Early Development of Silvetia babingtonii (Fucales, Phaeophyceae)
2014-05-05WANGGaogeWEIXiaojiaoSHUAILimeiLUBojunWANGShashaandKANGDongdong
WANG Gaoge, WEI Xiaojiao SHUAI Limei LU Bojun WANG Shasha, and KANG Dongdong
1) College of Marine Life Sciences, Ocean University of China, Qingdao 266003, P. R. China
2) Helmholtz-Zentrum für Ozeanforschung Kiel (GEOMAR), Düsternbrooker Weg 20 24105 Kiel, Germany
Early Development of Silvetia babingtonii (Fucales, Phaeophyceae)
WANG Gaoge1),*, WEI Xiaojiao1), SHUAI Limei1), LU Bojun1), WANG Shasha2), and KANG Dongdong1)
1) College of Marine Life Sciences, Ocean University of China, Qingdao 266003, P. R. China
2) Helmholtz-Zentrum für Ozeanforschung Kiel (GEOMAR), Düsternbrooker Weg 20 24105 Kiel, Germany
Silvetia babingtoniiis a potentially economic brown alga for sources of food and high-value added utilization. So far, sporeling nursery and field cultivation has not been successful. The lack of knowledge on development and life cycle of this alga hinder the development of techniques for the sporeings and cultivation. In this study, internal structure of oogonium and antherium ofS. babingtoniiwas observed with hematoxylin and eosin staining and through microscope. Meanwhile, early development from zygotes to juvenile sporelings was studied at 20℃ under 60–100 μmol photons m-2s-1. Zygotes germinated and divided into thallus and rhizoid cells. The larger thallus cells further divided and developed into juvenile sporelings; while the smaller rhizoid cells divided and elongated into rhizoid hairs. These findings documented the life cycle ofS. babingtoniiand provided fundamental knowledge for sporeling nursery in the near future.
Silvetia babingtonii; conceptacles; early development; life cycle; receptacles
1 Introduction
The cartilaginous algaSilvetia babingtonii(Fucaceae, Fucales, Phaeophyceae) commonly grows on intertidal rocks in warm-temperate waters in Japan, Korea and Russia (Lee and Kang, 2001; Nakaokaet al., 2006; Kozhenkova, 2009; Terasakiet al., 2009; Liuet al., 2011; Anastyuket al., 2012). It was formerly known asPelvetia babingtoniiand later transferred into genusSilvetiabased on the sequence of the internal transcribed spacer region (ITS-1, 5.8s and ITS-2) of nuclear ribosomal DNA (Serrãoet al., 1999).
S.babingtoniiis rich in the content of fucoidan, lipid and other compounds of economic values (Khotimchenko and Titlyanova, 1996; Terasakiet al., 2009; Skriptsovaet al., 2012). Extract ofS. babingtoniifeatures potent αglucosidase inhibiting activity and contains effective suppressors of postprandial hyperglycemia (Ohtaet al., 2002), promising for being used as pharmaceuticals. In addition,S. babingtoniicontains substances that inhibit dihydroorotate dehydrogenase (DHOD) activity of pathogen (Trypanosoma cruzi) and could prevent protozoan infection and mammalian cell proliferation (Naraet al., 2005; Spavieriet al., 2010). Therefore,S. babingtoniihas a very promising perspective for being used as nutraceuticals and pharmaceuticals in the near future. In order to protect the wild stock and provide the biomass ofS. babingtoniifor high-value added utilization, technologies related to sporeling nursery and field cultivation are in an urgent need. It is well known that knowledge of algal development and life cycle is the basis for developing techniques for sporeling nursery and cultivation. Unfortunately, life cycle ofS. babingtoniiis rather scarce so far.
Silvetia, along withFucus, are fucoid brown algae with the characteristic diplontic life cycles and the reduced gametophytic generation. Because fertilization is external and a large populations of synchronously developing zygotes can be obtained, fucoid algae are attractive and powerful models for investigating many aspects of cell biology for more than a hundred years (Nagasatoet al., 2010), such as embryogenesis (Bogaertet al., 2013), polarization (Kropfet al., 1999; Bisgrove and Kropf, 1998; Hable and Kropf, 1998; Kropfet al., 1998; Puet al., 2000; Bisgrove, 2007; Peterset al., 2007; Peters and Kropf, 2010) and asymmetric cell division (ACD) (Peterset al., 2008; Hable and Hart, 2010). So far, it has shown that both intrinsic and extrinsic cues (blue light, gravity, ion, electrical and osmotic gradients, presence of neighboring zygotesetc.) can regulate the polarization and ACD in fucoid zygotes (Sunet al., 2004). These findings added to our understanding on the progress controlling early embryo development, in particular polarization, axis formation and ACD.
With respect to the development ofS. babingtonii, oogenesis and cytokinesis have been well investigated. Nagasatoet al. (2001) reported the degeneration and ex-trusion of nuclei during oogenesis in detail inS. babingtonii,Cystoseira hakodatensisandSargassum confusumusing electron and immunofluorescence microscopy. According to their results, the number of nuclei was different during the oogenesis among the three genera and this could be added to phylogenetic relationships within the Fucales group. Recently, Nagasato and his colleagues (2010) further found that Golgi-derived vesicles (GVs) and flat cisternae (FCs) were involved in building the new cell partition membrane during the cytokinesis and clarified the structure of transient membrane compartments inS. babingtoniizygotes by electron tomography coupled with rapid freezing/freeze substitution (Nagasatoet al., 2014). However, the complete development involved in aspects of release of eggs and sperms, fertilization and zygote development remains largely unknown.
The most detailed descriptions related to the early development were conducted inSilvetia siliquosa(Liet al., 2007; Huanget al., 2008). Liet al. (2007) described the release and morphological changes of eggs and sperms, fertilization process and the development of zygotes inS. siliquosa. Huanget al. (2008) found that the optimal growth condition for the viable eggs and sperms was at 18.4℃ with salinity of 18. Moreover, it took 30 d for zygotes to develop into the healthy sporelings. Based on the developmental data, Huang and her colleagues established the techniques for sporeling nursery and obtained the young healthy sporelings successfully with a density of 60 per centimeter. Considering the potentially economic utilization ofS. babingtoniifor food and other higher-value products, it is in an urgent need to gain more understanding on its development. Therefore, the aim of this study was to investigate the early development from zygotes to sporelings ofS. babingtonii, our findings would not only enrich the knowledge on the life history of this alga, but also undoubtedly lay foundations for its high-value commercial utilization.
2 Materials and Methods
2.1 Algal Materials
S. babingtoniibearing receptacles were collected in the intertidal zone off the coast of Hakodate (Hokkaido Prefecture, Japan) (41˚45´N, 140˚49´E) in September 2011. The mature plants were rinsed in filtered seawater and wiped with moistened cotton tissue to remove epiphytes.
2.2 Histological Examination of Conceptacles
The receptacles were cut, and embedded into paraffin slices. The fixed receptacles were cut into pieces, which is 4–7 µm thickness, stained with hematoxylin and eosin (HE) (Ruiet al., 1980), and observed under a light microscope (Olympus CX31, Japan) equipped with a digital camera (Olympus E-620, Japan).
2.3 Settlement and Culture of Zygotes
The receptacles were washed thoroughly with sterile seawater three times, transferred to Petri dishes containing autoclaved seawater, and incubated at 8℃ under 60 μmol photons m-2s-1for 12 h. After the settlement of zygotes, the receptacles were removed and the zygotes were continuously cultured at 20℃ under 60–100 μmol photons m-2s-1. The culture medium was made of sterile seawater supplemented with 200 μmol L-1NO3--N (KNO3) and 20 μmol L-1PO43--P (KH2PO4) and exchanged weekly. The development of zygotes was examined and documented as were done for the histological examination of conceptacles.
2.4 Morphological Observations of Juvenile Sporelings
The juvenile sporelings were observed under a dissecting microscope (Zhu Guang GL-99BI 204199) when they were visible with naked eyes.
3 Results
3.1 Histological Observations of Conceptacles
The freshly collected mature sporophytes ofS. babingtonii(13 cm in height) consist of yellow-olive fronds, stipes and discoid-conical holdfasts. The fronds were smooth and regularly and dichotomously branched. While the holdfasts were short and subcylindrical. The angle of dichotomy was wide at bottom and narrow in upper parts (Fig.1a). The receptacles were rod-shaped and 4–7 cm in length, which mainly located in the upper parts of the fronds. The conceptacles occurred on the hollow surface of the receptacles (Fig.1b).

Fig.1 The mature sporophytes of S. babingtonii with receptacles. (a) A full view, bar = 2 cm; (b) Receptacles; conceptacles (arrows) on the hollow surface of receptacles, bar = 5 mm.
All specimens examined in this study were monoecious. The spherical conceptacles were composed of antheridia, oogonia and paraphyses (Fig.2a). The antheridia arose on stalk cells or branched paraphyses, bearing numerous spermatozoids. The antheridia were long ovoid. On average, they were 32.28 μm ± 75 μm (SD,n=10) in length and 8.42 μm ± 2 μm (SD,n=10) in diameter. The oogonia, which formed on the wall of conceptacles, were ovoid, and averagely 100.69 μm ± 5 μm (SD,n=10) in length and 50.99 μm ± 9 μm (SD,n=10) in diameter. The filamentous paraphyses surrounded the oogonia and the antheridia (Fig.2b).

Fig.2 The tissue slices of conceptacles of S. babingtonii. (a) Transverse section of a receptacle, including a conceptacle (c), bar = 200 μm; (b) Details of the conceptacle, showing antheridia (an), oogonia (o) and paraphyses (p), bar = 50 μm.
3.2 Early Development from Zygotes to Juvenile Sporelings
The zygotes were spheroid and hazel, 62.58 μm ± 2 μm (SD,n=10) in average diameter (Fig.3a). The zygotes started to divide and germinate within 24 h after settlement. Each zygote first divided transversely into two daughter cells,i.e., thallus and rhizoid cells in 2 d (Fig.3b). The larger thallus cells divided further and developed into juvenile sporeling on the 5th day. While the smaller rhizoid cells developed into elongated, colorless and transparent germ tubes (Fig.3c). During elongation, the germ tubes developed more rhizoid hairs. On the 23rdday, the juvenile sporelings were 80.45 μm ± 5 μm (SD,n=10) in length and 53.17 μm ± 2 μm (SD,n=10) in diameter; whereas the majority of juvenile sporelings had more than 10 rhizoid hairs with a mean length of 149.80 μm ± 3 μm (SD,n=10) (Fig.3d).
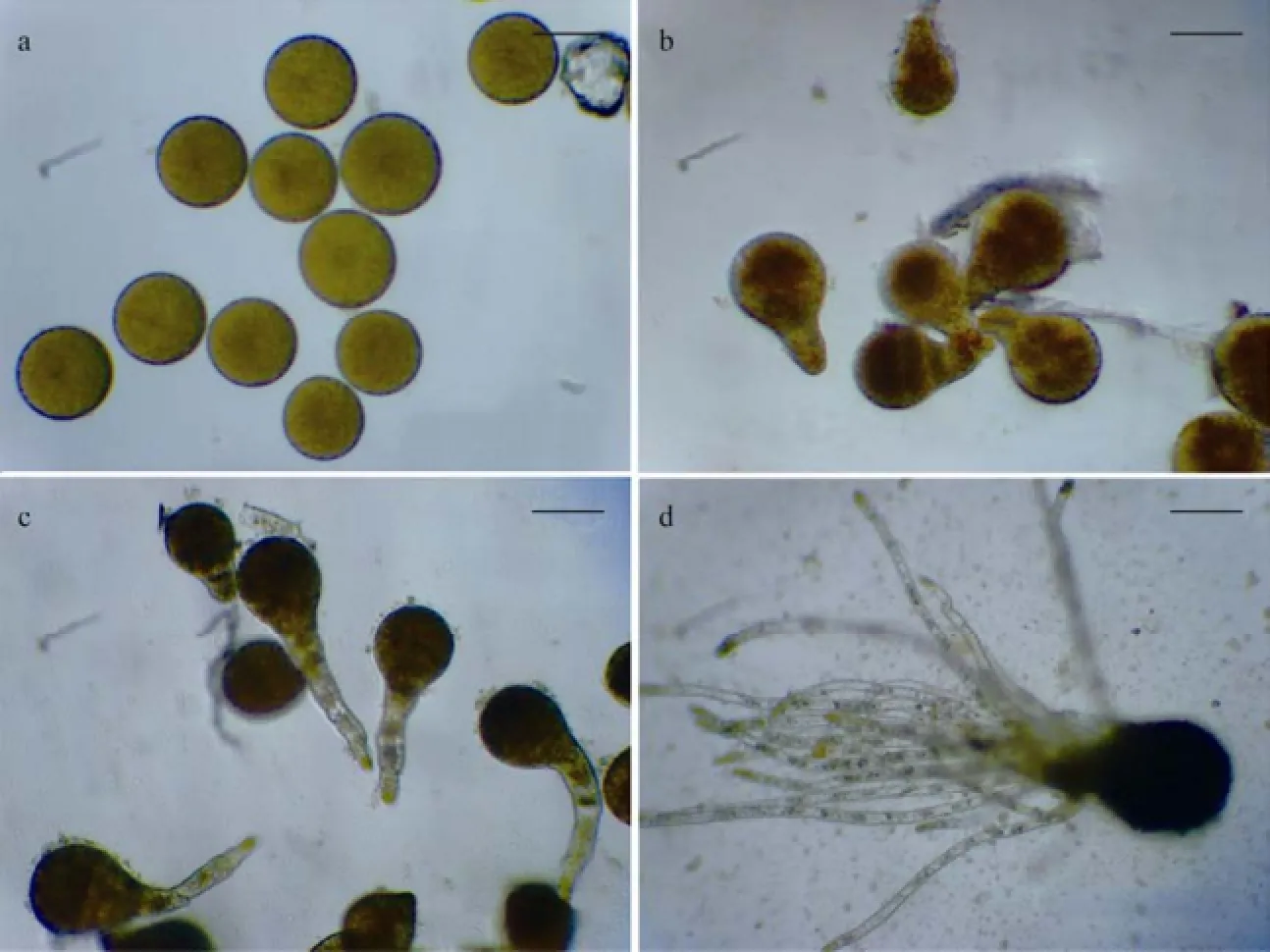
Fig.3 The early development of S. babingtonii from zygotes to juvenile sporelings. (a) Freshly settled zygotes, bar = 50 μm; (b) 2-day-old zygotes (divided and germinated), bar = 50 μm; (c) 5-day-old zygotes, developed into juvenile sporelings as well as long germ tubes, bar = 50 μm; (d) 23-day-old juvenile sporelings, bar = 50 μm.
3.3 Morphological Observations of Juvenile Sporelings
Approximately 3 month after zygote settlement, the juvenile sporelings became visible with naked eyes and were observed under a dissecting microscope (Fig.4). A few of juvenile sporelings were single (Fig.4a); while the majority developed in clusters (Fig.4b). During the development of juvenile sporelings, the basal portion elongated and developed continually, forming more rhizoidhairs (Figs.4c, 4d).
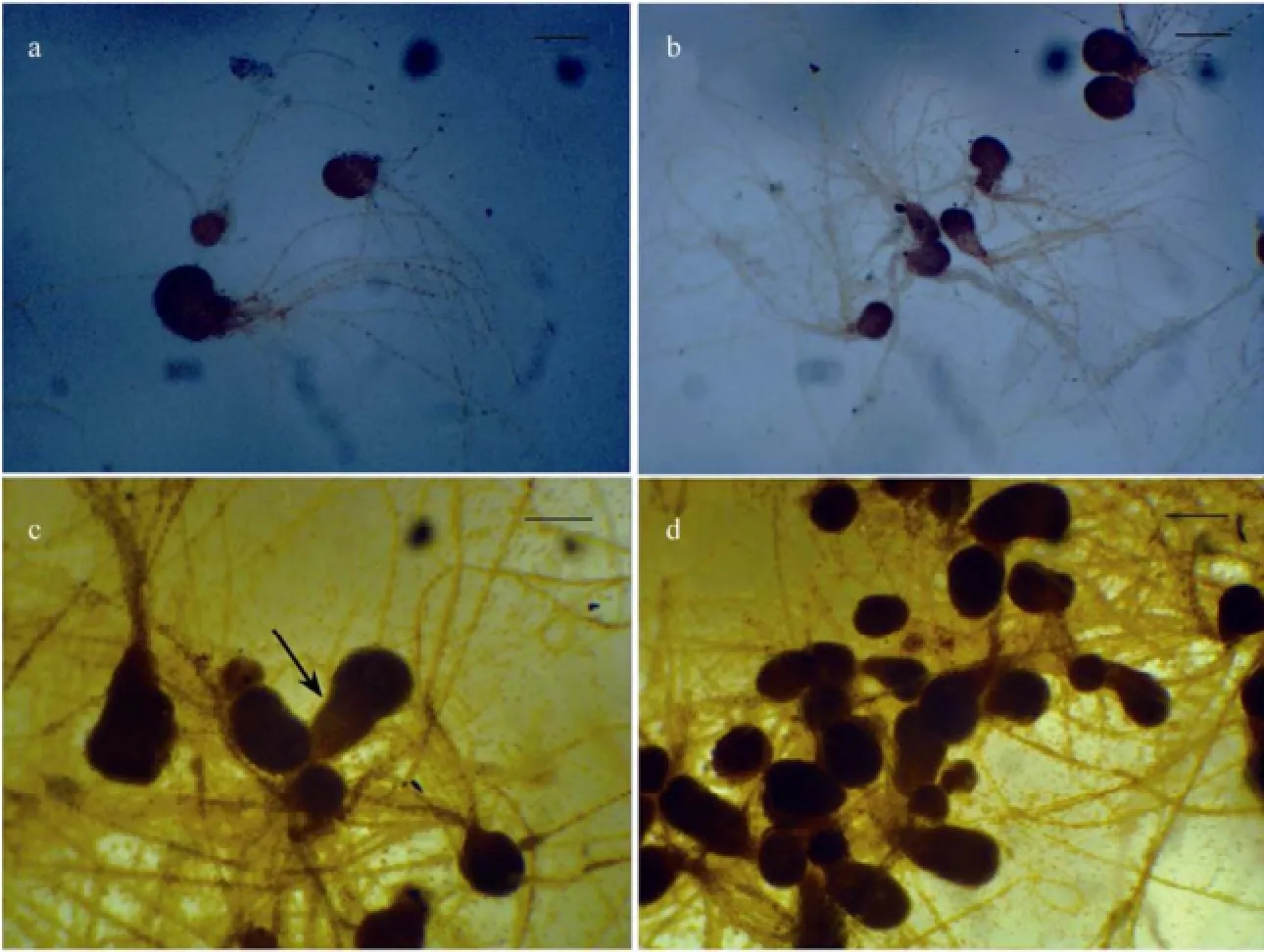
Fig.4 The juvenile sporelings of S. babingtonii. (a) Single 3-month-old juvenile sporeling, bar =150 μm; (b) A cluster of 3-month-old juvenile sporelings, bar = 300 μm; (c) A 4-month-old juvenile sporelings with elongation at its basal parts (arrows), bar = 200 μm; (d) A cluster of 4-month-old juvenile sporelings; bar = 200 μm.
3.4 The Life Cycle ofS.babingtonii
Based on our observations, life cycle ofS.babingtoniiwas outlined as in Fig.5. During the reproductive season, the reproductive plants are monoecious. The reproductive tissues-receptacles are produced on the specialized apices of the upper branches, which contain numerous spherical conceptacles on the subsided surface. The conceptacles are composed of antheridia, oogania and paraphyses. The freshly released eggs and sperms get fertilized externally. Zygotes germinate and divide into two daughter cells, named the thallus and rhizoid cells. The larger thallus cells further divide and develop into juvenile sporelings,while the smaller ones divide and elongated into rhizoid hairs. Finally, the juvenile sporelings further develop into the mature sporophytes.
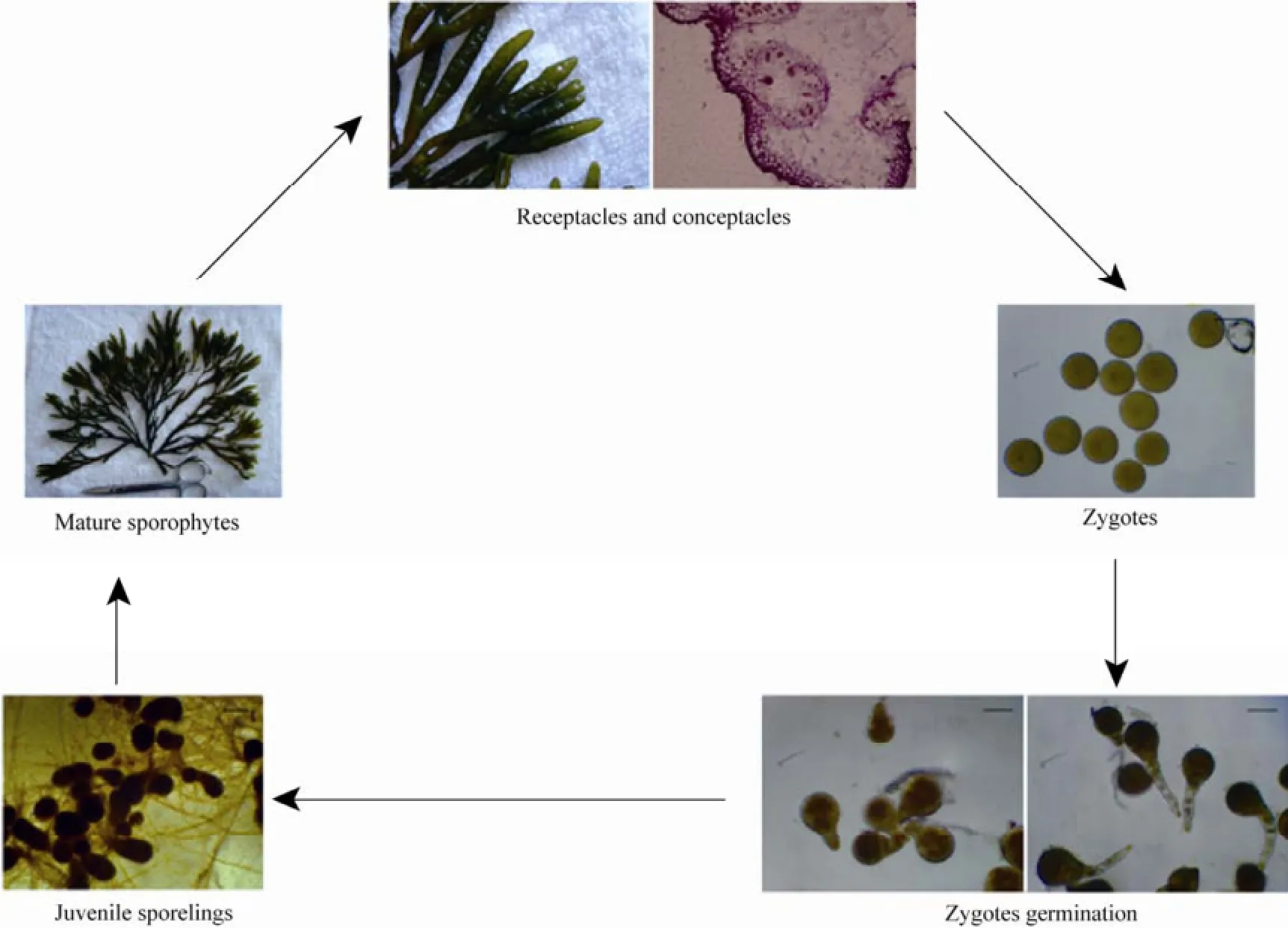
Fig.5 The life cycle of S. babingtonii.
4 Discussion
Previously, researches of the early development ofSilvetiamainly focused on polarization (Kropf, 1992), ACD (Bogaertet al., 2013), oogenesis (Nagasatoet al., 2001) and cytokinesis (Nagasatoet al., 2010, 2014).S. siliquosais the only species in genusSilvetiawhose early development was investigated integrally (Huanget al., 2008). The optimal condition for collecting zygotes ofS. siliquosais below 18.4℃ under natural light. Early development from zygotes to juvenile sporelings was recorded below 18℃ with a photon flux density of 30–50 μmol photons m-2s-1. Our results is somewhat similar to that ofS. siliquosa, however,there are some morphological difference between these two species. Firstly, the mean diameter of freshly settled zygotes ofS. babingtonii(62.58 μm ± 2 μm) is smaller than that ofS. siliquosa(90–100 μm). In addition, the majority ofS. babingtoniijuvenile sporelings have more than one rhizoid hair. In contrast, most of the juvenile sporelings ofS. siliquosahave single rhizoid hair during the early development. We speculate that the significant morphological changes may be resulted from the genetic difference between the two species and other influencing environmental factors, such as temperature, irradiance and nutrition.
Besides the morphological difference in early development, there are some morphological variations in fronds betweenS. babingtoniiandS. siliquosa. The color of mature fronds ofS. babingtoniiare yellow olive and darker than those ofS. siliquosa.Both species have regularly dichotomous fronds, but the number of branches ofS. babingtoniiis more than that ofS. siliquosa. Additionally, the fronds ofS. babingtoniiare turgid, while those ofS. siliquosaare compressed. Finally, the conceptacles ofS. babingtoniiare subsided and those ofS. siliquosaare on the flat surface of receptacles (Choet al., 2001). Although these two species have substantial difference in morphology, their internal structures of conceptacles are similar. In this study, we observed the structure of conceptacles ofS. babingtoniiand summarized the life cycle ofS. babingtoniifor the first time, thus providing fundamental data for researches on other species ofSilvetia.
The way of fertilization ofS. babingtoniiis external (Ladahet al., 2008), in which sperms and eggs are released from conceptacles and completed the fertilization externally. Due to the uncontrollability of egg and sperm release and the fertilization time, it remains difficult to observe and record the developmental process of fertilization, which needs further clarification.
Due to experimental limitations, this work only studied the zygote development ofS. babingtoniiat 20℃ with natural light. The conditions may not be optimal for the early development ofS. babingtonii. Future study will be conducted to determine the optimal conditions (e.g., temperature, irradiance and photoperiod) for the early development ofS. babingtonii.
Acknowledgements
This study was sponsored by the Shandong Agricultural Seedstock Breeding Project, and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, Ministry of Education of China (No. 2010-1561).
Anastyuk, S. D., Shevchenko, N. M., Dmitrenok, P. S., and Zvyagintseva, T. N., 2012. Structural similarities of fucoidans from brown algae Silvetia babingtonii and Fucus evanescens, determined by tandem MALDI-TOF mass spectrometry. Carbohydrate Research, 358 (1): 78-81.
Bisgrove, S. R., 2007. Cytoskeleton and early development in fucoid algae. Journal of Integrative Plant Biology, 49 (8): 1192-1198.
Bisgrove, S. R., and Kropf, D. L., 1998. Alignment of centrosomal and growth axes is a late event during polarization of Pelvetia compressa zygotes. Developmental Biology, 194 (2): 246-256.
Bogaert, K. A., Arun, A., Coelho, S. M., and Clerck, O. D., 2013. Brown algae as a model for plant organogenesis. Plant organogenesis: Methods and protocols. Methods in Molecular Biology, 959: 97-123.
Cho, T. O., Motomura, T., and Boo, S. M., 2001. Morphological review of Pelvetia and Silvetia (Fucaceae, Phaeophyta) with an emphasis on phylogenetic relationships. Journal of Plant Biology, 44 (1): 41-52.
Hable, W. E., and Hart, P. E., 2010. Signaling mechanisms in the establishment of plant and fucoid algal polarity. Molecular Reproduction and Development, 77: 751-758.
Hable, W. E., and Kropf, D. L., 1998. Roles of secretion and the cytoskeleton in cell adhesion and polarity establishment in Pelvetia compressa zygotes. Development Biology, 198 (1): 45-56.
Huang, L. J., Cai, H. B., Zhang, H. J., and Xu, X. Y., 2008. Development and utilization of seaweed natural resource-studies on seedling-rearing of Pelvetia siliquosa. Marine Fisheries Research, 29 (1): 70-75 (in Chinese with English abstract).
Khotimchenko, S. V., and Titlyanova, T. V., 1996. Distribution of an amino acid-containing phospholipid in brown algae. Phytochemistry, 41 (6): 1535-1537.
Kozhenkova, S. I., 2009. Retrospective analysis of the marine flora of Vostok Bay, Sea of Japan. Russian Journal of Marine Biology, 35 (4): 263-278.
Kropf, D. L., 1992. Establishment and expression of cellular polarity in fucoid zygotes. Microbilogy and Molecular Biology Reviews, 56 (2): 316-339.
Kropf, D. L., Bisgrove, S. R., and Hable, W. E., 1998. Cytoskeletal control of polar growth in plant cells. Current Opinion in Cell Biology, 10 (1): 117-122.
Kropf, D. L., Bisgrove, S. R., and Hable, W. E., 1999. Establishing a growth axis in fucoid algae. Trend in Plant Science, 4 (12): 490-494.
Ladah, L. B., Feddersen, F., Pearson, G. A., and Serrão, E. A., 2008. Egg release and settlement patterns of dioecious and hermaphroditic fucoid algae during the tidal cycle. Marine Biology, 155 (6): 583-591.
Lee, Y., and Kang, S., 2001. A Catalogue of the Seaweeds in Korea. Publishing Department of Cheju National University,Cheju, 662pp.
Li, M. Z., Ding, G., and Zhan, D. M., 2007. The preliminary experiment of reproduction and sporeling culture of Pelvetia siliquosa. Chinese Journal of Oceanology and Limnology phycology Branch of 7th Members of Congress and 14th Academic Thesis Abstracts. China Academic Journal Electronic Publishing House, Beijing, 284pp (in Chinese).
Liu, W., Li, M. Z., Zhan, D. M., Ding, G., and Wu, H. Y., 2011. Nucleotide analysis and molecular phylogenetic affinity of 18S rDNA of Silvetia siliquosa. Journal of Yantai University (Natural Science and Engineering Edition), 24 (1): 48-53.
Nagasato, C., Kajimura, N., Terauchi, M., Mineyuki, Y., and Motomura, T., 2014. Electron tomographic analysis of cytokinesis in the brown alga Silvetia babingtonii (Fucales, Phaeophyceae). Protoplasma, DOI: 10.1007/S00709-014-0635-y.
Nagasato, C., Inoue, A., Mizuno, M., Kanazawa, K., Ojima, T., Okuda, K., and Motomura, T., 2010. Membrane fusion process and assembly of cell wall during cytokinesis in the brown alga, Silvetia babingtonii (Fucales, Phaophyceae). Planta, 232 (2): 287-298.
Nagasato, C., Motomura, T., and Ichimura, T., 2001. Degeneration and extrusion of nuclei during oogenesis in Silvetia babingtonii, Cystoseira hakodatensis and Sargassum confusum (Fucales, Phaeophyceae). Phycologia, 40 (5): 411-420.
Nakaoka, M., Ito, N., Yamamoto, T., Okuda, T., and Noda, T., 2006. Similarity of rocky intertidal assemblages along the Pacific coast of Japan: effects of spatial scales and geographic distance. Ecological Research, 21 (3): 425-435.
Nara, T., Kamei, Y., Tsubouchi, A., Annoura, T., Hirota, K., Lizumi, K., Dohmoto, Y., Ono, T., and Aoki, T., 2005. Inhibitory action of marine algae extracts on the Trypanosoma cruzi dihydroorotate dehydrogenase activity and on the protozoan growth in mammalian cells. Parasitology International, 54 (1): 59-64.
Ohta, T., Sasaki, S., Oohori, T., Yoshikawa, S., and Kurihara, H., 2002. α-glucosidase inhibitory activity of a 70% methanol extract from Ezoishige (Pelvetia babingtonii de Toni) and its effect on the elevation of blood glucose level in rats. Bioscience Biotechnology and Biochemistry, 66 (7): 1552-1554.
Peters, A., F., Scornet, D., Ratin, M., Charrier, B., Monnier, A., Merrien, Y., Corre, E., Coelho, S. M., and Cock, J. M., 2008. Life-cycle-generation specific developmental processes are modified in the immediate upright mutant of the brown alga Ectocarpus siliculosus. Development, 135: 1503-1512.
Peters, N. T., and Kropf, D. L., 2010. Asymmetric microtubule arrays organize the endoplasmic reticulum during polarity establishment in the brown alga Silvetia compressa. Cytoskeleton, 67 (2): 102-111.
Peters, N. T., Logan, K. O., Miller, A. C., and Kropf, D. L., 2007. Phospholipase D signaling regulates microtubule organization in the fucoid alga Silvetia compressa. Plant and Cell Physiology, 48 (12): 1764-1774.
Pu, R., Wozniak, M., and Robinson, K. R., 2000. Cortical actin filaments form rapidly during photopolarization and are required for the development of calcium gradients in Pelvetia compressa zygotes. Development Biology, 222 (2): 440-449.
Rui, J. S., Du, Y. Q., Che, H. M., and Li, C. L., 1980. Tissue Section Technology. People Education Press, Shanghai, 1-108.
Serrão, E. A., Alice, L. A., and Brawley, S. H., 1999. Evolution of the Fucaceae (Phaeophyceae) inferred from nrDNA-ITS. Journal of Phycology, 35 (2): 382-394.
Skriptsova, A. V., Shevchenko, N. M., Tarbeeva, D. V., and Zyagintseva, T. N., 2012. Comparative study of polysaccharides from reproductive and sterile tissues of five brown seaweeds. Marine Biotechnology, 14 (3): 304-311.
Spavieri, J., Allmendinger, A., Kaiser, M., Casey, R., Hingley-Wilson, S., Lalvani, A., Guiry, M. D., Blunden, G., and Tasdemir, D., 2010. Antimycobacterial, antiprotozoal and cytotoxic potential of twenty-one brown algae (Phaeophyceae) from British and Irish waters. Phytotherapy Research, 24 (11): 1724-1729.
Sun, H., Basu, S., Brady, S., Luciano, R. L., and Muday, G. K., 2004. Interactions between auxin transport and the actin cytoskeleton in developmental polarity of Fucus distichus embryos in response to light and gravity. Plant Physiology, 135: 266-278.
Terasaki, M., Hirose, A., Narayan, B., Baba, Y., Kawagoe, C., Yasui, H., Saga, N., Hosokawa, M., and Miyashita, K., 2009. Evaluation of recoverable functional lipid components of several brown seaweeds (Phaeophyta) from Japan with special reference to fucoxanthin and fucosterol contents. Journal of Phycology, 45: 974-980.
(Edited by Qiu Yantao)
(Received November 11, 2012; revised January 3, 2013; accepted March 7, 2014)
© Ocean University of China, Science Press and Spring-Verlag Berlin Heidelberg 2014
* Corresponding author. Tel: 0086-532-82031809
E-mail wgaoge@ouc.edu.cn
杂志排行
Journal of Ocean University of China的其它文章
- Construction of Inorganic Elemental Fingerprint and Multivariate Statistical Analysis of Marine Traditional Chinese Medicine Meretricis concha from Rushan Bay
- A Comparative Study of Intensive Litopenaeus vannamei Culture on Four Bottom Substrates Without Water Change
- Characterization, Expression and Function Analysis of DAX1 Gene of Scallop (Chlamys farreri Jones and Preston 1904) During Its Gametogenesis
- Secondary Metabolites of a Deep Sea Derived Fungus Aspergillus versicolor CXCTD-06-6a and Their Bioactivity
- Preparation, Characterization and Pharmacokinetics of Fluorescence Labeled Propylene Glycol Alginate Sodium Sulfate
- A Comparison of Different Gracilariopsis lemaneiformis (Rhodophyta) Parts in Biochemical Characteristics, Protoplast Formation and Regeneration
