Secondary Metabolites of a Deep Sea Derived Fungus Aspergillus versicolor CXCTD-06-6a and Their Bioactivity
2014-05-05KONGXianglanCAIShengxinZHUTianjiaoGUQianqunLIDehaiandLUANYepeng
KONG Xianglan, CAI Shengxin, ZHU Tianjiao, GU Qianqun, LI Dehai, and LUAN Yepeng
Key Laboratory of Marine Drugs of Chinese Ministry of Education, School of Medicine and Pharmacy, Ocean University of China, Qingdao 266003, P. R. China
Secondary Metabolites of a Deep Sea Derived Fungus Aspergillus versicolor CXCTD-06-6a and Their Bioactivity
KONG Xianglan, CAI Shengxin, ZHU Tianjiao, GU Qianqun, LI Dehai*, and LUAN Yepeng*
Key Laboratory of Marine Drugs of Chinese Ministry of Education, School of Medicine and Pharmacy, Ocean University of China, Qingdao 266003, P. R. China
In order to obtain novel secondary metabolites, a deep sea inhabiting fungusAspergillus versicolorCXCTD-06-6a was investigated. One new diketopiperazine brevianamide W (1a), as well as five known diketopiperazine alkaloids, diketopiperazine V (1b), brevianamide Q (2), brevianamide R (3), brevianamide K (4), and brevianamide E (5), were isolated from the EtOAc extract of the fermentation broth. Their structures were elucidated by spectroscopy techniques (NMR, MS). The six compounds exhibited moderate radical scavenging activity against DPPH with clearance ratio of 55.0% (1a and 1b), 53.7% (2), 46.2% (3), 61.4% (4) and 19.3% (5) at a concentration of 13.9 μmol L-1, respectively; while the positive control ascorbic acid showed a ratio of 70.3% at the concentration of 28.4 μmol L-1.
deep sea fungus; secondary metabolite; diketopiperazine
1 Introduction
In the past decade, several interesting bioactive secondary metabolites were discovered including sterigmatocystins (Leeet al., 2010), nitrobenzyl esters (Belofskyet al., 1998), cyclopeptides (Zhuanget al., 2011), and chromones (Linet al., 2003) from the marine fungiAspergillus versicolor. These rich structure types indicated the powerful ability ofA. versicolorof producing diverse natural products.
In our screening of cytotoxic deep sea microorganisms (Caiet al., 2010; Liet al., 2007; Liet al., 2007; Duet al., 2009; Duet al., 2010), a fungus strain CXCTD-06-6aisolated from an underwater sample (depth, -800 m) was authenticated asAspergillus versicolor.Its extract exhibited potent cytotoxicity against the P388 cell line.

Fig.1 Structures of compounds 1-5.
In our previous investigations on the extract, three cytotoxic polyketides oxisterigmatocystins A-C (Caiet al., 2011) and two anthraquinones, averufin and averufanin (Jiet al., 2011), were isolated from this strain. The HPLC-UV profile of this extract suggested the existence of more types of compounds. For exploring the structural diversity, further isolation of this extract led to the discovery of six diketopiperazines, including brevianamide W (1a), brevianamide V (1b), brevianamide Q (2), brevianamide R (3), brevianamide K (4) and brevianamide E (5) (Fig.1). Brevianamides, a class of diketopiperazine alkaloids, are produced by fungi belonging to the generaAspergillus, mostlyAspergillus versicolor(Liet al., 2009; Liet al., 2010; Songet al., 2012; Steyn, 1973). Brevianamide W (1a) is the first case of brevianamide containing D-proline, which was identified by detailed spectroscopic and the advanced Marfey’s analysis (Luet al., 2012). In this paper, we reported the structure elucidation of the new metabolites1a, as well as the isolation and bioactivity of these six compounds.
2 Materials and Methods
2.1 General
Specific rotations were obtained on a JASCO P-1020 digital polarimeter. IR spectra were taken on a NICOLET NEXUS 470 spectrophotometer in KBr discs.1H,13C NMR and DEPT spectra and 2D-NMR were recorded on a JEOL JNM-ECP 600 spectrometer using TMS as the internal standard, and the chemical shifts were recorded asδvalues. ESI-MS was measured on a Q-TOF ULTIMA GLOBAL GAA076 LC mass spectrometer. Semiprepartive HPLC was performed using an ODS column (YMC-pak ODS-A, 10 mm×250 mm, 5 μm, 4 mL min-1).
2.2 Fungal Material
The fungal strainA. versicolorCXCTD-06-6a was isolated from an underwater sample (depth, -800 m) collected from the Pacific Ocean. The fungus strain was identified by Dr. Tianjiao Zhu and the voucher specimen was deposited in our laboratory at -20℃. Working stocks were prepared on Potato Dextrose agar slants and stored at 4℃.
2.3 Fermentation, Extraction and Isolation
Spores were directly inoculated into 500-mL Erlenmeyer flasks containing 100 mL fermentation medium (mannitol 20 g, maltose 20 g, glucose 10 g, monosodium glutamate 10 g, KH2PO40.5 g, MgSO4·7H2O 0.3 g, yeast extract 3 g, and corn steep liquor 1 g, dissolved in 1 L sea water, pH 6.5). The flasks were incubated on a rotatory shaker (165 r min-1, 28℃). After nine days of cultivation, 30 L of whole broth was filtered through cheesecloth to separate the broth supernatant and mycelia. The former was extracted with ethyl acetate; while the latter was extracted with acetone. The acetone extract was evaporated under reduced pressure to afford an aqueous solution, and then extracted with ethyl acetate. The two ethyl acetate extracts were combined and concentrated under reduced pressure to give a crude extract (35.0 g).
The crude extract (35.0 g) was subjected to a silica gel (300–400 mesh) CC and was separated into five fractions (Fr.1–Fr.5) using a step gradient elution of petroleum ether/CHCl3and CHCl3/CH3OH. The fraction Fr.3, eluted with 100:1 CHCl3/MeOH, was fractionated on a silica gel CC using a step gradient elution of petroleum ether/acetone and was separated into five fractions, Fr.3.1–Fr.3.5. Fr.3.3 was separated on Sephadex LH-20 CC using CHCl3/CH3OH (1:1) as the eluting solvent and silica gel column using elution of petroleum ether/acetone (4:1) to afford four fractions (Fr.3.3.2.1–Fr.3.3.2.4). Fr.3.3.2.2 was further purified by RP-18 semi-preparative HPLC (70:30 CH3OH/H2O) to obtain compound2(20.5 mg,tR15.5 min). Fr.3.3.2.4 was further purified by RP-18 semipreparative HPLC (65:35 CH3OH/H2O) to obtain compound3(3.5 mg,tR16.4 min). Fr.3.4 was separated on Sephadex LH-20 CC using CHCl3/CH3OH (1:1) as the eluting solvent to afford three fractions (Fr.3.4.1-Fr.3.4.3). Fr.3.4.2 was applied on a silica gel CC using a step gradient elution of petroleum ether/acetone and was separated into three fractions (Fr.3.4.2.1–Fr.3.4.2.3). Fr.3.4.2.1 was further purified by RP-18 semi-preparative HPLC (60:40 CH3OH/H2O) to obtain compound5(15.0 mg,tR20.4 min). Fr.3.4.2.2 was further purified by RP-18 semi-preparative HPLC (60:40 CH3OH/H2O) to obtain compound1aand1b(11.0 mg,tR18.6 min). Fr.3.4.2.3 was further purified by RP-18 semi-preparative HPLC (60:40 CH3OH/H2O) to obtain compound4(11.5 mg,tR16.5 min).
2.4 Physical-Chemical Property
Brevianamide W (1a) and V (1b): pale yellow amorphous powder; [α]25D+33.3 (c0.1, MeOH); UV (HPLC, mobile phase)λmax: 219, 340 nm; IR (KBr)νmax: 3307, 2970, 1688, 1628, 1435, 1385, 1314, 1235, 909, 745 cm-1;1H-NMR (CDCl3, 600 MHz) and13C-NMR (CDCl3, 150 MHz), see Table 1; HR-ESI-MSm/z: 350.1864 [M+H]+.
Brevianamide Q (2): colorless block crystal; [α]25D-8.3 (c0.1, MeOH); UV (HPLC, mobile phase)λmax: 215, 343 nm; IR (KBr)νmax: 3331, 2967, 2881, 1692, 1658, 1623, 1396, 1041, 745 cm-1; HR-ESI-MSm/z: 380.1964 [M+H]+.
Brevianamide R (3): pale yellow amorphous powder; [α]25D-14.0 (c0.1, MeOH); UV (HPLC, mobile phase)λmax: 217, 339 nm; IR (KBr)νmax: 3336, 2968, 1694, 1620, 1423, 1244, 1176, 1110, 1046, 746 cm-1; HR-ESI-MSm/z: 388.1625 [M+Na]+.
Brevianamide K (4): pale yellow amorphous powder; HR-ESI-MSm/z: 348.1706 [M+H]+.
Brevianamide E (5): pale yellow amorphous powder; ESI-MSm/z: 352.2 [M+H]+, 374.2 [M+Na]+.

Table 11H-NMR (600MHz) and13C-NMR (150MHz) data for brevianamide W(1a)†
2.5 Acid Hydrolysis of Compound 1
Pure brevianamides W and V (1aand1b) (1 mg) was hydrolyzed in 1 mL of 6 mol L-1HCl at 110℃ for 12 h in a 3 mL reaction vial. The cooled reaction mixture was evaporated to dryness, and traces of HCl were removed from the residual hydrolysate by repeated evaporation from H2O (3 × 1 mL) using N2gas.
2.6 HPLC Analysis of D/L-FDLA Derivatives
The acid hydrolysates of1was dissolved in 50 μL of H2O, and 25 μL of 1 mol L-1NaHCO3and 0.25 μmol ofN-R-(2,4-dinitro-5-fluorophenyl)-L-alanine amide (L-FD LA) in 100 μL of acetone were added. The mixture was then heated for 1 h at 40℃. After cooling to room temperature, 25 μL of 2 mol L-1HCl was added and the resulting solution was filtered through a small 4.5 μm filter and stored in the freezer until HPLC analysis. Amino acid standards were derivatized with L-FDLA in a similar manner. An 8 μL aliquot of the resulting mixture of L-FDLA derivative was analyzed by reversed-phase HPLC. A C18column (5 mm × 250 mm, 5 μm), with a linear gradient of (A) CH3CN and (B) 0.05% aqueous TFA from 5% to 45% (A) over 40 min at a flow rate of 1 mL min-1, was used to separate the L-FDLA derivatives, and the derivative of1was co-injected with D-pro and L-pro standards (D-pro (tR=36.51 min), L-pro (tR=35.31 min), FDLA (tR=36.00min)).
2.7 Biological Assay
The MTT assay (Mosmann, 1983), SRB assay (Skehanet al., 1990), DPPH scavenging assay (Chenet al., 2008) were processed according to the references.
3 Results and Discussion
3.1 Structure Determination
Brevianamide W (1a) and V (1b), a pale yellow amorphous powder, were isolated as an unisolated mixture. The molecular formula of1aand1bwere determined as C21H23N3O2based on HR-ESI-MS peak atm/z350.1864 [M+H]+(calcd. for C21H24N3O2, 350.1869), indicating twelve degrees of unsaturation. The NMR data (Table 1) and the UV absorption atλmax219, 340 nm of1were identical to those of brevianamide V (1b) (Songet al., 2012), and the planar structure of1was confirmed by the HMQC,1H-1H COSY and HMBC experiments (Fig.2).
In order to determine the absolute configuration of1, the Marfey’s analysis was performed. Treatment of the hydrolysates with L-FDLA gave diastereomeric derivatives and their configurations were identified by comparison with D/L-proline standards. The HPLC analysis showed that the sample derivatives contained both L-Pro and D-Pro with the ratio of 3:1 (Fig.3). The result indicated that1was a mixture of two compounds (1aand1b) with different absolute configurations. TheRenantiomer was named as brevianamide W (1a) and theSenantiomer was brevianamide V (1b) according to the reference. To isolate the racemate, the chiral column (Chiralpak IC column) was employed under different conditions, but the result indicated that this column is not fit for isolation of this compound. Due to the limitation of sample amount, further research was not attempted.

Fig.2 The selected1H-1H COSY and HMBC correlations of 1.
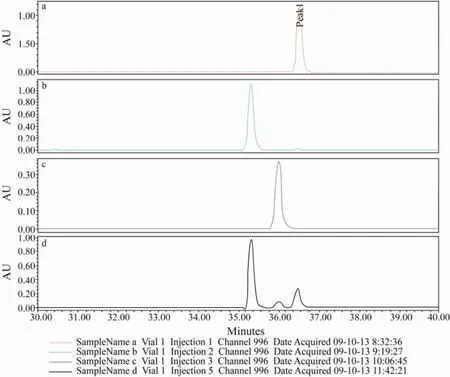
Fig.3 The HPLC analysis of the Marfey’s analysis of compound 1. a, The HPLC profile for L-FDLA derivative of D-Pro; b, The HPLC profile for L-FDLA derivative of L-Pro; c, The HPLC profile for L-FDLA; d, The HPLC profile for L-FDLA derivative of the acid hydrolysis product of 1.
3.2 Bioactivity
The cytotoxicities of compounds1a-5(on P388, BEL-7402 and MOLT-4 cell lines) were tested for cytotoxic effects and none of them showed cytotoxicity against the tested cell lines by MTT or SRB methods.
Compound1a-5exhibited moderate radical scavenging activity against DPPH with clearance ratio of 55.0% (1aand1b), 53.7%, 46.2%, 61.4% and 19.3% at a concentration of 13.9 μmol L-1, respectively. Ascorbic acid was applied as a positive control, and the ratio was 70.3% at the concentration of 28.4 μmol L-1.
Acknowledgements
This work was supported by Chinese National Science Fund (No. 41176120); Promotive Research Fund for Excellent Young and Middle-aged Scientists of Shandong Province (No. BS2010HZ027); Specialized Research Fund for the Doctoral Program of Higher Education (No. 20100132120026); and the Fifty First Postdoctoral Fund of China (2012M511552).
Belofsky, G. N., Jensen, P. R., Renner, M. K., and Fenical, W., 1998. New cytotoxic sesquiterpenoid nitrobenzoyl esters from a marine isolate of the fungus Aspergillus versicolor. Tetrahedron, 54 (9): 1715-1724.
Cai, S. X., Li, D. H., Zhu, T. J., Wang, F. P., Xiao, X., and Gu, Q. Q., 2010. Two new indole alkaloids from the marine-derived bacterium Aeromonas sp. CB101. Helvetica Chimica Acta, 93 (4): 791-795.
Cai, S. X., Zhu, T. J., Du, L., Zhao, B. Y., Li, D. H., and Gu, Q. Q., 2011. Sterigmatocystins from the deep-sea-derived fungus Aspergillus versicolor. The Journal of Antibiotics, 64 (2): 193-196.
Chen, L., Fang, Y. C., Zhu, T. J., Gu, Q. Q., and Zhu, W. M., 2008. Gentisyl alcohol derivatives from the marine-derived fungus Penicillium terrestre. The Journal of Natural Products, 71: 66-70.
Du, L., Li, D. H., Zhu, T. J., Cai, S. X., Wang, F. P., Xiao, X., and Gu, Q. Q., 2009. New alkaloids and diterpenes from a deep ocean sediment derived fungus Penicillium sp. Tetrahedron, 65 (5): 1033-1039.
Du, L., Feng, T., Zhao, B. Y., Li, D. H., Cai, S. X., Zhu, T. J., Wang, F. P., Xiao, X., and Gu, Q. Q., 2010. Alkaloids from a deep ocean sediment-derived fungus Penicillium sp. and their antitumor activities. The Journal of Antibiotics, 63 (4): 165-170.
Ji, C. Z., Sun, S. W., Cai, S. X., Zhu, T. J., Gu, Q. Q., and Li, D. H., 2011. Studies on the active secondary metabolites from the deep-sea-derived fungus Aspergillus sp. CXCTD-06-6a. Chinese Journal of Marine Drugs, 30 (3): 1-6.
Lee, Y. M., Li, H., Hong, J., Cho, H. Y., Bae, K. S., Kim, M. A., Kim, D., and Jung, J. H., 2010. Bioactive metabolites from the sponge-derived fungus Aspergillus versicolor. Archives of Pharmacal Research, 33 (2): 231-235.
Li, G. Y., Li, L. M., Yang, T., Chen, X. Z., Fang, D. M., and Zhang, G. L., 2010. Four new alkaloids, Brevianamides O-R, from the fungus Aspergillus versicolor. Helvetica Chimica Acta, 93: 2075-2080.
Li, D. H., Wang, F. P., Xiao, X., Fang, Y. C., Zhu, T. J., Gu, Q.Q., and Zhu, T. J., 2007. Trisorbicillinone A, a novel sorbicillin trimer, from a deep sea fungus, Phialocephala sp. FL30r, Tetrahedron Letter, 38 (46): 5235-5238.
Li, D. H., Wang, F. P., Cai, S. X., Zeng, X., Xiao, X., and Gu, Q. Q., 2007. Two new bisorbicillinoids isolated from a deep sea fungus, Phialocephala sp. FL30r. The Journal of Antibiotics, 60 (5): 317-320.
Li, G. Y., Yang, T., Luo, Y. G., Chen, X. Z., Fang, D. M., and Zhang, G. L., 2009. Brevianamide J, a new indole alkaloid dimer from fungus Aspergillus versicolor. Organic Letters, 11 (16): 3714-3717.
Lin, W. H., Brauers, G., Ebel, R., Wray, V., Berg, A., Sudarsono, and Proksch, P., 2003. Novel chromone derivatives from the fungus Aspergillus versicolor isolated from the marine sponge Xestospongia exigua. The Journal of Natural Products, 66 (1): 57-61.
Lu, Z. Y., Harper, M. K., Pond, C. D., Barrows, L. R., Ireland, C. M., and Van Wagoner, R. M., 2012. Thiazoline peptides and a Tris-phenethyl Urea from didemnum molle with anti-HIV activity. The Journal of Natural Products, 75 (8): 1436-1440.
Mosmann, T., 1983. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Journal of Immunological Methods, 65: 55-63.
Skehan, P., Storeng, R., Scudiero, D., Monks, A., McMahon, J., Vistica, D., Warren, J. T., Bokesch, H., Kenney, S., and Boyd, M. R., 1990. New colorimetric cytotoxicity assay for anticancer-drug screening. Journal of the National Cancer Institute, 82 (13): 1107-1112.
Song, F. H., Liu, X. R., Guo, H., Ren, B., Chen, C. X., Piggott, A. M., Yu, K., Gao, H., Wang, Q., Liu, M., Liu, X. T., Dai, H. D., Zhang, L. X., and Capon, R. J., 2012. Brevianamides with antitubercular potential from a marine-derived isolate of Aspergillus versicolor. Organic Letters, 14 (18): 4770-4773.
Steyn, P. S., 1973. Austamide, a new toxic metabolite from Aspergillus ustus. Tetrohedron Letters, 12: 3331-3334.
Zhuang, Y. B., Teng, X. C., Wang, Y., Liu, P. P., Wang, H., Li, J., Li, G. Q., and Zhu, W. M., 2011. Cyclopeptides and polyketides from coral-associated fungus, Aspergillus versicolor LCJ-5-4. Tetrahedron, 67 (37): 7085-7089.
(Edited by Qiu Yantao)
(Received November 7, 2012; revised January 7, 2013; accepted December 4, 2013)
© Ocean University of China, Science Press and Spring-Verlag Berlin Heidelberg 2014
* Corresponding authors. E-mail: dehaili@ouc.edu.cn
E-mail: luanouc@yahoo.com
杂志排行
Journal of Ocean University of China的其它文章
- Construction of Inorganic Elemental Fingerprint and Multivariate Statistical Analysis of Marine Traditional Chinese Medicine Meretricis concha from Rushan Bay
- A Comparative Study of Intensive Litopenaeus vannamei Culture on Four Bottom Substrates Without Water Change
- Characterization, Expression and Function Analysis of DAX1 Gene of Scallop (Chlamys farreri Jones and Preston 1904) During Its Gametogenesis
- Preparation, Characterization and Pharmacokinetics of Fluorescence Labeled Propylene Glycol Alginate Sodium Sulfate
- Early Development of Silvetia babingtonii (Fucales, Phaeophyceae)
- A Comparison of Different Gracilariopsis lemaneiformis (Rhodophyta) Parts in Biochemical Characteristics, Protoplast Formation and Regeneration
