SYNTHESIS AND STRUCTURE OF A MIXED-LIGANDS COMPLEX[CU(R-LACT)(O-PHEN)(H2O)]·4H2O
2014-06-01ZHANGQiangTANGDingxing
ZHANG QiangTANG Ding-xing
(1 Department of Material Engineering,Wuhu Institute of Technology,Wuhu Anhui 241002)
(2 Institute of Biochemical Engineering,Anhui Polytechnic University,Wuhu Anhui 241000)
SYNTHESIS AND STRUCTURE OF A MIXED-LIGANDS COMPLEX[CU(R-LACT)(O-PHEN)(H2O)]·4H2O
ZHANG Qiang1TANG Ding-xing2
(1 Department of Material Engineering,Wuhu Institute of Technology,Wuhu Anhui 241002)
(2 Institute of Biochemical Engineering,Anhui Polytechnic University,Wuhu Anhui 241000)
A mixed-ligands complex with the formula of[Cu(R-lact)(o-phen)(H2O)]·4H2O was synthesized by the reaction of copper carbonate with racemic lactic acid(RS-H2lact),and then with o-phenanthroline(o-phen)in ethanol-water(95%)solution.One of the two obtained compounds was dark blue column single crystal and was characterized by elemental analysis, IR spectrum and X-ray single crystal diffraction.The crystal belongs to triclinic system with space group P1,a=7.2825(5),b=11.7322(8),c=12.1194(8),α=65.8280(10)°,β=84.2120(10)°, γ=75.3290(10)°,V=913.88(11)3,Z=2,Dc=1533kg·m-3,Mr=421.89,μ(MoKα)=1.240mm-1, λ=0.71073,the final R=0.0483 and ωR=0.1199[I>2σ(I)]for 3120 observed reflections, F(OOO)=438.The copper atom is five coordinated with the distorted square pyramid environment.The five coordinated atoms are two oxygen atoms of depronated hydroxyl group and carboxyl of H2lact,two nitrogen atom of o-phen and one oxygen atom of water molecule.
copper(Ⅱ)complex;lactic acid;o-phenanthroline;crystal structure
1 Introduction
It is well known that lactic acid is a bio-active organic acid and its some transition metal and rare earth metal complexes have bio-catalytic activity[1~6].Furthermore some mixed ligands complexes containing lactate can be used as active component of anticancer agents[7,8].In recent years,the syntheses, structures and properties of transition metal complexes with α-hydroxycarboxylate have been reported widely[3,5,9~13], especially vanadium, molybdenum complexes.O-phenanthroline is one of the most useful neutral ligand in the design of mixed-ligand complexes[14,15].In this paper we reported the synthesis, crystal structure and some properties of the complex with the formula of[Cu (R-lact)(o-phen)(H2O)]·4H20.
2 Experimental

All reagents used were of analytical grade and without further purification.C、H、N analysis wasperformed on PE2400 analytical instrument,while Cu(Ⅱ)was measured by EDTA titration after the compound was burned at 873K.The infrared spectra(400~4000cm-1)were recorded by using KBr pellets on FTS-40 Fourier transform spectrometer.X-ray diffraction data were collected at 293K on Smart CCD X diffractometer with graphite monochromatized MoK/α radiation(λ=0.71073).
Copper carbonated(1.95g,excessive content)and RS-H2lact(1.80g)were mixed and stirred in 50ml ethanol-water(95%)solvent at 70℃for 30 h to react completely,the final pH≈6,then o-phen (2g)was added and stirred for 10 h at the same temperature,filtered the mixture to get blue solution.It was let to evaporate slowly at room temperature.Two complexes were obtained,of which one was dark blue column single crystal and was used for X-ray single crystal analysis.

A single crystal(0.64×0.40×0.18mm)of the complex was selected and mounted on a glass fiber for the X-ray diffraction analysis.All measurements were performed on a Simens SMART CCD X diffractometer equipped with graphite monochromatized MoK/α radiation(λ=0.71073)at 293(2)K.A total of 4774 reflections were collected,of which 3195(Rint=0.0224)reflections is independent and 3120 observed ones with I>2σ(I)were used in succeeding refinements.The data set was collected in the range of 1.84≤θ≤25.03 with the index ranges h=-8~7,k=-11~13,l=-13~14,with phi and omega scans technique and corrected for Lp effects.The data were processed by the SAINT package[16]and corrected for adsorption effects by the SADABS[17]procedure.The unit cell parameters were determined and refined by using SMART software[18].The primary atom sites was solved by direct methods and the secondary by difference Fourier electron density maps with SHELXS-97 program[19].The positional and anisotropic thermal parameters of all non-hydrogen atoms were refined by full-matrix least-squares techniques of SHELX-97 to the final R=0.0483 and ωR=0.1199[I>2σ(I)](w=1/[σ2(Fo2)+ (0.0492P)2+1.8022P]where P=(Fo2+2Fc2)/3),the largest parameter shift(Δ/σ)max=0.001 and the Goodness of fit on F2S=1.108.The hydrogen atoms were geometrically fixed at the calculated positions attached to their parent atoms and treated as riding atoms.In the final difference map, the residual maximum and minimum peaks are 0.716 and-0.706e/3,respectively.
3 Results and discussion
The important bands observed in the IR spectrum of the complex have been assigned as follows, the band of 3500~3237cm-1(s)to ν(O-H)of water molecules,the band of 1596cm-1(s)to ν(C=N)of o-phen,1655cm-1(s)to νas(COO),1427cm-1(s)to νs(COO)of monodentate fashion of carboxyl of lact, all agreement with those observed in the crystal structure analysis.

Crystal data and structure refinement details are listed in table 1,the atomic coordinates and equivalent isotropic displacement coefficients of the complex are listed in table 2, the selected bond distances and bond angles are listed in table 3,and the hydrogen bonding are listed in table 4.The structure of the complex is depicted in Fig.1,and the unit cell packing diagram of the complex is depicted in Fig.2.

Table 1 Crystal Data and Structure Refinement
Table 2 Atomic Coordinates(×104)and Equivalent Isotropic Displacement Parameters(2×103)

Table 2 Atomic Coordinates(×104)and Equivalent Isotropic Displacement Parameters(2×103)
Ueqis defined as one third of the trace of the orthogonalized Uijtensor.
Atom x Y z UeqAtom x y z UeqCu 5184(1) 6071(1) 2187(1) 32(1) C(11) 7004(6) 5597(5) 191(4) 34(1)N(1) 6420(6) 4319(4) 2228(4) 36(1) C(12) 7120(6) 4355(5) 1137(5) 35(1)N(2) 6193(6) 6615(4) 477(4) 35(1) C(13) 2602(7) 6238(5) 3918(5) 40(1)C(1) 6568(8) 3174(5) 3142(5) 44(1) C(14) 2542(17) 7567(6) 3033(9) 124(5)C(2) 7380(9) 2034(6) 3007(6) 56(2) C(15) 1412(15) 8650(7) 3242(9) 118(4)C(3) 8049(9) 2065(6) 1903(7) 56(2) 0(11) 3748(5) 5380(3) 3647(3) 42(1)C(4) 7962(7) 3244(5) 924(6) 45(1) 0(12) 1603(6) 5999(4) 4837(3) 52(1)C(5) 8667(8) 3405(6) -268(6) 53(2) 0(13) 3715(5) 7713(3) 2061(3) 39(1)C(6) 8571(8) 4580(7) -1159(6) 52(2) 0(14) 7869(5) 6134(4) 3002(3) 44(1)C(7) 7736(7) 5724(6) -963(5) 41(1) 0(21) 7939(6) -3210(4) 5505(4) 56(1)C(8) 7605(8) 6975(6) -1832(5) 51(1) 0(22) 6344(7) 8988(5) 2114(4) 63(1)C(9) 6804(8) 7999(6) -1540(5) 51(1) 0(23) 5879(9) -727(5) 4351(5) 85(2)C(10) 6118(8) 7791(5) -371(5) 44(1) 0(24) 1911(8) 9811(6) 56(5) 85(2)

Symmetry transformations used to generate the equivalent atoms
Table 4 Bond Lengths(nm)and Bond Angles()of Hydrogen Bonds

Table 4 Bond Lengths(nm)and Bond Angles()of Hydrogen Bonds
Symmetry transformations used to generate the equivalent atoms:#1-x+1,-y+1,-z+1 #2 x+1,y-1,z #3-x+1,-y,-z+ 1 #4 x,y+1,z #5 x,y-1,z #6-x+1,-y+2,-z
D-H...A d(D-H) d(H...A) d(D...A) <(DHA)0(14)-H(1)...0(22) 0.93(3) 2.21(4) 3.002(6) 142(5)0(14)-H(2)...0(12)#1 0.94(3) 1.85(3) 2.760(5) 165(6)0(21)-H(3)...0(12)#2 0.92(3) 1.84(3) 2.750(5) 174(7)0(21)-H(4)...0(11)#3 0.92(3) 1.96(3) 2.869(6) 169(6)0(22)-H(5)...0(13) 0.93(3) 1.82(3) 2.732(6) 168(7)0(22)-H(6)...0(23)#4 0.92(3) 2.08(6) 2.841(7) 139(7)0(23)-H(7)...0(21) 0.94(3) 1.84(4) 2.751(7) 164(8)0(23)-H(8)...0(22)#5 0.93(3) 2.00(5) 2.841(7) 149(8)0(24)-H(9)...0(22)#6 0.94(3) 1.85(3) 2.777(7) 172(8)0(24)-H(10)...0(13) 0.94(3) 1.87(3) 2.792(6) 168(8)
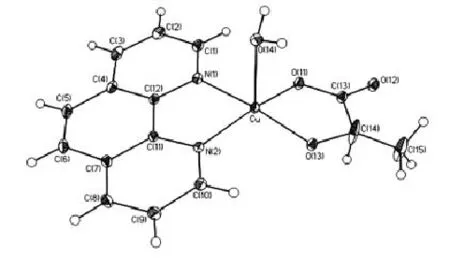
Fig.1 Structure of the Complex
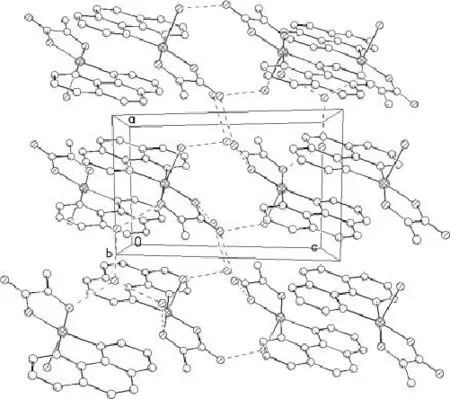
Fig.2 The unit cell packing diagram of the complex
The compound is composed of the complex of Cu (R-lact)(o-phen)(H2O)and crystal water molecules.The Cu(Ⅱ) atom of the complex is five-coordinated with distorted square pyramid environment.It is to say that the Cu(Ⅱ)atom coordinated with N(1)and N(2)atoms of o-phen,depronated hydroxyl 0(13) atom and depronated carboxyl 0(11)atom and 0(14)atom of water.The angle of N(1)-Cu-N(2)is 81.74°,N(1)-Cu-0(11)92.64°,0(11)-Cu-0(13)85.83°,0(13)-Cu-N(2)97.71°,with the sum of 357.92°.Therefore the plane consisting of CuN(1)N(2)0(11)0(13)is uncoplanar.The bond angles of 0(14)-Cu-N(1), 0(14)-Cu-N(2),0(14)-Cu-0(11),0(14)-Cu-0(13)are 90.92°,94.59°,100.41°,96.90°respectively,in-dicating that 0(14)located at the top of the pyramid while N(1)N(2)0(11)0(13)composed the bottom with mean deviation from plane 0.0646.The distances of Cu and 0(14)to the bottom are 0.1977and 2.5005respectively.
The uncoordinated water molecules are all crystal ones.In the compound there is no obvious π-π stacking interaction between o-phen.But all water molecules act as proton donor to form moderate hydrogen bonding with the other oxygen atom of water and the oxygen of lact-2to give a 3D supramolecule.
The single crystal of mixed-ligands complex of [Cu(R-lact)(o-phen)(H2O)]·4H2O was obtained and was characterized by elemental analysis,IR spectrum and X-ray single crystal diffraction.The crystal belongs to triclinic system with space group P1and unit cell dimensions of a=7.2825(5),b= 11.7322(8),c=12.1194(8),α=65.8280(10)°, β=84.2120(10)°,γ=75.3290(10)°.The copper atom is five coordinated with the distorted square pyramid environment.The five coordinated atoms are two oxygen atoms of depronated hydroxyl group and carboxyl of H2lact,two nitrogen atom of o-phen and one oxygen atom of water molecule.The water molecules act as proton donor to form moderate hydrogen bonding with the other oxygen atom of water and the oxygen of lact-2 to give a 3D supramolecule.
[1]Zhu G M,Liu Z R.Shape-memory polymers for biomedical engineering applications[J].Journal of Biomedical Engineering, 2005,(5):1082-1084.(in chinese)
[2]Jiang S B,Luo S Z.Aqueous thermodynamic equilibrium speciation simulation of Sm-NTMP[J].Chemical Research and Application,2004,(3):336-339.(in chinese)
[3]Zhou Z H,Hou S Y,Cao Z X,et al.Syntheses,crystal structures and biological relevance of glycolato and S-lactato molybdates[J].Journal of Inorganic Biochemistry,2004,(6):1037-1044.
[4]Yu K C,Wang F X,Zhang Y,et al.Synthesis and MRI relaxation enhancement of mono-exter-amido gadolinium complexes [J].Chinese Journal of Inorganic chemistry,2004,(4):389-393.(in chinese)
[5]Schwendt P,Svancarek P,Smatanova I,et al.Stereospecific formation of alpha-hydroxycarboxylato oxo peroxo complexes of vanadium(V).Crystal structure of(NBu4)2[V2O2(O2)2(L-lact)2]·2H2O and(NBu4)2[V2O2(O2)2(D-lact)(L-lact)]·2H2O[J].Journal of Inorganic Biochemistry.2000,(1-2):59-64.
[6]Lin M L,Liu X M,Cui X L,et al.Synthesis,characterizition and biological activity of rare earth complexes with benzimidazole-semidiethylenetriamine Schiff base[J].Chinese Rare Earths,2004,(6):38-40.(in chinese)
[7]Sinisterra R D,Shastri V P,Najjar R,et al.Encapsulation and release of rhodium(II)citrate and its association complex with hydroxypropjl-beta-cyclodextrin from biodegradable polymer microspheres[J].Journal of Pharmaceutical Sciences,1999,(5):574-576.
[8]Wang L H,Liu Y,Yuan F P,et al.Synthesis,Characterization and Antitumor Activities of a Series of Platinum(II)Complexes with 1R,3S-1,2,2-Trimethylcyclopentanediamine[J].Chinese Jouanal of Inorganic Chemistry.2004,(7):775-780.(in chinese)
[9]Elena B,Rosa C.,Alfonso C.et al.Coordination of α-hydroxycarboxylic acids with first-row transition ions[J].Coordination Chemistry Reviews,2013,(257):2639-2651.
[10]Chen X.Y.,George S.Goff,William C.,et al.Solid-State and Solution-State Coordination Chemistry of Lanthanide(III)Complexes with α-Hydroxyisobutyric Acid[J].Inorg.Chem.2012,(24):13254-13263.
[11]Susana B.,Rosa C.,Alfonso C.,et al.Structural features of palladium(II)complexes with a-hydroxycarboxylate and aromatic α,α’-diimine ligands[J].Polyhedron 2013,(50):512-523.
[12]M.Kaliva,C.Gabriel,C.P.Raptopoulou et al.A Unique Dinuclear Mixed V(V)0xo-peroxo Complex in the Structural Speciation of the Ternary V(V)-Peroxo-citrate System.Potential Mechanistic and Structural Insight into the Aqueous Synthetic Chemistry of Dinuclear V(V)-Citrate Species with H2O2[J].Inorg.Chem.,2011,(22):11423-11436
[13]Hati S,Batchelor R J,Einstein F W B,et al.Vanadium(V)Complexes of α-Hydroxycarboxylic Acids in Aqueous Solution [J].Inorganic Chemistry,2001,(24):6258-6265.
[14]Li J,Zhang F X,Tang Z X,et al.Synthesis crystal structure and properties of binuclear manganese complex[(phen)2Mn2(μ-O)(μ-Ac)2(H2O)2](ClO4)2·4H2O[J].Chemical Journal of Chinese Universities,2000,(6):836-839.(in chinese)
[15]Carballo R,Castineiras A,Covelo B,et al.Solid state coordination chemistry of mononuclear mixed-ligand complexes of Ni(II), Cu(II)and Zn(II)with α-hydroxycarboxylic acids and imidazole[J].Polyhedron,2004,(9):1505-1508.
[16]Siemens,SAINT Software Reference Manual,Siemens Energy&Automation Inc.,Madison,Wisconsin,USA,1994.
[17]Sheldrick G M.SADABS,Absorption Correction Program,University of Gottingen,Germany,1996.
[18]Siemens,SMART Software Reference Manual,Siemens Energy&Automation Inc.,Madison,Wisconsin,USA,1996.
[19]Sheldrick G.M.SHELXS-97 and SHELXL-97,Program for Crystal Structure Solution,University of Gottingen,Germany,1997.
责任编辑:陈澍斌
0614.121
AArtiqcle ID:1672-2868(2014)06-0061-07
Received:2014-06-21
Biography:ZHANG Qiang(1975-),male,born in Wuwei City,Anhui Province,Wuhu Institute of Technology,associate professor,master,major in applied chemistry.
