High-solid Anaerobic Co-digestion of Food Waste and Rice Straw for Biogas Production
2014-07-02PeiZhanjiangLiuJieShiFengmeiWangSuGaoYabingandZhangDalei
Pei Zhan-jiang , Liu Jie, Shi Feng-mei, Wang Su, GaoYa-bing, and Zhang Da-lei
1 College of Engineering, Shenyang Agricultural University, Shenyang 110161, China
2 Rural Energy Institute, Heilongjiang Academy of Agricultural Sciences, Harbin 150086, China
3 Liaoning Institute of Energy Resource, Yingkou 115003, Liaoning, China
Introduction
Food waste (FW), mainly coming from residential,restaurants, school cafeterias and factory lunchrooms,is expected to increase continuously in the next decade due to increasing population and improved standards of living (Dai et al., 2013). In China, the production of FW attained 10 million tons/year and occupied 50% of the municipal solid waste, almost 60% of FW has not reached necessary stabilization (Lin et al., 2011). Most of FW is landf i lled or untreated and may cause many environmental problems in the transportation and storage, such as contamination of soil and groundwater, generating greenhouse gases and odor due to volatile organic compounds of FW rapid decomposition (Zhang et al., 2011).
Anaerobic digestion (AD)of FW has been proven to be an eff i cient and green technology for solids reduction and biogas production in recent years (Shahriari et al., 2012; Zhang et al., 2013). However, anaerobic digestion was found unstable, when FW was used as mono-substrate due to high moisture, organic contents and biodegradability, which may encounter various potential inhibitors, including fast VFAs production from starch and free ammonia from protein (Liu et al.,2009; Chen et al., 2014). The main components of rice straw (RS)are lignin, cellulose and hemicellulose and it is difficult to degrade as mono-substrate (Gu et al., 2014). Recently, more researchers turned to study anaerobic co-digestion. Anaerobic co-digestion is simultaneous digestion of a mixture of two or more substrates and can enhance volumetric biogas production, improve system stability, dilute toxic chemicals, provide a better nutrient balance, manage mixed wastes easily and improve fertilizer value of digested residues (Shi et al., 2014; Zhu et al., 2014).The main purpose of this work was to study the effect of FW and RS co-digestion on biogas production and stability of the anaerobic co-digestion system, with TS contents ranging from 8% to 15%. Co-digestion of FW and RS was carried out under mesophilic conditions with six different mixing ratios and the effects of FW to RS ratio were investigated.
Materials and Methods
Feedstocks and eff l uent
FW was collected from Jinquan Environmental Protection Co., Ltd. FW was cut into particles with average size of 6.0 mm using a kitchen blender(ML-C620). The pieces of FW were flushed with nitrogen gas and stored in 20 L plastic containers at–20℃ until thawed and kept at 4℃ for use as a feedstock (Shin et al., 2010; Frigon et al., 2012). FW mixture consisted of (75%of wet weight)35% vegetable roots, 6% meats, 20% rice, 8% flour and 31%others (Table 1). RS was obtained from a local farm in Minzhu District, Harbin, and ground through a 10-mm sieve with a grinder. The ground RS was stored in air tight containers until use. The anaerobic seeding sludge, used as inoculum for the experiments,was collected from an anaerobic fermentation tank of rural energy institute of Heilongjiang Academy of Agricultural Sciences. The inoculum was incubated at 35℃ and an agitation at 2 Hz with no substrate for 48 h prior to the start-up of the assays (Frigon et al., 2012).
Co-digestion of FW and RS
Batch anaerobic digestion (AD)with complete premixing was carried out at six FW/RS (1 : 0, 4 : 1,3 : 1, 2 : 1, 1 : 1 and 0 : 1), which referred to as T1 to T6. Each batch AD system consisted of a 5 L digestion glass bottle (4 L reactive volume), a 5 L gas collection glass bottle (filled with saturated salt water)and a 5 L liquid collection beaker. The batch AD systems were operated at (35±1)℃. All the tests were run with duplicate reactors.

Table 1 Characteristics of food waste, rice straw and seeding sludge
Analytical methods
Biogas was collected daily by saturated salt water displacement method (Zhang et al., 2013). The content of CH4, CO2and H2S in biogas was analyzed by gas chromatography (GC-7890A, Agilent in USA). GC applied two thermal conductivity detectors (TCD).Argon and nitrogen were used as carrier gas at pressure of 0.2 MPa and a flow rate of 20 mL · min-1. The temperatures of injection port, column oven,front TCD and back TCD were 125, 50, 150 and 150℃, respectively. VFAs content of effluents was analyzed by gas chromatography (GC-6890A, Agilent in USA), which applied a flame ionization detector(FID)and equipped with a 30 m×250 μm×1.4 μm capillary column (Agilent, DB-624UI). Nitrogen was used as carrier at a pressure of 25 kPa and an injection rate of 400 mL · min-1. The oven temperature was programmed to rise from 40℃ to 200℃ at a rate of 10℃ · min-1. COD, total ammonia nitrogen (TAN), VS and TS were measured according to the procedures outlined by APHA Standard Methods (APHA, 1995).For sodium determination, the samples were digested with HNO3, followed by elemental analysis using inductively coupled plasma optical emission spectrometry(Optima2100 DV, PerkinElmer, US)(Da et al., 2013).
Results
Effect of FW/RS ratios on biogas production yields and CH4 content
The batch anaerobic digestion test was carried out efficiently and stably at six different FW/RS mixture ratios (1 : 0, 4 : 1, 3 : 1, 2 : 1, 1 : 1, and 0 : 1). The biogas production and CH4content at different time are summarized in Figs. 1 and 2, respectively. It can be seen that FW/RS ratio had a signif i cant effect on biogas yields and CH4contents. The maximum production yield of the biogas was 60.55 mL · g · VS-1· d-1with the mixture ratio of 3 : 1 (T3), which were 178% and 70%higher than those of mono-digestion FW (T1)and RS(T6), respectively. Fig. 1 showed that the daily biogas yield of T2 to T5 increased rapidly in the initial eight days, and 75% of the final biogas production was produced in the initial 10 days. After 60 days of the digestion, the maximum biogas yield of T2, T3, T4 and T5 were 50, 60.55, 55.25 and 47.36 mL · gV · S-1·d-1, respectively. From Fig. 2, the maximum CH4contents of T2, T3, T4 and T5 were 62, 70, 67 and 58,respectively. For the reactors with 100% of FW, the failure of the anaerobic digestion could be observed after the 6th day. While the biogas yield of monodigestions RS stayed stable at a low level during the 60-day digestion, and CH4contents were the lowest of all the tested ratios except T1. Compared with monodigestions of T1 and T6, CH4contents of the codigested reactors (T2, T3, T4, and T5)were all enhanced. The results showed that system stability and biogas yield of co-digestions of FW and RS were improved. It had been widely reported that anaerobic co-digestion of two or more substrates could improve system stability and increase total biogas production yield (Zhang et al., 2013). For instance, at levels up to 60% of the initial volatile solids, adding the food waste into a manure digester signif i cantly increased the methane yields for 20 days of the digestion (El-Mashad,2010). A methane yield of 0.85 L CH4· L-1· d-1at 36℃and 0.82 CHH4· L-1· d-1at 55℃ were obtained in continuously stirred-tank reactors containing 70%cattle manure, 20% food waste and 10% sewage sludge(Quiroga et al., 2014). Anaerobic co-digestion of the food waste and yard waste at specif i c ratios could improve digester operating characteristics (Brown, 2013).
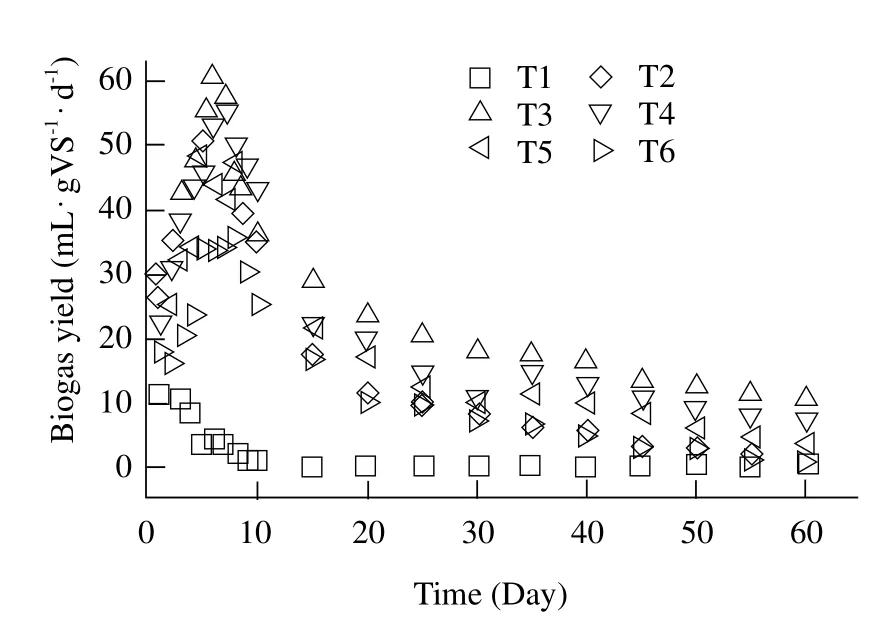
Fig. 1 Daily biogas yield based on VS

Fig. 2 CH4 content in biogas
Concentrations of VFAs in biogas slurry
The variation of the effluent concentration of VFAs with time during the anaerobic co-digestion period is shown in Fig. 3. VFAs concentration of T1-T6 increased and then decreased rapidly during the initial 5 days except mono-digestion of FW (T1), in which the maximum VFA concentration (35 600 mg · L-1)was obtained at the 5th day. During the initial 5 days of fermentation stage, the total VFAs concentration of T2-T5 reached 28 000 mg · L-1. VFAs concentration of T2-T5 was slightly lower than that of T1. Fig. 3 also revealed that the eff l uent concentration of VFAs increased smoothly with the increase concentration of FW, accordingly, resulting in acidification when the concentration of FW was too high in the system.FW contained soluble materials and biodegradable matter, such as proteins, starches and lipids and so on. It meant that FW had more acidogenic organic materials. During anaerobic mono-digestions of FW,biodegradable organic materials could be microbiologically degraded rapidly. It could be concluded that the hydrolysis of FW was the main reason for VFAs concentration increase (Zhang et al., 2013).Nagao et al. (2012)also found that the rate of methanogenesis would be lower than the rate of acidogenesis, acetogenesis in mono-digestion of FW,leading to VFAs accumulation.
The composition of VFAs was important as it could provide useful information regarding the degree of the hydrolysis and fermentation (Wang et al., 2014). VFAs were mainly composed of the acetate, propionate and butyrate. The content of acetate played a dominant role during anaerobic digestion (Zhang et al., 2013). Fig. 4 indicated that the contents of the acetate of all the treatments increased rapidly at the initial 5 days. The highest acetate concentrations of T2-T5 were 15 000,13 560, 12 480 and 9 800 mg · L-1, respectively. Therefore, it hinted that the higher FW concentration,the higher content of the acetate. The content of the acetate of T2-T5 decreased smoothly after the 6th day. The content of acetate of T2 to T5 was more than 2 000 mg · L-1after the 6th day in mono-digestions of FW and it was the result of the failure of anaerobic digestion. It could be explained that FW contained much more biodegradable matter and more acidogenic bacteria under anaerobic condition, leading to acidification of the system. It was disadvantageous to biogas production because methanogens were inhibited under acidic conditions (Wang et al., 2014).
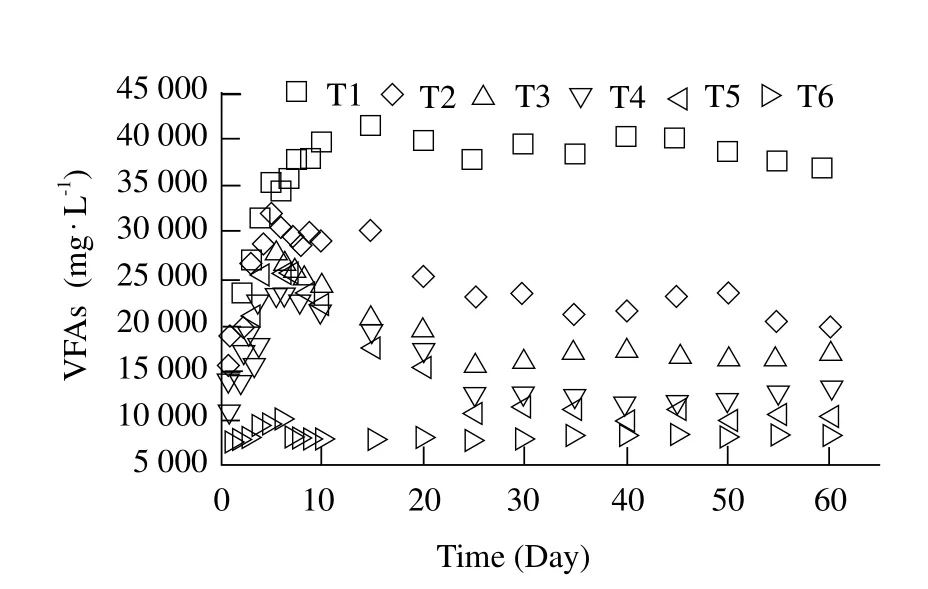
Fig. 3 Variation of eff l uent content of VFAs
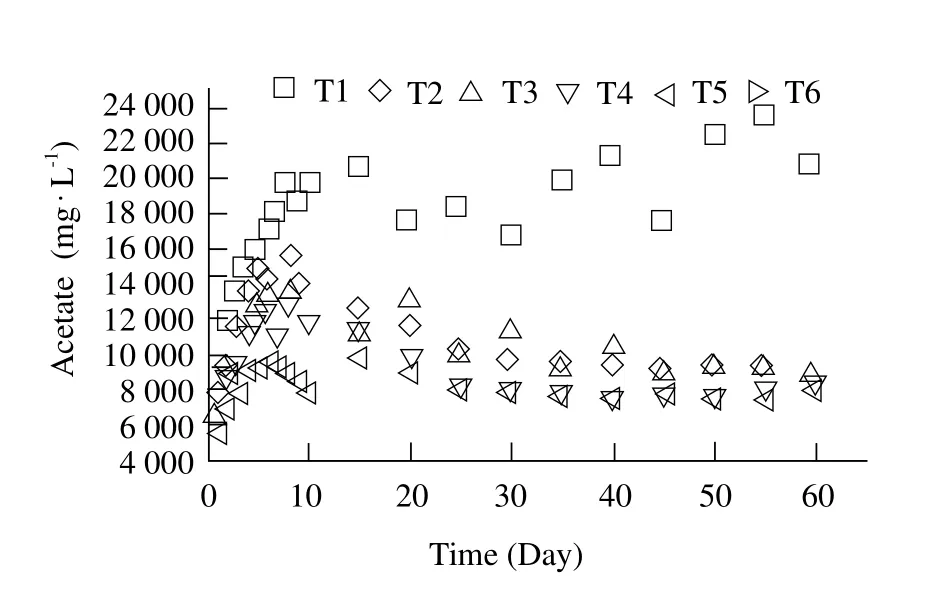
Fig. 4 Variation of eff l uent content of acetate
Propionate was a main intermediate in the stage of hydrolysis and it was an unfavorable substrate for the anaerobic digestion due to it was difficult to be directly decomposed into biogas (Meng et al., 2013).So the content of the propionate was very important during the anaerobic digestion. The concentration of propionate is shown in Fig.5. The propionate concentration of T2-T6 had been rose slowly at the initial 5 days and then maintained between 3 000 and 4 000 mg · L-1. The biogas productions of all the treatments were also relatively stable except that of T1, which showed that no acidification occurred when the pro-pionate concentration below 4 000 mg · L-1. Qiao et al.(2013)also confirmed that propionate accumulation could result in the system unstable due to a drop in pH,if the digester was not managed properly and carefully.
Butyrate, another component of the main VFAs, was converted into acetate during the anaerobic digestion production, which was considered the rate-limiting step of the anaerobic digestion. The concentration of butyrate is shown in Fig. 6. Compared to the variation of propionate with time, the content of butyrate increased dramatically and higher than that of the propionate.But the concentration of the butyrate in the reactors was stable from startup and throughout the period except T1. The highest butyrate concentration of T2-T5 were 7 850, 7 468, 6 850 and 6 047 mg · L-1, respectively.After startup, the contents of a butyrate of T2-T5 decreased smoothly between 3 000-4 000 mg · L-1, due to butyrate decomposed by methanogenic bacteria.
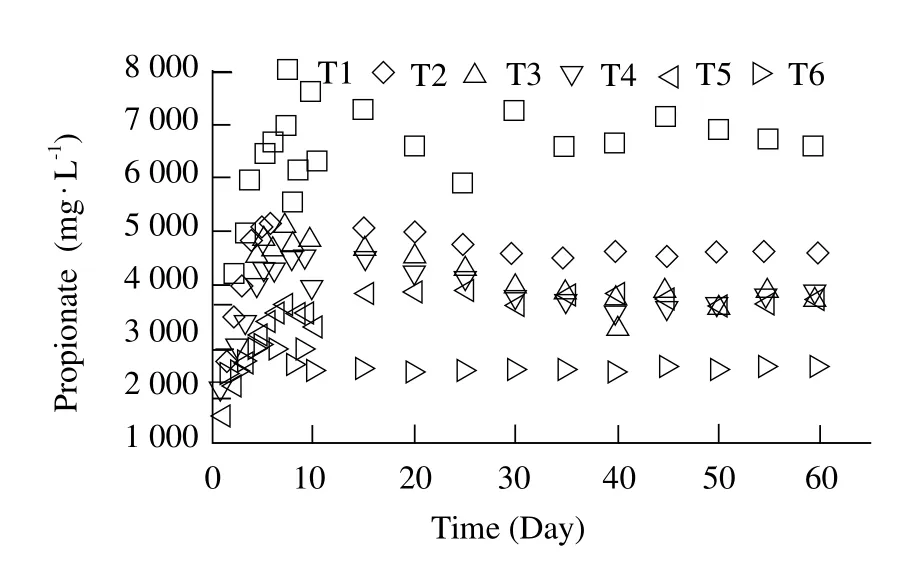
Fig. 5 Variation of eff l uent content of propionate
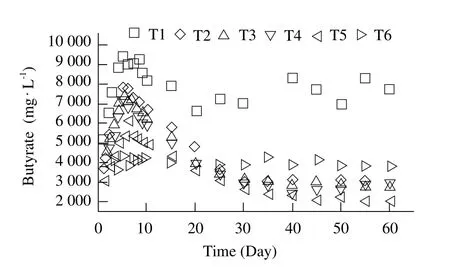
Fig. 6 Variation of eff l uent content of butyrate
Concentrations of ammonia nitrogen
Ammonia played a vital role on the performance and stability of anaerobic digestion of organic feedstock especially FW (Rajagopal et al., 2013). Yenigün(2013)pointed out proper content of ammonium was beneficial for methanogenic bacterial growth,but undesirably excessive ammonia nitrogen may be reached and may inhibit biogas production during the breakdown of proteins available in the substrate.Optimal ammonia concentration was usually used to evaluate the degree of the protein degradation optimal ammonia concentration and ensure sufficient buffer capacity on the system of the anaerobic digestion and resulted in increasing of the biogas production in the digestion process. The contents of ammonia nitrogen in different reactors are presented in Fig. 7. It was very similar in the variation of ammonia nitrogen concentration in eff l uent for all the treatments during fermentation. The contents of ammonia nitrogen increased linearly with fermentation time for initial 5 days. After the 6th day, NH4+-N concentrations decreased smoothly then remained between 900-1 500 mg · L-1and kept stable. From Fig. 7 it could be also founded that the more RS percent content, the higher content of ammonia nitrogen. It was clear that co-digestion of FW and RS could enhance the system stability of high-solid anaerobic digestion. The results suggested that co-digestion of FW and RS reduced concentrations of ammonia, thus better system stability could be achieved.
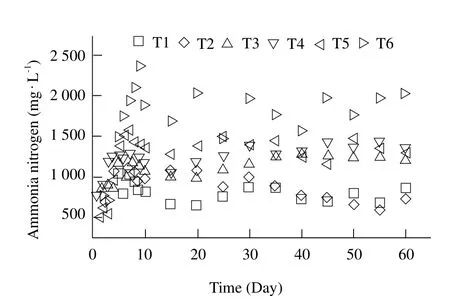
Fig. 7 Variation of eff l uent contents of ammonia nitrogen
Conclusions
From the results obtained, it could be concluded that the anaerobic co-digestion of FW and RS not only improved system stability but also greatly enhanced volumetric biogas production in comparison with mono-digestions. The highest methane yield of 60.55 mL · gV · S-1· d-1VS was obtained with a FW to RS of 3 : 1 based on total solids (TS), which was 178% and 70% higher than that of the mono-digestions of FW and RS, respectively. A high VFAs production could be obtained during anaerobic co-digestion, which increased smoothly with the increase of FW content,accordingly, resulting in acidification when FW content was too high.
APHA AWWA WEF. 1995. Aggregate organic constituents. In:Eaton A D, Clesceri L S, Greenberg A E. Standard methods for the examination of water and wastewater. Washington, DC, USA.pp.1-72.
Brown D, Li Y B. 2013. Solid state anaerobic co-digestion of yard waste and food waste for biogas production. Bioresour Technol,127C: 275-280.
Chen X, Yan W, Sheng K C, et al. 2014. Comparison of high-solids to liquid anaerobic co-digestion of food waste and green waste.Bioresour Technol, 154: 215-221.
Dai X H, Duan N N, Dong B, et al. 2013. High-solids anaerobic codigestion of sewage sludge and food waste in comparison with mono digestions: stability and performance. Waste Manag, 33(2): 308-316.El-Mashad H M, Zhang R H. 2010. Biogas production from co-digestion of dairy manure and food waste. Bioresour Technol, 101(11):4021-4028.
Frigon J-C, Mehta P, Guiot S R. 2012. Impact of mechanical, chemical and enzymatic pre-treatments on the methane yield from the anaerobic digestion of switchgrass. Biomass Bioenergy, 36: 1-11.
Gu Y, Chen X H, Liu Z G, et al. 2014. Effect of inoculum sources on the anaerobic digestion of rice straw. Bioresour Technol, 158C:149-155.
Lin J, Zuo J E, Gan L L, et al. 2011. Effects of mixture ratio on anaerobic co-digestion with fruit and vegetable waste and food waste of China. J Environ Sci, 23(8): 1403-1408.
Liu G Q, Zhang R H, El-Mashad H M, et al. 2009. Effect of feed to inoculum ratios on biogas yields of food and green wastes. Bioresour Technol, 100(21): 5103-5108.
Meng X S, Zhang Y B, Li Q, et al. 2013. Adding Fe0 powder to enhance the anaerobic conversion of propionate to acetate. Biochem Eng J, 73: 80-85.
Nagao N, Tajima N, Kawai M, et al. 2012. Maximum organic loading rate for the single-stage wet anaerobic digestion of food waste.Bioresour Technol, 118: 210-218.
Qiao W, Takayanagi K, Niu Q G, et al. 2013. Long-term stability of thermophilic co-digestion submerged anaerobic membrane reactor encountering high organic loading rate, persistent propionate and detectable hydrogen in biogas. Bioresour Technol, 149: 92-102.
Quiroga G, Castrillon L, Fernandez-Nava Y, et al. 2014. Effect of ultrasound pre-treatment in the anaerobic co-digestion of cattle manure with food waste and sludge. Bioresour Technol, 154: 74-79.
Rajagopal R, Masse D I, Singh G. 2013. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour Technol, 143: 632-641.
Shahriari H, Warith M, Hamoda M, et al. 2012. Effect of leachate recirculation on mesophilic anaerobic digestion of food waste. Waste Manag, 32(3): 400-403.
Shi X S, Yuan X Z, Wang Y P, et al. 2014. Modeling of the methane production and pH value during the anaerobic co-digestion of dairy manure and spent mushroom substrate. Biochem Eng J, 244: 258-263.
Shin S G, Han G, Lim J, et al. 2010. A comprehensive microbial insight into two-stage anaerobic digestion of food waste-recycling wastewater. Water Res, 44(17): 4838-4849.
Wang K, Yin J, Shen D S, et al. 2014. Anaerobic digestion of food waste for volatile fatty acids (VFAs)production with different types of inoculum: effect of pH. Bioresour Technol, 161: 395-401.
Yenigün O, Demirel B. 2013. Ammonia inhibition in anaerobic digestion: a review. Process Biochem, 48(5/6): 901-911.
Zhang C S, Su H J, Tan T W. 2013. Batch and semi-continuous anaerobic digestion of food waste in a dual solid-liquid system. Bioresour Technol, 145: 10-16.
Zhang C S, Xiao G, Peng L Y, et al. 2013. The anaerobic co-digestion of food waste and cattle manure. Bioresour Technol, 129: 170-176.
Zhang L, Lee Y W, Jahng D. 2011. Anaerobic co-digestion of food waste and piggery wastewater: focusing on the role of trace elements.Bioresour Technol, 102(8): 5048-5059.
Zhu J Y, Zheng Y, Xu F Q, et al. 2014. Solid-state anaerobic codigestion of hay and soybean processing waste for biogas production.Bioresour Techno, 154: 240-247.
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Recent Progress of Commercially Available Biosensors in China and Their Applications in Fermentation Processes
- Wheat Generation Adding in Xundian County of Yunnan Province in Summer
- Screening of Optimal Differentiation Medium to Lonicera edulis
- A Corpus-based Study on Collocation and Colligation of "Soil" in Agricultural English
- Microsatellite Analysis of Genetic Diversity Between Loach with Different Levels of Ploidy
- Rapid Non-destructive Detection for Molds Colony of Paddy Rice Based on Near Infrared Spectroscopy
