Screening of Optimal Differentiation Medium to Lonicera edulis
2014-07-02LiFuhengXuQinghuaQuDiZhuHuijieLiNandingandLiuZengbing
Li Fu-heng, Xu Qing-hua, Поляков А В, Qu Di, Zhu Hui-jie, Li Nan-ding, and Liu Zeng-bing
1 College of Life Sciences, Northeast Agricultural University, Harbin 150030, China
2 All Russian Research Institute of Vegetable Crops RAAS, Ramensky District, Moscow Region, 140153, Russia
Introduction
Lonicera edulis (Lonicera caerulea L. var. edulis Turcz. ex Herd), belonged to Caprifoliaceae Lonicera L.,also known as black bear fruit, Yama Ko, is a perennial deciduous shrub, and is mainly distributed in the northeast of China, Inner Mongolia and Far East of Russian (Huang et al., 1982; Li et al., 2002).Its fruit is rich in sugar, organic acid, mineral, vitamin and various microelements. It could be eaten raw, for processing and landscaping, and also could be used to extract the natural edible red pigment. This fruit has a high medicinal value so that is enjoyed "the 3rd generation fruit" (Xiang et al., 2004). Now, the main problem of the restricting the development of Lonicera edulis is the depletion of the wild resources and the lack of the good artif i cial cultivated varieties.
Therefore, conducting the system research by combining the tissue culture rapid propagation technology and breeding new varieties of Lonicera edulis gains the important significance in theory and practice to improve seedling breeding efficiency, accelerate the breeding process, protect and rational use of Lonicera edulis resources, improve the economic value and the level of production, increase the income of the peasants and so on.
Some researches on Lonicera edulis tissue culture and the rapid propagation technology have been made by researchers at home and abroad, which had made important progresses, such as the disinfection of the explants and the screening of optimum induction medium (Ding et al., 2006; Jin et al., 2011). Some researchers succeed in the induction of the callus and tube seedlings when using stem segments as explants,which survival rate is over 90% (Zhao et al., 2003;Liang et al., 2011). Some researchers succeed in screening the suitable medium by using axillary buds as explants for tissue culture and rapid propagation,with 3.3 multiplication coeff i cient (Yang et al., 2012;Li et al., 2012).
Nevertheless, there are few reports on differentia-tion medium which combined the tissue culture rapid propagation and the cultivation of the novel breeds.Therefore, an excellent Lonicera edulis strain L1-8 was used as material in this research. The tissue culture differentiation medium of L1-8 was optimized by a four-factor and four-level orthogonal test. The study was based on the rapid propagation technology and breeding new varieties, had a strong pertinence, and can provide theory basis and technical support for the development of the industrialization of Lonicera edulis.
Materials and Methods
Materials
An excellent Lonicera edulis strain, L1-8 that was bred by Northeast Institute of Geography and Agroecology, Chinese Academy of Sciences, was used as material in this research. The experiments were carried out from the year 2012 to 2013 in the Northeast Institute of Geography and Agro-ecology, Chinese Academy of Sciences.
Preparation of culture medium
Added pre formulated liquor (major elements, trace elements, inositol, Fe salt, VB1, VB6, nicotinic acid,and glycine)according to the culture medium formula.Added 30 g sucrose and 7 g agar into 1 000 mL solution, adjusted pH to 5.8, packed into triangle bottle,and sealed the bottle by sealing fi lm, and sterilized for 20 min at 121℃.
Obtain explants and disinfection methods
The Lonicera edulis dormant branches were collected and washed for 3 h with running water, then hydroponics to axillary bud germination. Took a 0.5 cmlong axillary bud, and made it wash by sterile water,and treated with 75% ethanol for 10 s. The four disinfection methods treated 5 min respectively with 10% sodium hypochlorite, 10% calcium chloride, 0.1%mercuric chloride and 6% hydrogen peroxide solution,washed 4-5 times with sterile water, and put onto the sterile fi lter paper to dry the surface (Table 1).optimum disinfection method for the explants.
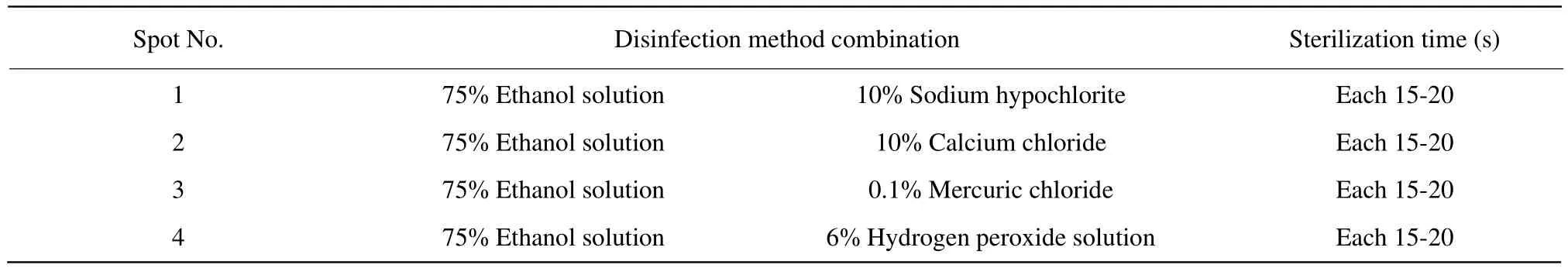
Table 1 Different disinfection methods
Screening of explant disinfection methods
The sterile axillary buds were inoculated to the medium containing 2.0 mg · L-16-BA and 0.2 mg · L-1NAA on the super clean bench, there were 50 bottles of the disinfection treatment and each bottle with one explant. The experiment repeated for three times.The inoculated explants were cultured in the tissue culture chamber. The data of the number of the explant contamination, death and germination after 20 days was calculated for statistics analyses to screen the
Aseptic seedling culture
The axillary buds were disinfected with the above optimum disinfection method and were inoculated to the culture medium. The seedlings were removed and cut into segments for the differentiation culture before its growth reached the top of the triangle bottle.
Differentiation culture
Four basic medium (MS, White, WPM, and A3)and four concentrations of the three hormones (IBA,6-BA, and GA3)were used as factors and levels to design a four-factor and four-level orthogonal test that based on L16 (45)orthogonal chart. The aseptic bud seedlings were cut into 1.5 cm stems, and inoculated to the differentiation medium with 10 bottles of each treatment and each bottle with five stems. The differentiation numbers were statistic after 30 days and calculated the differentiation rate. Range analysis was carried out in order to screen the optimum differentiation medium composition.
Culture conditions
The culture temperature was (24±2)℃. The illumination time was 12 h · d-1(6: 00 a.m.-18: 00 p.m. light,18: 00 p.m.-6:00 a.m. dark). Each culture rack had two fl uorescent lamps with 40 W.
Calculation formulas and data processing
Contamination rate (%)=Number of contamination axillary buds/Total number of axillary buds×100
Death rate (%)=Number of death axillary buds/Total number of axillary buds×100
Germination rate (%)=Number of no contamination axillary buds/Total number of axillary buds×100
Differentiation rate (%)=Number of differentiation bud seeding stems/Total number of bud seeding stems×100
The test data was analyzed by using Excel 2007 and SPSS 10.0.
Results
Comparison of different explant disinfection effects
The contamination rate, death rate and germination rate of the four kinds of the explant disinfection methods were statistics and calculated in Table 2.
As shown in Table 2, treatment 4 (75% ethanol solution and 6% hydrogen peroxide solution)was the most undesirable method that the contamination rate(38.03%)and death rate (33.30%)were significantly higher than those of other treatments, and the germination rate (28.67%)was significantly lower than that of other treatments. The disinfection effect of treatment 3 (75% ethanol solution and 0.1% mercuric chloride)was the most desirable method that the contamination rate and death rate were 6.33% and 8% which were significantly lower than those of other three treatments and the germination rate was 85.67% which was signif i cantly higher than that of other three treatments. treatments 1 (75% ethanol solution and 10% sodium hypochlorite)and 2 (75%ethanol solution and 10% calcium chloride)were similar and the difference between their germination rates (56% and 62%)didn't reach significant level.Therefore, the optimum disinfection method of L1-8 axillary bud explants was 75% ethanol solution treatment for 15-20 s and 0.1% mercuric chloride treatment for 15-20 s.
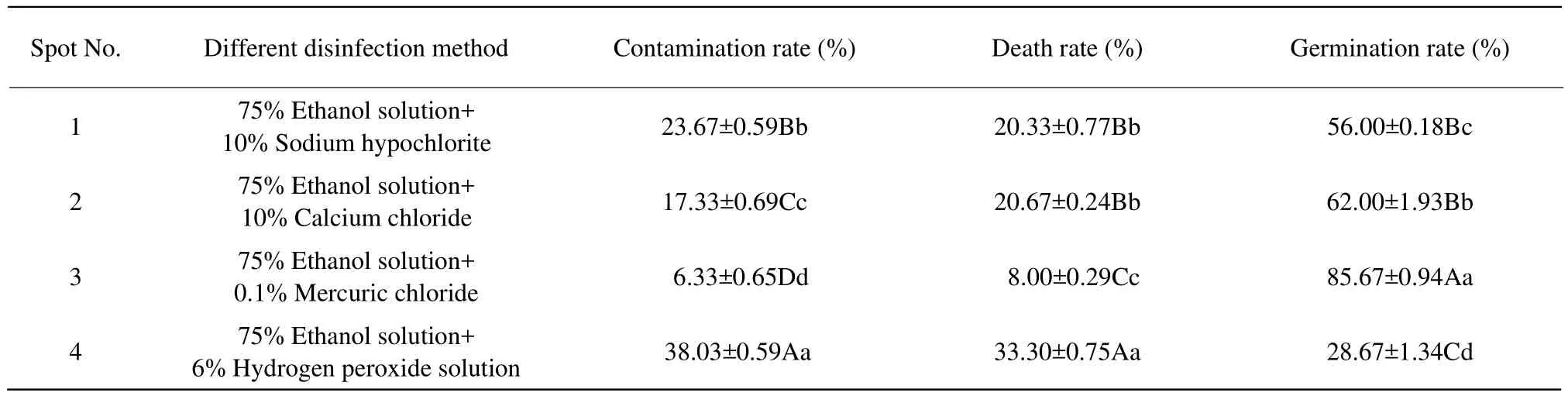
Table 2 Comparison of different treatments on disinfection effect of L1-8 explants
Effects of different basic media and hormone treatments on L1-8 differentiation rate
The differentiation rates of each treatment combination and the range analysis value of the orthogonal test results are shown in Table 3.
From Table 3, different kinds of the basic culture media and the three kinds of hormones with different concentrations of the treatment combinations could induce the differentiation of the explant and the differentiation rates were between 31.67%-95.92%. The differentiation rate of treatment 3 was the highest(95.92%)and it was higher than the lowest differentiation rate value of the treatment combination 13 64.25%.The range of each factor (R)followed by the size of the basic culture medium (25.97), 6-BA (24.68), IBA(14.07)and GA3(13.39)so that the primary and secondary order of the factor to differentiation rate was the basic culture medium>6-BA>IBA>GA3. The optimal levels of each factor are: MS culture medium,2.0 mg · L-16-BA, 0.3 mg · L-1IBA and 2.0 mg · L-1GA3.The optimal combination by range analysis was basically same with the highest differentiation rate of three treatments of the orthogonal test. The difference between the two lies was in the concentration of GA3,the former was 2.0 mg · L-1, and the latter was 1.5 mg · L-1.Considering the effect of GA3on the differentiation rate was minimal, and treatment 3 could save 25% GA3usage so that the cost could be reduced. Therefore, the complementary experiment was unnecessarily to be added, and the three treatment combinations could be determined as the optimal combination.

Table 3 Effect of different media and hormone combinations on L1-8 tissue culture seedlings' differentiation rate
Discussion
Attention paid to analysis results of orthogonal test
The orthogonal test design is composed of some representative levels of the experimental factors, and uses the partial tests instead of the comprehensive tests. The comprehensive test condition was obtained through the analysis of the partial experiment result,thus finding out the optimum level combination(Ming, 2005). It is need to pay attention that the most desirable level combination obtained by analyses is only a theoretical one. This level combination would not appear in orthogonal test design in general.Therefore, the complementary experiment will be added in order to validate the optimal level combination. Didn't add the complementary experiment and determine the optimal directly as the three treatment combinations in this research was a special case that did not have the general signif i cance.
What should be also realized that the optimal combination of the levels obtained from the analysis of the orthogonal test was relatively correlated to this study,not the absolutely "optimal". It's only a preliminary result, which needs to be further discussed and to improve the test ideas and refine the test condition in order to obtain the better culture medium formula. However, this point is easy to be negleced by researchers.
Screening and optimization of culture medium
The basic culture medium is an important medium in plant tissue culture. Different plants require various nutrients so different medium components are needed because of the difference of the biology and genetics characteristics (Zhong et al., 2001), and in each period of the organogenesis requirements for the medium of plants are also not the same.
Four kinds of the basic culture media, MS, White,WPM and A3(medium for strawberry)(Han et al.,1997)were used for the research of the differentiation in axillary bud explants of Lonicera edulis L1-8 strain. Each of the basic medium containing organic and inorganic salt components was not the same. The results showed that MS basic medium was the most suitable medium for differentiation of the axillary bud explants of L1-8 strain, which might be related to the number and proportion of the nutrients, the higher concentration of the inorganic salt and ion.In the prospective research of Lonicera edulis tissue culture, an approach that appropriate to add some ion concentrations can be used to optimize the selection medium for the purpose of improving the training effect and the proliferation coeff i cient.
Endogenous and exogenous hormones play an important role in regulating tissue culture process. The reasonable collocations of the auxin and cytokinin are the most factors that affected the explant differentiated.It is reported that the reasonable collocation of the auxin and cytokinin could promote the differentiation and proliferation of the shoots better than a single hormone. In the proliferation culture of the medicinal plants Huazelan, the reasonable collocation of 6-BA and IBA could yield the higher coefficient of bud germination with a rapid germination of the little bud and grow well in the later period (Liang et al., 2012).The results of this research showed that the optimum combination of differentiation culture of Lonicera caerulea axillary bud explants was MS contained 2.0 mg · L-16-BA, 0.3 mg · L-1IBA and 1.5 mg · L-1GA3,which differentiation rate was higher and the shoot grew well. The reason of lower differentiation rates of other hormone combinations might be due to different kinds of hormone physiological effects caused by mutual antagonism (Cui et al., 2000).
Conclusions
The optimum disinfection method of L1-8 axillary bud explants was 75% ethanol solution treatment for 15-20 s and 0.1% mercuric chloride treatment for 15-20 s, which contamination rate was the lowest. The best differentiation culture was MS culture medium+2.0 mg · L-16-BA+0.3 mg · L-1IBA+2.0 mg · L-1GA3,which differentiation rate was up to 95.92%.
Li Fu-heng and Qu Di contuibuted equally to this work.
Cui K R, Dai R L. 2000. Molecular biology of plant somatic embryogenesis. Beijing Science Press, Beijing. pp. 56-59.
Ding M, Feng R, Wang SY. 2006. Cyanidin-3-glucoside, anatural product derived from black bery, exhibits chemopreventive and chemotherapeutic activity. Joumal of Biological Chemistry, 281:17359-17368.
Han X M. 1997. Optimization of culture medium for differentiation of strawberry plantlets in vitro. Biological Technology, 7(2): 27-29.
Huang P H, Hao S W, Zhuo L H. 1982. Preliminary study of Lonicera edulis in the northeast of China. Research on Natural Resources, 1:57-62.
Jin C, Cao H N, Zong C W, et al. 2011. Research on tissue culture and rapid propagation technology of superior individuals of Lonicera edulis Turcz. Agricultural Science & Technology, 12(11): 1585-1588.Liang Z J, Pan C M, Lai Z Z, et al. 2012. Culture and rapid propagation of medicinal plant tissue Huazelan. Plant Physiology, 48(1):85-89.
Li G J, Li Y X, Lu H Y, et al. 2012. Russia hardy culture technology of Lonicera edulis tissue. Forestry Science and Technology, 37(4):219-220.
Li H, Xing G J, Lian M D. 2002. Medicinal plant Lonicera edulis.China Forest by Products, 10: 45-49.
Liang Q L, Zhang Q C, Yang Z G, et al. 2012. Technology of tissue culture of Lonicera edulis. Journal of Beihua University, 7(6): 25-27.Ming D X. 2005. Field experiment and biological statistics. Beijing Science Press, Beijing. pp. 247-251.
Teng H Y, Zhu G Q, Huang P, et al. 2005. Analysis of orthogonal test design examples. Pharmaceutical Care and Research, 8(1): 75-76.
Xiang Y J, Wang D W. 2004. Current situation and development prospect of Lonicera caerulea utilization. Journal of Tarim Land Reclamation University, 16(4): 26-29.
Xiao Y, Zhang C H, Zhi Y X, et al. 2001. Induction and plantlet regeneration of grape fl ower organ somatic embryo. Journal of Fruit Science, 28(5): 888-892.
Yang Z G, Zhang Q C, Sun G R. 2012. Study on rapid propagation technology of Lonicera edulis tissue culture. Hubei Agricultural Sciences, 52(3): 706-707.
Zhao Y, Huo J W, Wang L J. 2003. Tissue culture and plantlet regeneration of Lonicera edulis. Plant Physiology Communications,39(5): 468-470.
Zhong Y, Zhang J, Luo C D, et al. 2001. Study on the technique system of tissue culture of Rhododendron (I)- basic medium and explant selection. Journal of Sichuan Agricultural University, 19(1): 37-39.
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Recent Progress of Commercially Available Biosensors in China and Their Applications in Fermentation Processes
- Wheat Generation Adding in Xundian County of Yunnan Province in Summer
- High-solid Anaerobic Co-digestion of Food Waste and Rice Straw for Biogas Production
- A Corpus-based Study on Collocation and Colligation of "Soil" in Agricultural English
- Microsatellite Analysis of Genetic Diversity Between Loach with Different Levels of Ploidy
- Rapid Non-destructive Detection for Molds Colony of Paddy Rice Based on Near Infrared Spectroscopy
