acidic montmorillonite coated onto the monolith cordierite support was measured by an ultrasonic method. Five monolithic catalysts of different acidic montmorillonite loadings were submerged in five beakers containing alcohol,and then beakers are placed in an ultrasonic oscillator containing water.With ultrasonic power of 160 W of 25 kHz exerted for 60 min,the mass loss of the coating was less than 3%(expulsion rate in Table 1).
X-ray diffraction(XRD)patterns were recorded on a Bruker D8 advanced diffractometer,with Nidetector side filtered Cu-Kαradiation over a 2θrange from 5°to 50°at 40 kV and 40 mA using a step size of 5°and a step time of1 min.
Scanning electron microscopy(SEM)micrographs were recorded on a JEOL JSM-6701F microscope working at 5.0 kV accelerating voltage. Before observation,the sample was sprayed with gold by an ion sputtering instrument in order to make the sample conductive.
The BET surface area,pore volume and pore size distribution were determined by using a Micromeritics ASAP-2020 surface area analyzer. Before each adsorption/desorption,the samples were degassed for at least 5 h at 350°C under vacuum.Surface area was calculated with the BET equation,while pore volume and pore size distribution were obtained using the BJH method.
2.4.Catalytic activity measurements
Catalytic activity was measured using a conventional fixed-bed reactor apparatus(8 mm in inner diameter and 300 mm in length packed with 5 cylindrical monolithic catalysts above)at atmospheric pressure.A schematic diagram of the experimental apparatus is shown in Fig.1.Five monolithic catalysts were placed in the reaction tube,separated by quartz sand and quartz wool,and the reaction temperature was maintained at 60°C-90°C by a water bath.
In the comparison experiment,the acid resins(Amberlyst 15)and five monolithic units(mass of each unit is 1.4 g and the acidic montmorillonite loading is 32.5%,by mass)were placed in the reactor.2.275 g acid resin(Amberlyst 15,dried at 80°C for 24 h before use),an equal amount to the acidic montmorillonite on the five monolithic units, mixed with 0.45 mm-0.8 mm glass beads uniformly was placed in thesame reactor.Atypicalliquid mixture consisting of CHP in cumene as solvent was pumped into the reactor from the bottom.On the top of the reactor,part of liquid left as the products and the resetliquid circled and mixed with the fresh feed.Reaction products were withdrawn periodically and analyzed with a gas chromatograph(GC 4000A,East &West Analytical Instruments,Inc.,Beijing)equipped with a flame ionization detector(FID),and the chromatographic column was a DB-Wax capillary column.Phenoland acetone were the main reaction products, and the catalytic activity was represented by the conversion of CHP.
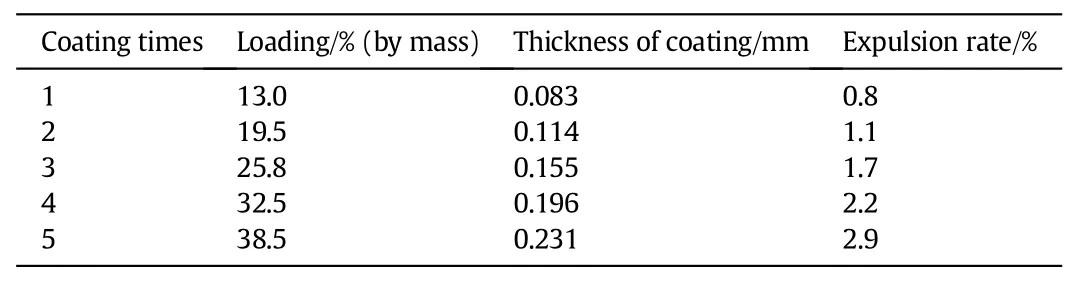
Table 1 Properties of monolithic catalyst

Fig.1.Experimental apparatus for CHP cleavage.1—raw materialvessel;2—metering pump;3—preheater;4—fixed-bed reactor;5—temperature controlslot;6—condenser;7—gas chromatograph.
3.Results and Discussion
3.1.X-ray diffraction studies
Fig.2 shows the XRD patterns of cordierite substrate and monolithic catalysts with different acidic montmorillonite loadings.As observed in the figure,cordierite has characteristic diffraction peaks at 10.6°,18.2°, 26.5°,28.6°,29.6°and 34.1°.These characteristic peaks are in agreement with the literature[25].For the acidic montmorillonite/cordierite samples several new diffraction peaks are identified,while the peaks at 21.9°, 25.4°and 26.6°demonstrate the appearance of acidic montmorillonite in the cordierite substrate.The diffraction peaks corresponding to the acidic montmorillonite are relatively weak when the acidic montmorillonite loadings are 13.0%and 19.5%(by mass),probably due to the acidicmontmorillonite uniform dispersion on the surface of the cordierite. With increasing acidic montmorillonite loadings,the intensity of diffraction peaks of acidic montmorillonite increased gradually.When the loading of acidic montmorillonite increased to 32.5%(by mass)the diffraction peak of acidic montmorillonite increased significantly,but the position of the acidic montmorillonite peak does not substantially change,indicating that the basic skeleton of acidic montmorillonite does not have major changes.
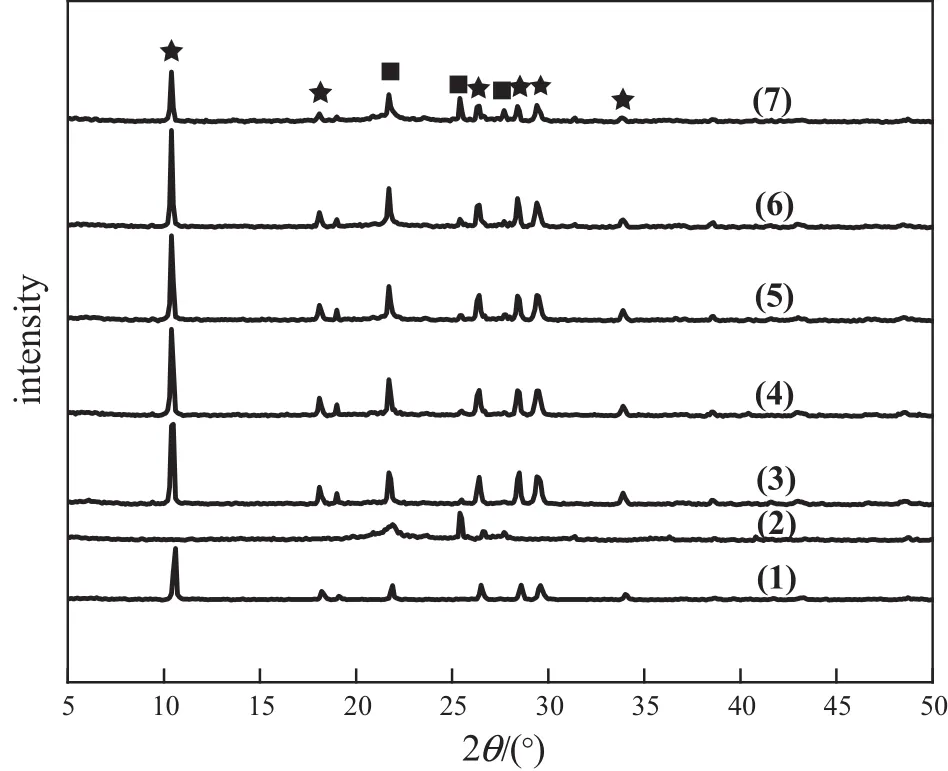
Fig.2.XRD patterns of bare cordierite and monolithic catalysts with different acidic montmorillonite loadings.(1)Bare cordierite substrate;(2)acidic montmorillonite; (3)13.0%(by mass)acidic montmorillonite loading;(4)19.5%(by mass)acidic montmorillonite loading;(5)25.8%(by mass)acidic montmorillonite loading;(6)32.5%(by mass) acidic montmorillonite loading;(7)38.5%(by mass)acidic montmorillonite loading.■acidic montmorillonite;★bare cordierite.
3.2.N2adsorption-desorption isotherms
The pore structure parameters for bare cordierite and acidic montmorillonite/cordierite samples with different acidic montmorillonite loadings are listed in Table 2.The cordierite unpretreated with acid has the smallest specific surface area[26],and after pretreatment with oxalic acid the specific surface area of cordierite has increased to 23.7 m2·g-1and the average pore size has increased to 3.41 nm,indicating the generation of mesoporous structures.After montmorillonite acidification for4 h,the layered structure is destroyed,and the structural aluminum is dissolved and removed,thereby reforming the porous structure and increasing surface area,pore volume and pore size[27] compared with montmorillonite without acidification.It is found that the surface area,pore volume and pore size of acidic montmorillonite/ cordierite catalysts are enhanced greatly comparing with the uncoated cordierite.The values for the uncoated cordierite are 23.7 m2·g-1, 0.023 cm3·g-1,and 3.41 nm,and they increase to 31.5 m2·g-1, 0.045 cm3·g-1,and 5.67 nm after being coated.This is attributed to the acidic montmorillonite and SiO2in silica sol.With increasing acidic montmorillonite loading,the specific surface area and pore volume increase gradually,but with smaller and smaller extent,that is in agreement with the change of acidic montmorillonite.
3.3.Morphology ofcatalysts
Fig.3 shows the SEMmorphologies of bare cordierite and monolithic catalysts with different acidic montmorillonite loadings.It can be seen that the surface of cordierite is smooth,fl at,and less mesoporous. After pretreatment with oxalic acid,the surface of cordierite partially becomes much coarser and has more macropores,and pore size and pore volume also increase,which facilitates the coating of acidic montmorillonite.After acidic montmorillonite coated onto cordierite the surface is covered,and the pores are blocked.Fig.3(c)shows that the surface of acidic montmorillonite/cordierite is uniform within a certain loading range,highly dispersed,no cracks,and no apparent interface between acidic montmorillonite and cordierite.It also shows that acidic montmorillonite combines with cordierite closely and acidic montmorillonite is not easily detached from the cordierite surface.With the increase of the acidic montmorillonite loading,acidic montmorillonite particles gradually increase,and significant aggregation phenomena appear.As shown in Fig.3(e)and(f),the increment of acidic montmorillonite thickness is due to the increment of acidic montmorillonite loading accompanied by a significant cracking of the surface.Some cracks can be observed,which may be brought on by acidic montmorillonite loading,and on the corners and porous walls,accumulation appeared. Moreover the interface between acidic montmorillonite and cordierite carrier is slightly obvious.
3.4.Effect of acidic montmorillonite loading
Acidic montmorillonite loading is important in a heterogeneous catalytic system,and it will influence the local reaction rate directly and the quality of products finally.Fig.4 illustrates the influence of acidic montmorillonite loading on the conversion of CHP.It can be seen that the conversion of CHP increases with the increase of the acidic montmorillonite loading under the same operating condition,and it reaches the maximum at the loading of32.5%(by mass).This result can be mainly attributed to the proportional increase in the number of active sites. However,when the acidic montmorillonite loading is over 32.5%(by mass),the conversion of CHP decreased with further increasing in the acidic montmorillonite loading.In the case of acidic montmorillonite loading at32.5%(by mass),the results of SEM and BET characterization indicate that the monolithic catalyst exhibited a relative large specific surface area,the appropriate pore size and pore volume,and high dispersion of the cordierite surface,which are allfavorable factors to the CHP chemical adsorption on the surface of catalysts.Neither the lower nor higher loading will benefit the catalytic activity.In addition, the specific surface area,pore volume and pore size of32.5%(by mass) and 38.5%(by mass)acidic montmorillonite loading catalysts are similar. Furthermore,the expulsion rate of38.5%(by mass)loading monolithic catalysts is greater than 32.5%(by mass)loading(Table 1).This explains that the excessive loading leads to low firmness and poor stability due to agglomeration,which is not beneficial to the catalytic performance of the monolithic catalysts[28].Consequently the optimum value of acidic montmorillonite loading in monolithic catalysts is 32.5%(by mass)for the highest catalytic activity and in this case the conversion of CHP achieves the maximum value.
3.5.Effect of the reaction temperature
The effects of reaction temperature on the conversion of CHP and the selectivity of phenol using monolithic catalysts as well as using solid acid catalyst(acid resins)[29]are investigated in this work,and the results are illustrated in Figs.5 and 6,respectively.It can be seen that the conversion of CHP increases substantially with increasing reaction temperature for both monolithic catalysts and acid resins,but the selectivity of phenol shows an inverse trend,i.e.,the selectivity decreases as reaction temperature increases.However,phenol selectivity using monolithic catalysts is higher than that using acid resins.In addition, the thermal cleavage of CHP enhanced and the generation of byproducts increases as the reaction temperature increases,resulting in a decrease of phenol selectivity.Thus,a trade-off between the CHP conversion and phenol selectivity should be considered for the selection of the optimum reaction temperature.Consequently,80°C is an optimal reaction temperature for the CHP cleavage reaction using monolithic catalysts.
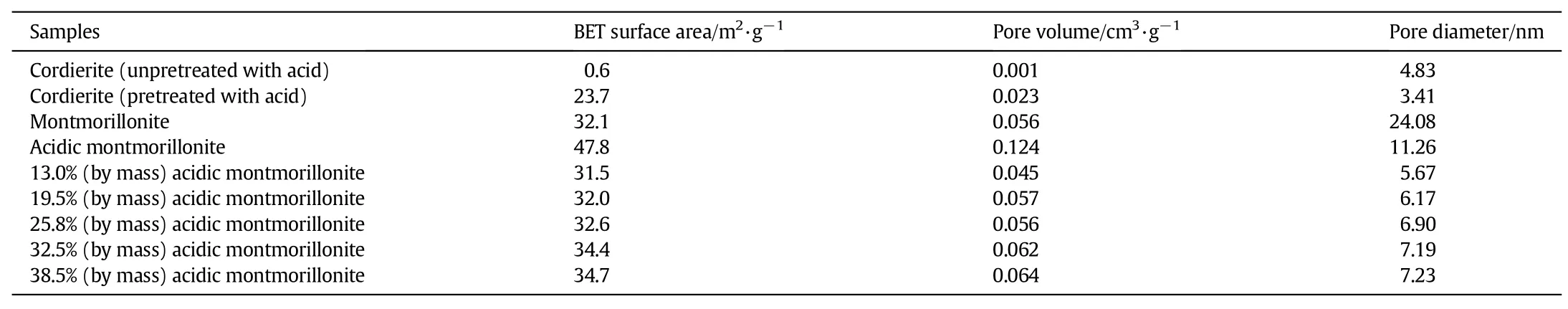
Table 2 Pore structure parameters of samples
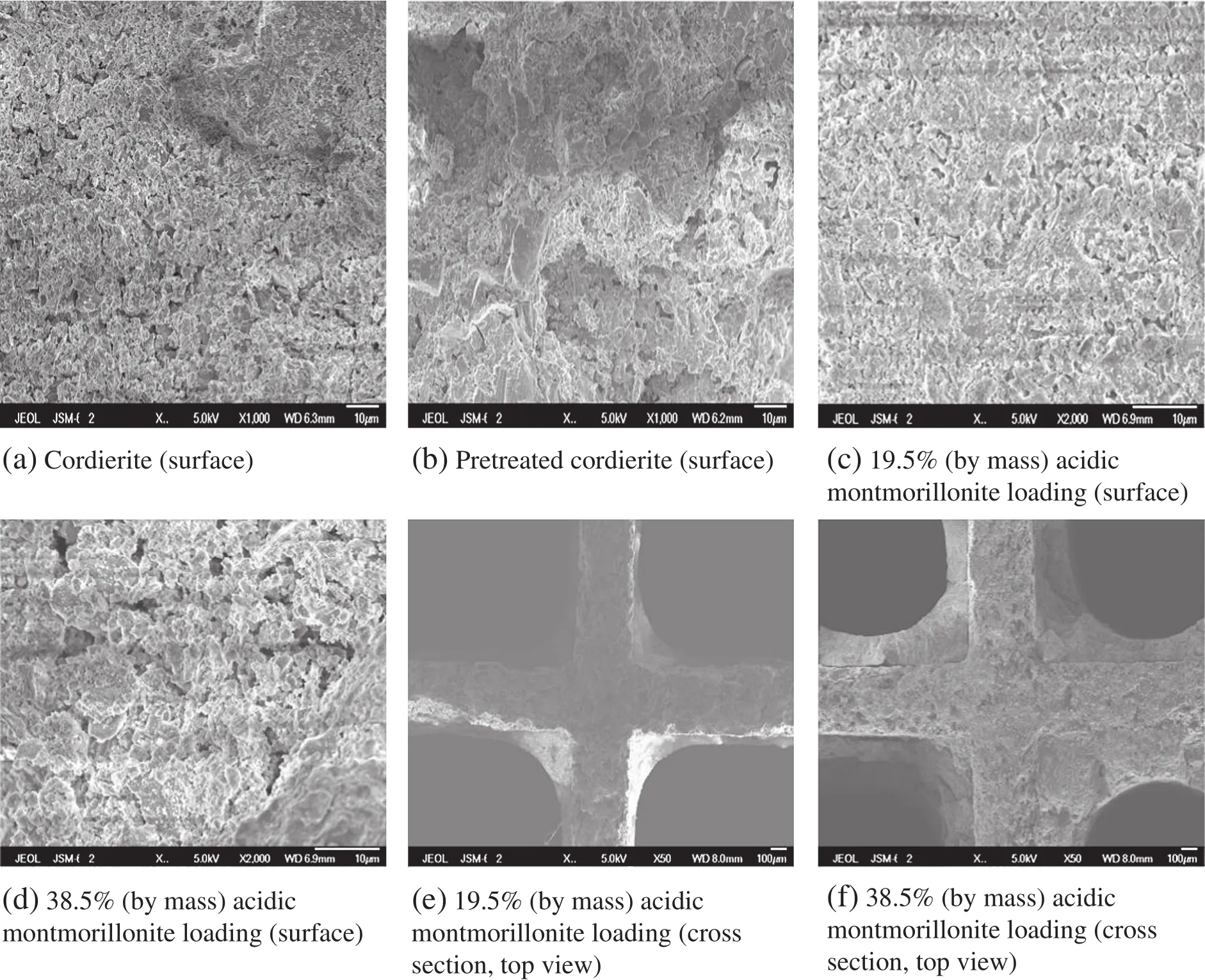
Fig.3.SEM microphotographs of monolithic catalyst surface.
3.6.Effect of the concentration of CHP
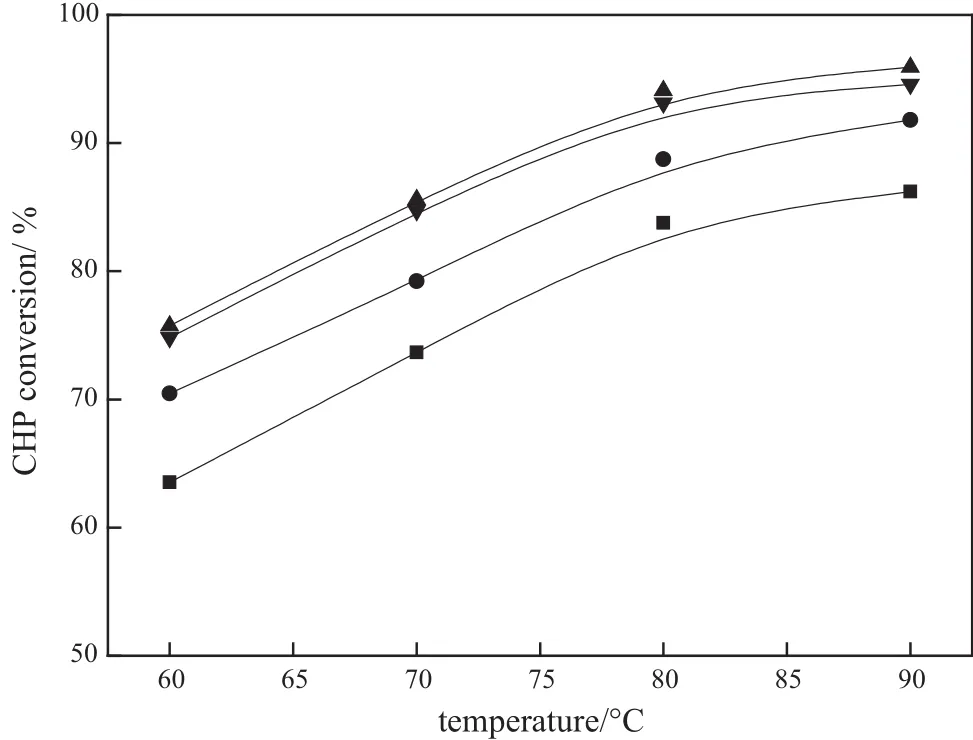
Fig.4.Effect of acidic montmorillonite loading on CHP conversion.Experimental conditions: CHP:acetone=1:3 and WHSV=90 h-1.■19.5%(by mass)acidic montmorillonite loading;●25.8%(by mass)acidic montmorillonite loading;▲32.5%(by mass)acidic montmorillonite loading;▼38.5%(by mass)acidic montmorillonite loading.

Fig.5.Effectof the reaction temperature on CHPconversion.Experimental conditions:32.5% (by mass)acidic montmorillonite loading,CHP:acetone=1:3,and WHSV=90 h-1.■acid resins;●monolithic catalyst.
Figs.7 and 8 present the influence of the concentration of CHP on the conversion of CHP and the selectivity of phenol.The mass ratio of CHP to acetone was investigated to be in the range from 1:2 to 1:4 using monolithic catalysts.The results show that the conversion of CHP decreased with increasing the concentration of CHP,and the conversion reaches 100%at 80°C when the mass ratio is 1:4.Under the same reaction conditions,the number of the active sites is roughly constant,however,the number ratio of the active sites to the CHP moleculars at high CHP concentration is lower than that at low CHP concentration.It can be deduce that the high number ratio of CHP to active sites leads to high conversion at the same residence time.As shown in Fig.8,higher CHP concentration is not conducive to the selectivity of phenol.However, the selectivity of phenol reduces slowly and is high in general when the ratio of CHP to acetone is inferior to 1:3.However,the extra acetone will also exacerbate the burden of the condensation system.Our work reveals that a mass ratio of CHP to acetone ofabout1:3 is the optimum choice for the cleavage of CHP on the acidic montmorillonite/cordierite monolithic catalysts.
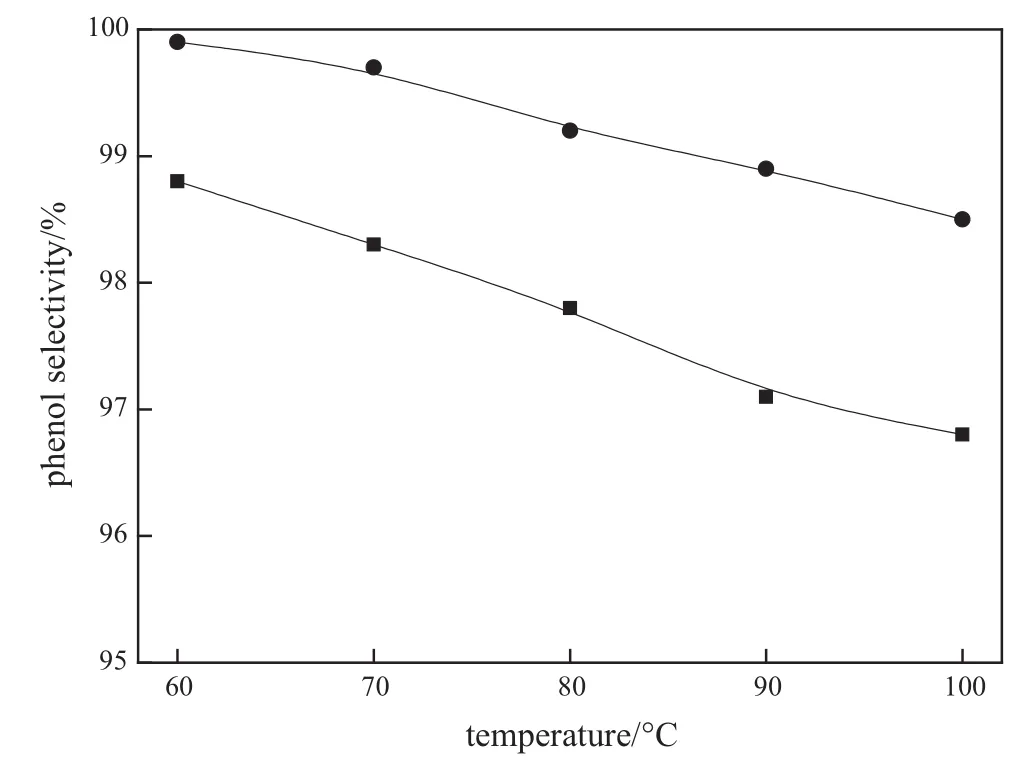
Fig.6.Effect of the reaction temperature on phenolselectivity.Experimentalconditions: 32.5%(by mass)acidic montmorillonite loading,CHP:acetone=1:3,and WHSV= 90 h-1.■acid resins;●monolithic catalyst.
3.7.Effect of the weight hourly space velocity
The weight hourly space velocity(WHSV)of CHP,which is de fi ned as the mass flow rate of pure CHP per unit mass of acidic catalysts,is also an important parameter for reactor design and operation.As can be seen from Fig.9,the influence of reaction temperature on the conversion of CHP is more signi fi cantthan the in fl uence of WHSV.The conversion of CHP was basically unchanged when the WHSV increases from 10 h-1to 90 h-1,indicating that the monolithic catalysts can handle a larger capacity.When the WHSV exceeds 90 h-1,the conversion of CHP emerges decreasing tendency with increasing WHSV,possibly due to the insufficient contact time between CHP and catalyst active centers [30].The influence of WHSV(from 10 h-1to 90 h-1)on the selectivity of phenol is shown in Fig.10,in which phenols electivity is lower than 99.5%.As WHSV increases further,the selectivity of phenol increases slightly to approach 100%.According to the conversion of CHP and phenol selectivity for the acidic montmorillonite/cordierite monolithic catalysts,the appropriate WHSV can be about 90 h-1.

Fig.7.Effect of the concentration of CHP on CHP conversion.Experimental conditions: 32.5%(by mass)acidic montmorillonite loading and WHSV=90 h-1.■1:2;●1:3;▲1:4.

Fig.8.Effect of the concentration of CHP on phenolselectivity.Experimental conditions: 32.5%(by mass)acidic montmorillonite loading and WHSV=90 h-1.■1:2;●1:3;▲1:4.
4.Conclusions
The acidic montmorillonite/cordierite monolithic catalysts were prepared by slurry wetcoating using silica sol as the binder,and characterized by XRD,N2adsorption/desorption isotherms and SEM techniques.It was found that acidic montmorillonite was dispersed firmly on the surface of cordierite substrate,and the surface area of the monolithic catalysts significantly increased with an increase in the acidic montmorillonite.
The cleavage of cumene hydroperoxide(CHP)was used as the model reaction to investigate the reaction performance of an acidic montmorillonite/cordierite monolithic catalyst.On the basis of the experiments,severaloperation parameters were investigated to find the optimum reaction conditions,which are acidic montmorilloniteloading of 32.5%(by mass),reaction temperature of 80°C,WHSV of 90 h-1,and mass ratio of CHP to acetone of1:3.
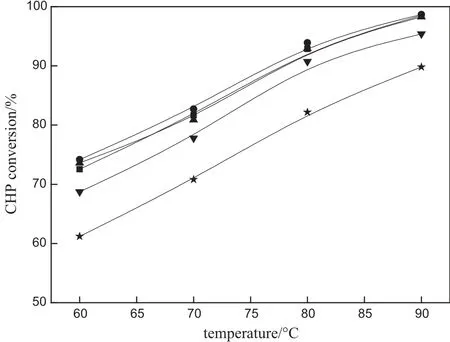
Fig.9.EffectofWHSVon CHP conversion.Experimental conditions:32.5%(by mass)acidic montmorillonite loading and CHP:acetone=1:3.■10 h-1;●30 h-1;▲90 h-1;▼200 h-1;★300 h-1.
The higher catalytic activity and selectivity make monolithic catalysts more competitive.Meanwhile,the monolithic catalysts can also be recycled for severaltimes withoutobvious decreasing of the activity. Further work exploring the utility of these novelcatalysts is on-going in our laboratory.
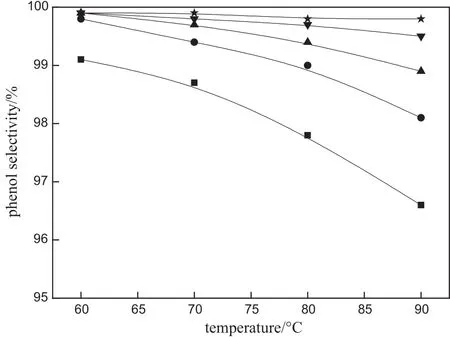
Fig.10.Effect of WHSV on phenolselectivity.Experimentalconditions:32.5%(by mass) acidic montmorillonite loading and CHP:acetone=1:3.■10 h-1;●30 h-1;▲90 h-1;▼200 h-1;★300 h-1.
[1]G.Cao,Production of Phenol/Acetone Using Cumene Process,Chemical Industry Press,Beijing,1983.(in Chinese).
[2]J.H.Jian,Y.F.Liu,J.J.Yang,Research of catalysts for the decomposition of cumene hydroperoxide,Petrochem.Technol.27(2)(1998)136-139(in Chinese).
[3]Y.F.Li,Phenolproduction technology progress at home and abroad,Chin.Petrochem. 8(2004)40-42(in Chinese).
[4]X.M.Cui,Progress of phenolproduction techniques and analysis domestic market, Sichuan Chem.Eng.7(1)(2004)19-23(in Chinese).
[5]Y.Y.Wang,Catalytic technology of cumene peroxidation method,Prog.Petrochem. Technol.5(6)(2004)48-52(in Chinese).
[6]J.F.Knifton,N.J.Grice,“Method for production phenol/acetone from cumene hydroperoxide”,U.S.Pat.4876397(1989).
[7]C.Distiller,“Catalysts for the cleavage of cumene hydroperoxide”,Bri Pat.676771 (1948).
[8]Y.Z.Yu,H.Y.Jiang,B.Xiao,W.J.Tao,Y.Zhao,L.Wang,X.C.Wu,H.R.Song,Process of solid acid catalysts for cumene hydroperoxide decomposition producing phenoland acetone,Sci.Technol.Chem.Ind.15(1)(2007)58-62(in Chinese).
[9]Z.H.Ye,Analysis of composition of sediment in acetone re fi ne device of cumene process,Petrochem.Technol.9(12)(1980)709-712.
[10]K.F.Shi,Production ofphenol/acetone using decomposition ofcumene hydroperoxide catalyzed by ion-exchange resin,Petrochem.Technol.9(8)(1980)449-453(in Chinese).
[11]V.Holler,I.Yuranow,L.Kiwi-Minsker,Structured multiphase reactors based on fibrous catalysts:nitrite hydrogenation as a case study,Catal.Today 69(2001) 175-181.
[12]E.Z.Min,Development and countermeasures of petrochemical catalytic materials for the 21st century,OilGas J.29(5)(2000)215-220.
[13]I.Yuranov,L.Kiwi-Minsker,A.Renken,Structured combustion catalysts based on sintered metal fibre filters,Appl.Catal.43(2003)217-227.
[14]J.M.Victor,D.Campos,M.Rosa,Structured catalysts for partial oxidations,Catal. Today 169(2001)121-129.
[15]S.Roy,A.K.Heibel,W.Liu,Design of monolithic catalysts for multiphase reactions, Chem.Eng.Sci.59(5)(2004)957-966.
[16]H.Mei,C.Li,H.Liu,S.F.Ji,Simulation of catalytic combustion of methane in a monolith honeycomb reactor,Chin.J.Chem.Eng.14(1)(2006)56-64.
[17]Y.Zhao,Y.F.Zheng,F.Xin,Properties and applications of monolithic catalysts,Chem. React.Eng.Technol.20(4)(2004)357-361(in Chinese).
[18]G.Groppi,E.Tronconi,Design of novel monolith catalyst supports for gas/solid reactions with heat exchange,Chem.Eng.Sci.55(12)(2000)2161-2171.
[19]F.M.Dautzenberg,M.Mukherjee,Process intensification using multifunctional reactors,Chem.Eng.Sci.56(2)(2001)251-267.
[20]T.A.Nijhuis,M.T.Kreutzer,A.C.J.Romijn,Monolithic catalysts as efficient threephase reactors,Chem.Eng.Sci.56(3)(2001)823-829.
[21]A.M.Hilmen,E.Bergene,O.A.Lindvg,Fischer-Tropsch synthesis on monolithic catalysts of different materials,Catal.Today 69(2001)227-232.
[22]Q.B.Zhang,L.H.Zhao,B.T.Teng,Y.L.Xie,L.Yue,Pd/Ce0.8Zr0.2O2/substrate monolithic catalyst for toluene catalytic combustion,Chin.J.Catal.29(4)(2008)373-378.
[23]T.A.Nijhuis,A.E.W.Beers,T.Vergunst,I.Hoek,F.Kapteijn,J.A.Moulijn,Preparation of monolithic catalysts,Catal.Rev.43(4)(2001)345-380.
[24]J.W.Geus,J.C.Giezen,Monoliths in catalytic oxidation,Catal.Today 47(1)(1999) 169-180.
[25]X.X.Sun,J.Zhang,G.H.Zeng,Research ofsynthesis of cordierite,Ceramics 7(2005) 22-24(in Chinese).
[26]C.B.Chen,P.Li,Z.J.Sui,Preparation of cordierite honeycomb coating with large surface area and high adhesive capacity,Ind.Catal.18(3)(2010)40-45.
[27]H.C.James,Environmentally friendly chemistry using supported reagent catalysts: structure-property relationships for clayzic,J.Chem.Soc.Perkin Trans.6(1994) 1117-1129.
[28]G.L.Zhao,J.W.Teng,Z.K.Xie,Characterization of ammonium fluorosilicate modified HZSM-5 catalyst and its catalytic performance of hydrocarbon cracking,J.Catal.26 (12)(2005)1083-1087.
[29]D.G.Huang,M.H.Han,J.F.Wang,Y.Jin,Catalytic decomposition process of cumene hydroperoxide using sulfonic resins as catalyst,Chem.Eng.J.88(1)(2002)215-223.
[30]T.Takeguchi,S.Aoyama,J.Ueda,Catalytic combustion of volatile organic compounds on supported precious metal catalysts,Top.Catal.23(1-4)(2003) 159-162.
☆Supported by the National Natural Science Foundation of China(21121064, 21076008,21206008),the Projects in the National Science&Technology Pillar Program during the 12th Five-Year Plan Period(2011BAC06B04),and the Research Fund for the Doctoral Program of Higher Education of China(20120010110002).
*Corresponding author.
E-mailaddress:zhangjie@mail.buct.edu.cn(J.Zhang).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Separation Science and Engineering Performance Prediction of Structured Packing Column for Cryogenic Air Separation with Hybrid Model☆
- Enhanced Photocatalytic Properties of Silver Oxide Loaded Bismuth Vanadate☆
- Recovery and Recycling of Ti Supported Bimodal Mesoporous Catalysts Prepared via Ship-in-a-bottle Method in the Epoxidation of Cyclohexene☆
- Promoting Xylene Production in Benzene Methylation using Hierarchically Porous ZSM-5 Derived from a Modified Dry-gel Route☆
- Energy,Resources and Environmental Technology Experimental and Modeling Study on de-NOxCharacteristics of Selective Non-catalytic Reduction in O2/CO2Atmosphere☆
- Energy,Resources and Environmental Technology CO2Removal from Biogas by Water Washing System☆