A Comparative Study of Intensive Litopenaeus vannamei Culture on Four Bottom Substrates Without Water Change
2014-05-05SHANHongweiZHANGLiGAOLeiSUYuepengBAOWeiyangandMAShen
SHAN Hongwei, ZHANG Li, GAO Lei, SU Yuepeng, BAO Weiyang, and MA Shen,
1) Key Laboratory of Mariculture of Ministry of Education, Ocean University of China, Qingdao 266003, P. R. China
2) College of Environmental Science & Engineering, Yangzhou University, Yangzhou 225127, P. R. China
3) Provincial Key Laboratory of Marine Fishery Molecular Biology of Liaoning, Liaoning Ocean and Fishery Science Institute, Dalian 116023, P. R. China
4) College of Oceanography and Environmental Science, Xiamen University, Xiamen 361005, P. R. China
A Comparative Study of Intensive Litopenaeus vannamei Culture on Four Bottom Substrates Without Water Change
SHAN Hongwei1), ZHANG Li2), GAO Lei3),1), SU Yuepeng4), BAO Weiyang2), and MA Shen1),*
1) Key Laboratory of Mariculture of Ministry of Education, Ocean University of China, Qingdao 266003, P. R. China
2) College of Environmental Science & Engineering, Yangzhou University, Yangzhou 225127, P. R. China
3) Provincial Key Laboratory of Marine Fishery Molecular Biology of Liaoning, Liaoning Ocean and Fishery Science Institute, Dalian 116023, P. R. China
4) College of Oceanography and Environmental Science, Xiamen University, Xiamen 361005, P. R. China
The effect of four bottom substrates, oyster shell powder (OP), sugarcane bagasse (SB), a mixture of OP and SB (OS) and fresh soil (FS), on the water quality and bacterial and zooplankton density of intensive shrimp (Litopenaeus vannamei) culture tanks without water change and the growth performance of cultured shrimp were compared in this study. At the end of a 110 days culturing trial, the total ammonium-N (TAN) of the water on SB and the nitrite nitrogen (NO2-N) on OS was significantly lower than that on the other substrates (P<0.05), which coincided with the high density of ammonium- and nitrite-oxidizing bacteria in the water on SB and OS, respectively. The concentration of chlorophyll a (Chla) increased slowly on OP, SB and OS but remained low on FS. The density of total bacteria on OP, SB and OS was one order of magnitude higher than that on FS, and the density of zooplankton on SB and OS was significantly higher than that on FS or OP (P<0.05). The improved water quality and increased density of bacteria and zooplankton on SB and OS may have had a synergistic effect on shrimp culture, improving its growth performance (high survival rate and yield and low feed conversion rate). SB and OS were more effective for improving the growth performance of intensively culturedL. vannameiwithout water change than OP and FS. To our knowledge, this study presents the first evidence regarding the effect of different bottom substrates on intensive shrimp culture.
bottom substrate; oyster shell powder; sugarcane bagasse; intensive shrimp culture
1 Introduction
The rapid intensification and expansion of shrimp culture has raised environmental concerns because these activities discharge a large amount of nutrient rich wastewater (Nayloret al., 1998; Nayloret al., 2000). As shown early in the intensive culture system of black tiger shrimp (Penaeus monodon) in Thailand, only 24% of feed nitrogen (N) was recoverable by shrimp harvesting, whereas the remainder was retained in the water and finally exported to the surrounding environment (Briggs and Funge-Smith, 1994). Jacksonet al. (2003) reported that approximately 57% of N input to an intensive culture pond ofP.monodonin Australia was discharged into the environment. In addition, it was reported that the amount of phosphorus, N and suspended solids reached 321668 and 215000 kg ha-1, respectively, in the intensive culture ponds ofP.monodonin Thailand in a four-month production cycle (Dierberg and Kiattisimkul, 1996). Thus, culturing shrimp intensively with less or no water change is highly appreciated; it reduces the effluent, making aquaculture more responsible and sustainable (Avnimelechet al., 1994).
Several water treatment techniques have been studied for their efficiency in reducing the need for water change in aquaculture systems. Of them, water recirculation and biofloc techniques are most effective. A water recirculation nsystem has been used in culturingP.monodonandLitopenaeus vannamei(Tsenget al., 1998; Menasvetaet al., 2001; Linet al., 2005; Reid and Arnold, 1992). Biofloc material absorbs excess nutrients, thus maintaining water quality (Hargreaves, 2006; De Schryveret al., 2008; Crabet al., 2012). It may also serve as a potential food source for shrimp (Thompsonet al., 2002; Burfordet al., 2004; Xuet al., 2012a). In contrast, the studies on the contribution of bottom substrates to shrimp culture are scarce.
In this study, several cheap materials suitable for microbial growth,i.e., OP, SB and OS, were used as the bottom substrate for the intensive culture ofL.vannamei.Oyster shell is porous and favorable for microbial colonization. It has been used as the biofilm carrier of ammonium oxidizing bacteria (AOB) in a biofiltration system of shrimp broodstock cultivation (Ivanovet al., 2006) and as the adsorption and filtration medium for phosphate removal in an integratively constructed wetland system for living sewage treatment (Park and Polpraser, 2008). SB is a favorable carrier of bacteria, on which biofilm may develop and serve as food for carp (Cyprinus carpio) and rohu (Labeo rohita) (Rameshet al., 1999); as it is rich in cellulose and hemicellulose, the carbon (C) source of microbes (Pandeyet al., 2000).
In this study, we compared the effect of OP, SB and OS, the potential bottom substrate, and FS, the most commonly used bottom substrate, on the intensive culture ofL.vannamei. The compared included 1) TAN, NO2-N and Chla, the indicators of water quality; 2) the density of total and N-cycle-related bacteria in bottom substrate and water; 3) zooplankton density; and 4) shrimp growth performance, including average body length, yield, survival rate and feed conversion ratio (feed quantity to shrimp yield, FCR).
2 Materials and Methods
2.1 Experimental Design
The juvenile shrimp (L.vannamei) was cultured in Lvyuan Aquatic Cultivation Co., Ltd., Zhoushan City, Zhejiang Province, China, from July 11 to October 29, 2009. The individual shrimp, 1.3 cm ± 0.1 cm in body length, were divided into FS, OP, SB and OS group and stocked separately, 3 tanks each group and 25 individuals each tank. The tank was 185 L in volume with about 0.17 m2of water surface area, 0.93 m of water depth and a bottom substrate depth of 5–10 cm. FS was applied directly, whereas the other substrates were rinsed thoroughly with clean seawater before setup. The OP and SB substrate were 0.1–0.3 cm and 0.1–0.5 cm in particle size and approximately 1.0 kg and 0.2 kg in weight, respectively.
During a 110 d culture period, the tank was aerated with air stones and covered with a cloth filtering out 50% of sunlight. The seawater in the tank was kept unchanged, but evaporation was reimbursed with fresh water for a loss of water in order to keep salinity constant. The shrimps were fed with a commercial feed (crude protein ≥42.0%, crude fiber ≤ 5.0%, crude ash ≤ 16.0%, moisture ≤12.0%, Ca2+≥ 1.5%, total phosphorus ≥ 1.0%, NaCl ≤3.0%, lysine ≥ 2.1%) twice a day at 06:00 and 18:00 and a rate of 10% body weight at beginning, which was gradually reduced to 4% body weight late in experiment based on an estimated survival rate of 80%. The shrimp growth performance was characterized based on the average body weight, yield, survival and FCR.
2.2 Sample Analysis
The concentration of TAN, NO2-N, and Chlawas determined every ten days according to the National Specification for Marine Monitoring of China (http://www.spsp. gov.cn/page/CN/2007/GB%2017378.4-2007.shtml). The density of total bacteria (TB) in bottom substrate and water was determined every ten days with a method modified from Hobbieet al. (1977). The N-cycle-related bacteria in bottom substrate and water, including AOB, nitrite-oxidizing bacteria (NOB) and denitrifying bacteria (DB), were enumerated using fluorescentin situhybridization (FISH) (Pernthaleret al., 2001; Pernthaleret al., 2002). After hybridization and color development, the bacteria were counted under a fluorescent microscope (Moter and Göbel, 2000). The probes specifically targeting to the 16S rRNA gene of AOB, NOB, and DB were NSO190 (Kindaichiet al., 2004), NIT3/CNIT3 (Schrammet al., 1996) and DEN67 (Ginigeet al., 2004), respectively. The density of zooplankton was determined every ten days according to the National Specifications for Oceanographic Surveys of China (http://www.spsp.gov. cn/page/CN/2007/GBT%2012763.6-2007.shtml). The collected zooplankton were preserved in 5% buffered formaldehyde, identified and counted under a stereomicroscope (Olympus, BX41) as described by Rosset al. (2008).
2.3 Statistical Analysis
The data were presented as mean ± standard deviation (n=3). All of the data were checked for normality, with the percentage arcsine transformed prior to analysis. Data comparison was made by one-way analysis of variation followed by Tukey’s test using SPSS 13.0. Difference was considered significant whenP< 0.05.
3 Results
3.1 Variation in the Concentration of Water TAN, NO2-N and Chla
Water quality parameters (TAN, NO2-N and Chla) were effectively improved by the addition of new bottom substrates. TAN concentration was less than 0.51 mg L-1in the first 60 d and varied later during culture (Fig.1A). A similar trend was found on FS, OP and OS. TAN sharply increased late during culture. In contrast, on SB, TAN was barely detectable on most days during culture, showing a significantly lower level than on other substrates (P< 0.05). NO2-N in water on all substrates was extremely low before day 60, but it increased rapidly late during culture (Fig.1B). At the end of culture (day 110), no significant difference was found among FS, OP and SB. However, the concentration on OS was significantly lower than that on other substrates (P< 0.05). Chlain water on OP, SB and OS slowly increased, whereas it remained low on FS (Fig.1C). From day 50 onward, Chlaon OP, SB, and OS was significantly higher than that on FS (P< 0.05).
3.2 Variation in Bacterial Density of Bottom Substrate
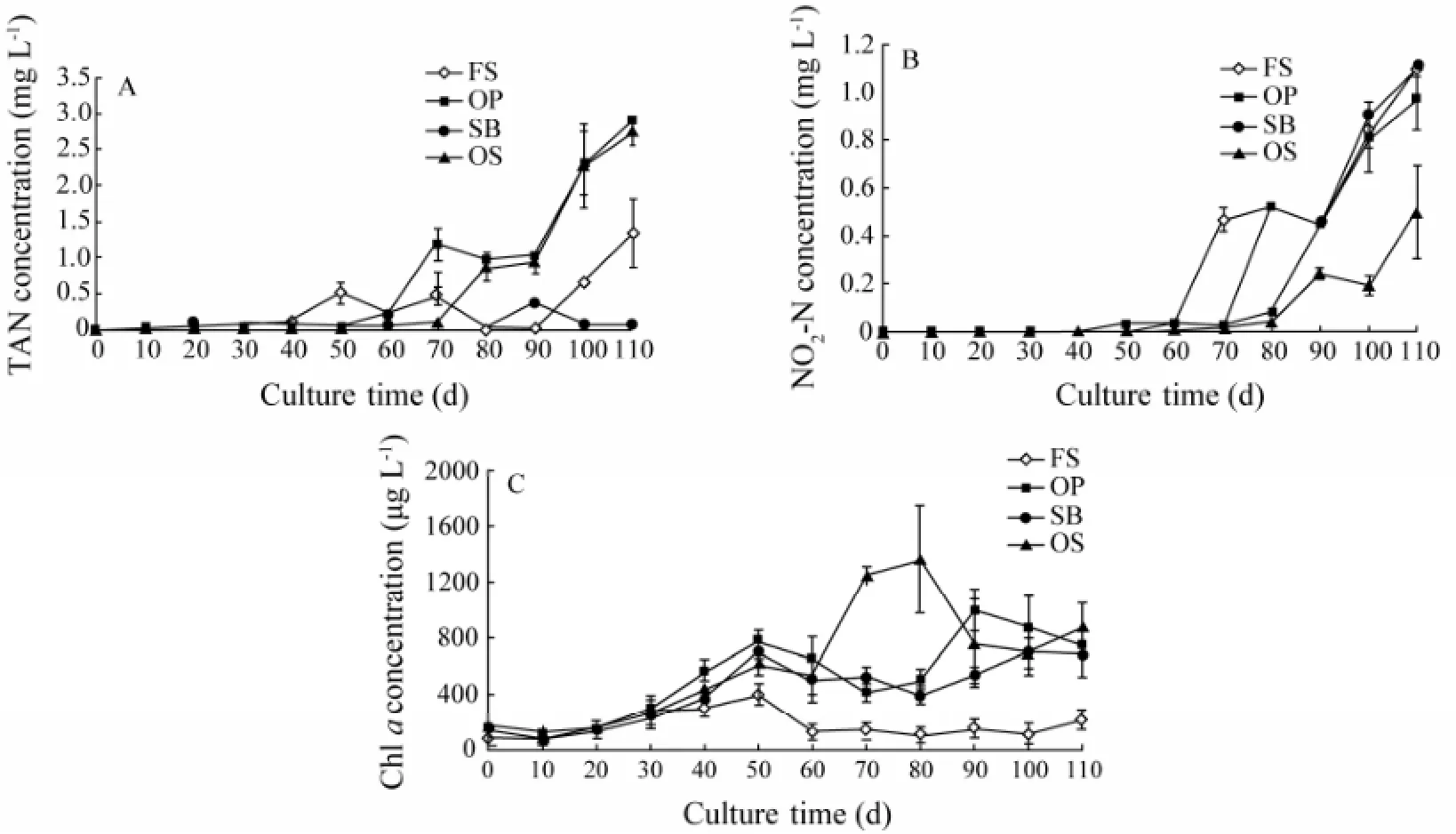
Fig.1 The variation in TAN (A), NO2-N (B) and Chl a (C) in water on four bottom substrate. Values are means ± SD (n=3).
The new bottom substrates (OP, SB and OS) effectively increased bacterial metabolism. The TB of FS remained < 8.74 × 109cells g-1during culture (Fig.2A); while that of OP, SB and OS increased gradually in the first 40 d and later stabilized at a density one order of magnitude higher than that of FS (P< 0.05). The density of N-cycle-related bacteria (AOB, NOB, and DB) exhibited a similar trend, gradually increasing early and stabilizing late (Figs.2B, C and D). The AOB of FS was below 1.52 × 104cells g-1, significantly lower than that of OP, SB or OS (P< 0.05, Fig.2B).
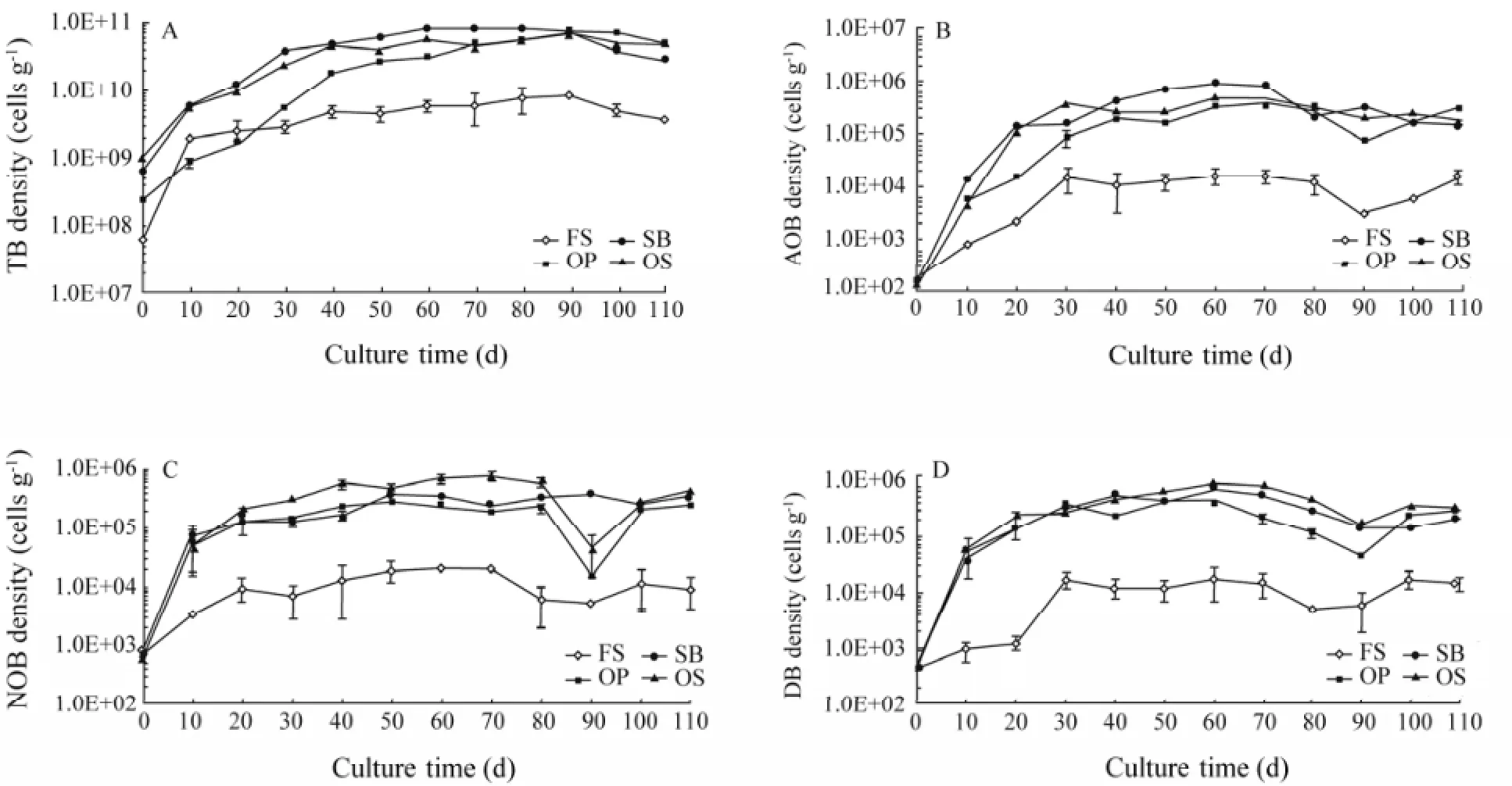
Fig.2 The density variation of TB (A), AOB (B), NOB (C) and DB (D). Values are means ±SD (n=3).
3.3 Variation in Bacterial Density of Water
The bacterial metabolism in culture water was effectively increased by the addition of new bottom substrates. The density of TB in water on all substrates gradually increased during culture (Fig.3A). The density of TB in water on SB was higher than that on other substrates, and no significant difference was found among FS, OP and OS. The density of N-cycle-related bacteria, AOB, NOB and DB, in water on all substrates gradually increased early and then quickly decreased with slight fluctuation late during culture (Figs.3B, C and D). The density of AOB on FS and OP was extremely low during culturing (Fig.3B), whereas that on SB and OS was generally one order of magnitude higher (P< 0.05, Fig.3B). The density of NOB on FS was lower than that on other substrates late during culture (days 90–110,P< 0.05, Fig.3C). The density of DB on OP, SB and OS was significantly higher than that on FS from day 10 to day 90 (P< 0.05, Fig.3D).
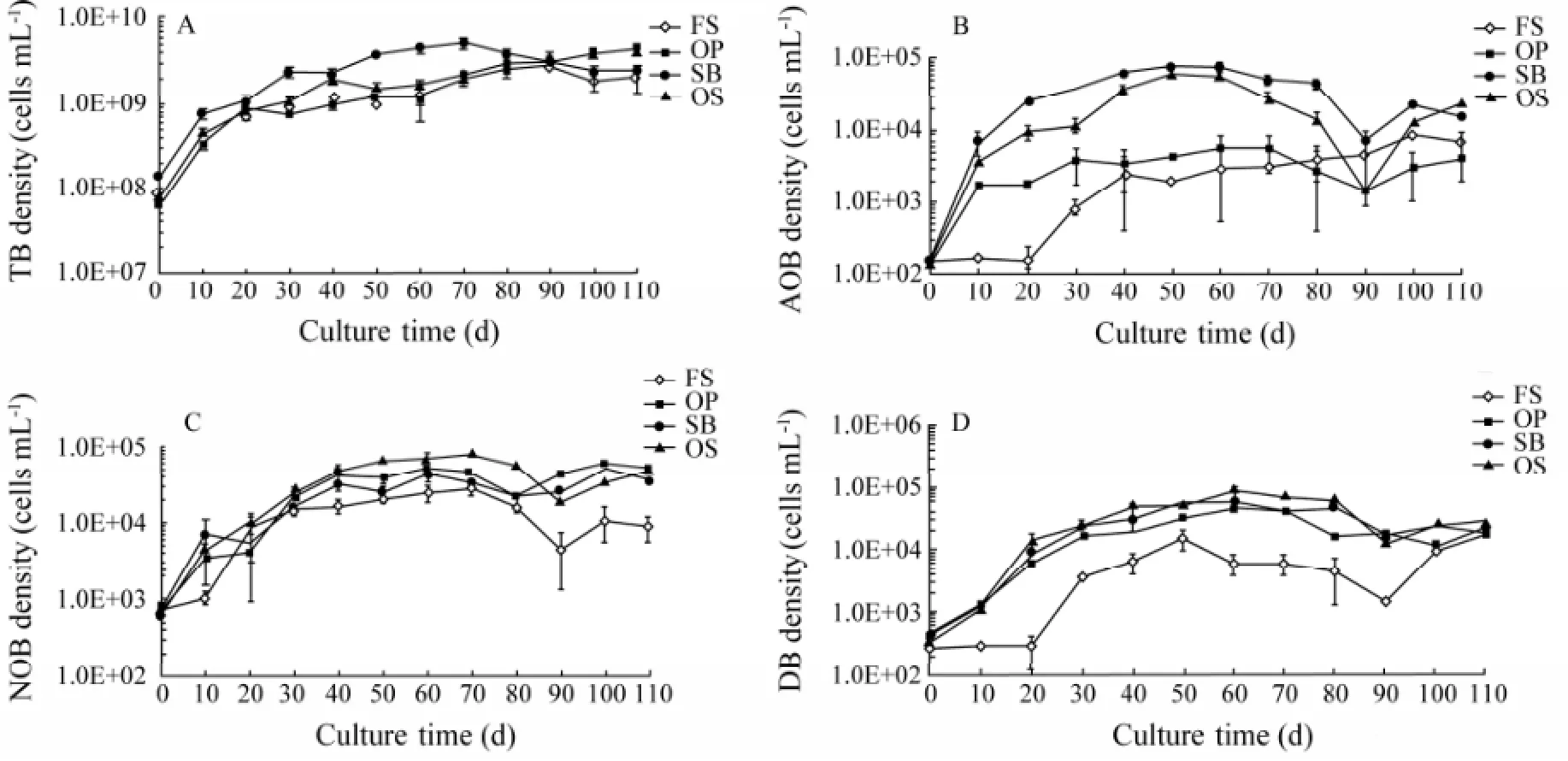
Fig.3 The variations of TB (A), AOB (B), NOB (C) and DB (D) in water on four bottom substrates. Values are mean ± SD (n=3).
3.4 Density Variation of Zooplankton
The SB increased the zooplankton density in culture water. The density of zooplankton on FS and OP remained less than 2 ind. L-1during culture (Fig.4). In contrast, that on SB and OS increased quickly early, reaching the maximum, 16 and 14 ind. L-1, respectively, in the middle of culture. The density of zooplankton on SB and OS exhibited a similar trend late during culture and decreased to 2 ind. L-1at the end. The density of zooplankton on SB-containing substrates, SB and OS, was significantly higher than that on substrates without SB (FS and OP) (P< 0.05).
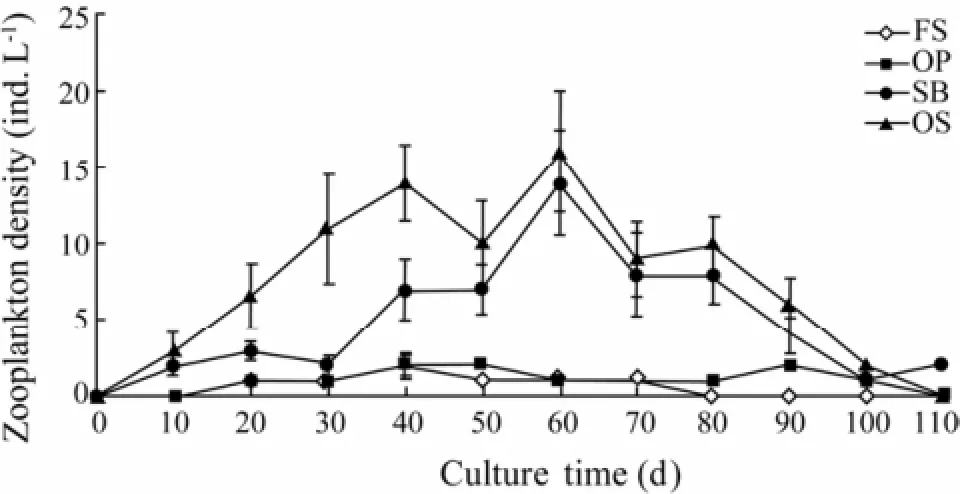
Fig.4 The density variation of zooplankton in water. Values are mean ± SD (n=3).
3.5 Effect of Bottom Substrates on the Growth Performance of Shrimp
No significant difference in average body length, survival rate, yield and FCR of shrimp was found among four substrates (Table 1). The average body length of shrimp on FS was 10.03 cm, somewhat longer than that on other substrates. The survival rate and yield on OS were 72% ± 7% and 1.21 ± 0.32 kg m-2, respectively, higher than those on FS, OP and SB. In addition, FCR on OS was 1.89 ± 0.58, lower than that on other substrates.
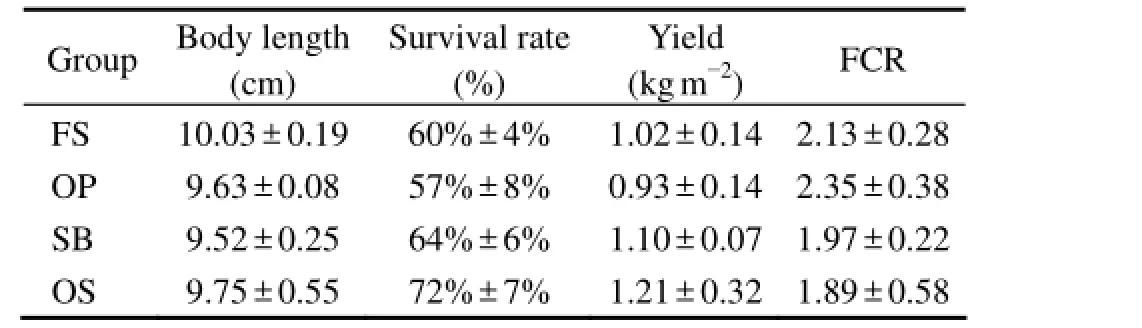
Table 1 The body length, yield, survival rate and FCR of L. vannamei at the end of culture
4 Discussions
Bottom substrate is very important for meeting success in shrimp culture, especially for semi-intensive to super-intensive culture (Hopkinset al., 1994; Bergheim and Åsgård, 1996; Avnimelech and Ritvo, 2003); as shrimp spend most of their lifetime at bottom, burrowing into and ingesting bottom substrate (Boyd, 1989). Therefore, it is essential to evaluate the effect of bottom substrate on the growth performance of shrimp so that an appropriate substrate can be identified.
TAN and NO2-N concentration were significantly improved by SB and OS (Figs.1A, B), which may be attributable to the high density of AOB and NOB in water on SB and OS, as AOB can convert TAN into NO2-N and NOB can oxidize NO2-N into NO3-N (Botheet al., 2000). Our findings are consistent with those reported previously (Raoet al., 2000). Studies have shown that oyster shell can absorb phosphate as was found in sewage treatment (Namasivayamet al., 2005; Park and Polpraser, 2008). Thus, OP is considered suitable for absorbing and aggregating organic matter. It may degrade into diverse compounds, especially nitrogenous ones, wich may significantly elevate the concentration of TAN and NO2-N. The water TAN concentration was higher on OP and OS than on FS and SB, which may be due to the higher content of organic N compound in oyster shell and their decomposi-tion (Lianget al., 2006). Ivanovet al. (2006) also reported that oyster shell has ecological advantages for microbial biofilm formation.
Previous studies showed that phytoplankton plays an important role in aquaculture; as a healthy phytoplankton bloom may reduce toxic substances by consuming NH4+and binding heavy metals (Chien, 1992; Daviset al., 2003; Bradleyet al., 2010; Liet al., 2010). In this study, we found that the content of Chlaincreased significantly in middle and late phase of culture on SB, OP and OS. Biofilm formation on SB (Rameshet al., 1999) or organic matter absorption by OP (Namasivayamet al., 2005) may result in a massive amount of organic aggregates under aeration, though such an effect requires further investigation. These aggregates may be a nutrient source for phytoplankton (Granéliet al., 1999), thus elevating the content of Chlaas was observed in this study. A high density of phytoplankton should aid to removing nutrients from water.
In this study, the density of TB and N-cycle-related bacteria on OP, SB and OS substrate was significantly higher than that on FS, indicating that the alternative bottom substrates effectively increased bacterial metabolism. The TB density on SB showed a different extent of increase with the addition of new substrate. This result could be attributed to adequate mixing by aeration of water column and the surface of SB, which benefited relevant bacterial growth (Pandeyet al., 2000; Rameshet al., 1999). Aeration may also break a small portion of SB and associating surface biofilms into particles, leading to biofloc growth in water column. Bioflocs have been demonstrated to maintain excellent water quality as well as favorable growth performance and protein conversion of fish and shrimp (Avnimelech, 2007; De Schryveret al., 2008; Crabet al., 2009; Gaoet al., 2012; Xuet al., 2012b). Further study is needed to investigate the potential promotion by SB of biofloc growth and the role of bioflocs in shrimp growth in intensive shrimp culture systems.
The zooplankton density in culture water was significantly higher on SB and OS substrate than that on other two substrates. Studies have shown that zooplankton can graze directly on microflora growing on pebbles, bottom sediment, detritus and plant substrate (Moriarty, 1997). Therefore, the biofilms and/or bioflocs around the particles in SB and OS might provide a food source for zooplankton and lead to the higher zooplankton density observed. A high density of zooplankton can contribute to the conversion efficiency of microbial protein into shrimp body protein (Andersonet al., 1987; Martínez-Córdova and Peña-Messina, 2005). The increased involvement of zooplankton on SB and OS substrate might also improve food web stability and the efficiency of material/energy flow through food chain.
Although there were no statistically significant differences, a higher shrimp survival rate and yield and a lower shrimp FCR were found on the SB and OS substrates. These may have been due to the synergistic effects of water quality and bacterial and zooplankton densities that were improved by the addition of SB in these two treatments.
In conclusion, this study provided the first evidence regarding the effect of different bottom substrates on intensive shrimp culture. SB and OP were two favorable bottom substrates; as they enhanced bacterial metabolism and improved iwater quality in intensiveL. vannameiculture without water change. Additionally, SB was beneficial for zooplankton growth, and its combination with OP was more effective for improving the growth performance of shrimp and maximizing the profit of intensive aquaculture systems.
Acknowledgements
This study was supported by National Science and Technology Supporting Program of the Twelfth Five-Year Plan of China (2011BAD13B10) and the Special Fund for Agro-scientific Research in the Public Interest, China (201103034). The authors are grateful to the reviewers for their critical comments on the manuscript.
Anderson, R. K., Parker, P. L., and Lawrence, A., 1987. A13C/12C tracer study of the utilization of presented feed by a commercially important shrimp Penaeus vannamei in a pond grow-out system. Journal of the World Aquaculture Society, 18 (3): 148-155.
Avnimelech, Y., Kochva, M., and Diab, S., 1994. Development of controlled intensive aquaculture systems with a limited water exchange and adjusted C to N ratio. Israeli Journal of Aquaculture-Bamidgeh, 46: 119-131.
Avnimelech, Y., and Ritvo, G., 2003. Shrimp and fish pond soils: Processes and management. Aquaculture, 220: 549-567.
Avnimelech, Y., 2007. Feeding with microbial flocs by tilapia in minimal discharge bio-flocs technology ponds. Aquaculture, 264: 140-147.
Bergheim, A., and Åsgård, T., 1996. Waste production from aquaculture. In: Aquaculture and Water Resource Management. Baird, D. J., et al., eds., Blackwell, Oxford, 50-80.
Bothe, H., Jost, G., Schloter, M., Ward, B. B., and Witzel, K. P., 2000. Molecular analysis of ammonia oxidation and denitrification in natural environments. FEMS Microbiology Reviews, 24: 673-690.
Boyd, C. E., 1989. Water quality management and aeration in shrimp farming. Fisheries and Allied Aquacultures Departmental Series No. 2. Alabama Agricultural Experiment Station. Auburn University, Alabama, 83pp.
Bradley, P. B., Lomas, M. W., and Bronk, D. A., 2010. Inorganic and organic nitrogen use by phytoplankton along Chesapeake Bay, measured using a flow cytometric sorting approach. Estuaries and Coasts, 33: 971-984.
Briggs, M. R. P., and Funge-Smith, S. J., 1994. A nutrient budget of some intensive marine shrimp ponds in Thailand. Aquaculture and Fisheries Management, 25: 789-811.
Burford, M. A., Thompson, P. J., Mcintosh, R. P., Bauman, R. H., and Pearson, D. C., 2004. The contribution of flocculated material to shrimp (Litopenaeus vannamei) nutrition in a high-intensity, zero-exchange system. Aquaculture, 232: 525-537.
Chien, Y. H., 1992. Water quality requirements and managementfor marine shrimp culture. In: Proceedings of the Special Session on Shrimp Farming. Wyban, J., ed., World Aquaculture Society, Baton Rouge, LA, 144-156.
Crab, R., Kochva, M., Verstraete, W., and Avnimelech, Y., 2009. Bio-flocs technology application in over-wintering of tilapia. Aquacultural Engineering, 40: 105-112.
Crab, R., Defoirdt, T., Bossier, P., and Verstraete, W., 2012. Biofloc technology in aquaculture: Beneficial effects and future challenges. Aquaculture, 356-357: 351-356.
Davis, T. A., Volesky, B., and Mucci, A., 2003. A review of the biochemistry of heavy metal biosorption by brown algae. Water Research, 37: 4311-4330.
De Schryver, P., Crab, R., Defoirdt, T., Boon, N., and Verstraete, W., 2008. The basics of bio-flocs technology: The added value for aquaculture. Aquaculture, 277: 125-137.
Dierberg, F. E., and Kiattisimkul, W., 1996. Issues, impacts, and implications of aquaculture in Thailand of shrimp. Environmental Management, 20: 649-666.
Gao, L., Shan, H. W., Zhang, T. W., Bao, W. Y., and Ma, S., 2012. Effects of carbohydrate addition on Litopenaeus vannamei intensive culture in a zero-water exchange system. Aquaculture, 342-343: 89-96.
Ginige, M. P., Hugenholtz, P., Daims, H., Wagner, M., Keller, J., and Blackall, L. L., 2004. Use of stable-isotope probing, full-cycle rRNA analysis, and fluorescence in situ hybridization-microautoradiography to study a methanol-fed denitrifying microbial community. Applied and Environmental Microbiology, 70 (1): 588-596.
Granéli, E., Carlsson, P., and Legrand, C., 1999. The role of C, N and P in dissolved and particulate organic matter as a nutrient source for phytoplankton growth, including toxic species. Aquatic Ecology, 33: 17-27.
Hargreaves, J. A., 2006. Photosynthetic suspended-growth systems in aquaculture. Aquacultural Engineering, 34: 344-363.
Hobbie, J. E., Daley, R. J., and Jasper, S., 1977. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Applied and Environmental Microbiology, 33: 1225-1228.
Hopkins, J. S., Sandifer, P. A., and Browdy, C. L., 1994. Sludge management in intensive pond culture of shrimp: Effect of management regime on water quality, sludge characteristics, nitrogen extinction, and shrimp production. Aquacultural Engineering, 13: 11-30.
Ivanov, V., Stabnikova, O., Sihanonth, P., and Menasveta, P., 2006. Aggregation of ammonia-oxidizing bacteria in microbial biofilm on oyster shell surface. World Journal of Microbiology and Biotechnology, 22: 807-812.
Jackson, C., Preston, N., Thompson, P. J., and Burford, M., 2003. Nitrogen budget and effluent nitrogen components at an intensive shrimp farm. Aquaculture, 218: 397- 411.
Kindaichi, T., Ito, T., and Okabe, S., 2004. Ecophysiological interaction between nitrifying bacteria and heterotrophic bacteria in autotrophic nitrifying biofilms as determined by microautoradiography-fluorescence in situ hybridization. Applied and Environmental Microbiology, 70 (3): 1641-1650.
Li, X., Hu, H. Y., Gan, K., and Yang J., 2010. Growth and nutrient removal properties of a freshwater microalga Scenedesmus sp. LX1 under different kinds of nitrogen sources. Ecological Engineering, 36: 379-381.
Liang, Y., Leonard, J. J., Feddes, J. J. R., and McGill, W. B., 2006. Influence of carbon and buffer amendment on ammonia volatilization in composting. Bioresource Technology, 97: 748-761.
Lin, Y. F., Jing, S. R., Lee, D. Y, Chang, Y. F., Chen, Y, M., and Shih, K. C., 2005. Performance of a constructed wetland treating intensive shrimp aquaculture wastewater under high hydraulic loading rate. Environmental Pollution, 134: 411-421.
Martínez-Córdova, L. R., and Peña-Messina, E., 2005. Biotic communities and feeding habits of Litopenaeus vannamei (Boone 1931) and Litopenaeus stylirostris (Stimpson 1974) in monoculture and polyculture semi-intensive ponds. Aquacutlture Research, 36: 1075-1084.
Menasveta, P., Panritdam, T., Sihanonth, P., Powtongsook, S., Chuntapa, B., and Lee, P., 2001. Design and function of a closed, recirculating seawater system with denitrification for the culture of black tiger shrimp broodstock. Aquacultural Engineering, 25: 35-39.
Moriarty, D. J. W., 1997. The role of microorganisms in aquaculture ponds. Aquaculture, 151: 333-349.
Moter, A., and Göbel, U. B., 2000. Fluorescence in situ hybridization (FISH) for direct visualization of microorganisms. Journal of Microbiological Methods, 41: 85-112.
Namasivayam, C., Sakoda, A., and Suzuki, M., 2005. Technical note removal of phosphate by adsorption onto oyster shell powder-kinetic studies. Journal of Chemical Technology and Biotechnology, 80: 356-358.
Naylor, R. L., Goldburg, R. J., Mooney, H., Beveridge, M. C. M., Clay, J., Folke, C., Kautsky, N., Lubchenco, J., Primavera, J., and Williams, M., 1998. Nature’s subsidies to shrimp and salmon farming. Science, 282: 883-884.
Naylor, R. L., Goldburg, R. J., Primavera, J. H., Kautsky, N., Beveridge, M. C. M., Clay, J. Folke, C., Lubchenco, J., Mooney H., and Troell, M., 2000. Effect of aquaculture on world fish supplies. Nature, 405: 1017-1024.
Pandey, A., Soccol, C. R., Nigam, P. Q., and Soccol, V. T., 2000. Biotechnological potential of agro-industrial residues. I: Sugarcane bagasse. Bioresource Technology, 74: 69-80.
Park, W. H., and Polprasert, C., 2008. Roles of oyster shells in an integrated constructed wetland system designed for P removal. Ecological Engineering, 34: 50-56.
Pernthaler, A., Pernthaler, J., and Amann, R., 2002. Fluorescence in situ hybridization and catalyzed reporter deposition for the identification of marine bacteria. Applied and Environmental Microbiology, 68: 3094-3101.
Pernthaler, J., GIöckner, F., Schönhuber, W., and Amann, R., 2001. Fluorescence in situ hybridization (FISH) with rRNA-targeted oligonucleotide probes. Methods in Microbiology, 30: 207-226.
Ramesh, M. R., Shankar, K. M., Mohan, C. V., and Varghese, T. J., 1999. Comparison of three plant substrates for enhancing carp growth through bacterial biofilm. Aquacultural Engineering, 19: 119-131.
Rao, P. S. S., Karunasagar, I., Otta, S. K., and Karunasagar, I., 2000. Incidence of bacteria involved in nitrogen and sulphur cycles in tropical shrimp culture ponds. Aquaculture International, 8: 463-472.
Reid, B., and Arnold, C. R., 1992. The intensive culture of the Penaeid shrimp Penaeus vannamei Boone in a recirculating raceway system. Journal of the World Aquaculture Society, 23: 146-153.
Ross, R. M., Quetin, L. B., Martinson, D. G., Iannuzzi, R. A., Stammerjohn, S. E., and Smith, R. C., 2008. Palmer LTER: Patterns of distribution of five dominant zooplankton species in the epipelagic zone west of the Antarctic Peninsula, 1993–2004. Deep-Sea Research II, 55: 2086-2105.
Schramm, A., Larsen, L. H., Revsbech, N. P., Ramsing, N. B., Amann, R., and Schleifer, K. H., 1996. Structure and function of a nitrifying biofilm as determined by in situ hybridizationand the use of microelectrodes. Applied and Environmental Microbiology, 62 (12): 4641-4647.
Thompson, F. L., Abreu, P. C., and Wasielesky, W., 2002. Importance of biofilm for water quality and nourishment in intensive shrimp culture. Aquaculture, 203: 263-278.
Tseng, K. F., Su, H. M., and Su, M. S., 1998. Culture of Penaeus monodon in a recirculating system. Aquacultural Engineering, 17: 138-147.
Xu, W. J., Pan, L. Q., Zhao, D. H., and Huang, J., 2012a. Preliminary investigation into the contribution of bioflocs on protein nutrition of Litopenaeus vannamei fed with different dietary protein levels in zero-water exchange culture tanks. Aquaculture, 350-353: 147-153.
Xu, W. J., Pan, L. Q., Sun, X. H., and Huang, J., 2012b. Effects of bioflocs on water quality, and survival, growth and digestive enzyme activities of Litopenaeus vannamei (Boone) in zero-water exchange culture tanks. Aquaculture Research, 2012: 1-10.
(Edited by Qiu Yantao)
(Received November 9, 2012; revised December 20, 2012; accepted December 4, 2013)
© Ocean University of China, Science Press and Spring-Verlag Berlin Heidelberg 2014
* Corresponding author. E-mail: mashen@ouc.edu.cn
杂志排行
Journal of Ocean University of China的其它文章
- Construction of Inorganic Elemental Fingerprint and Multivariate Statistical Analysis of Marine Traditional Chinese Medicine Meretricis concha from Rushan Bay
- Characterization, Expression and Function Analysis of DAX1 Gene of Scallop (Chlamys farreri Jones and Preston 1904) During Its Gametogenesis
- Secondary Metabolites of a Deep Sea Derived Fungus Aspergillus versicolor CXCTD-06-6a and Their Bioactivity
- Preparation, Characterization and Pharmacokinetics of Fluorescence Labeled Propylene Glycol Alginate Sodium Sulfate
- Early Development of Silvetia babingtonii (Fucales, Phaeophyceae)
- A Comparison of Different Gracilariopsis lemaneiformis (Rhodophyta) Parts in Biochemical Characteristics, Protoplast Formation and Regeneration
