Biodiversity of endophytic fungi from seven herbaceous medicinal plants of Malnad region, Western Ghats, southern India
2014-04-19ShankarNaikKrishnappaKrishnamurthy
B.Shankar Naik • M.Krishnappa • Y.L.Krishnamurthy
Introduction
Endophytes are the microbes that colonize living internal tissues of plants without causing any immediate overt symptoms (Petrini 1986).They are found in almost all plants studied, including liverworts, hornworts, mosses, lycophytes, equisetopsids, ferns and seed plants from arctic to the most biologically diverse tropical forests (Bacon and White 2000).Investigations of endophytes over the past four decades have increased in number due to characteristics of endophytes including cryptic life style, ubiquitous presence and contributions to host plant survival or vigor (Fisher et al.1986).The host-fungal symbiosis may be beneficial to hosts in conferring resistance to insects, pathogens and herbivores (White and Cole 1985; Clay 1988; Shankar et al.2006), reducing physiological costs in terms of water relations, photosynthesis, stress and drought tolerance (Redman et al.2002; Marquez et al.2007; Rodrigues et al.2009), and enhanced vegetative growth (Ernst et al.2003).Plant-associated microbes have also been recognized for their ecological roles influencing host populations, plant communities (Clay and Hollah 1999; Rudgers and Clay 2007), biosynthesis, biotransformation and biodegradation (Koide et al.2005; Yu and Dai 2011).Individual plants can harbor dozens of endophytic fungal species (Arnold and Lutzoni 2007) and these endophytes contribute to the hyper diversity of fungi (Hawksworth 2001).Surveys in tropical moist forests suggest that most of the undiscovered endophyte diversity occurs in tropical plants (Frohlich and Hyde 1999; Arnold 2008).Endophytic fungi are repositories of novel metabolites of pharmaceutical importance (Strobel et al.2004).The ability of endophytes to secrete substances in vitro that limit the growth of other microbial species including pathogens has contributed to current enthusiasm for bioprospecting and biological control with endophytic fungi (Gunatilaka 2006; Strobel and Daisy 2003).Many natural products have been isolated from endophytes (Pelaez 2005), including alkaloids, terpenoids, steroids, quinons, isocoumarins, lignans, phenyl propanoids, phenols and lactones (Zhou et al.2009; Aly et al.2010).In this context, endophytes might contribute to solving plant, animal and human health problems in response to the increasing threats from drug resistant strains of plant and human pathogens.
Medicinal herbs are an important group of hosts for endophytic fungi (Huang et al.2008).Endophytes from Chinese medicinal plants show efficacy as pharmaceutical and agricultural compounds (Shentu et al.2007; Kusari et al.2008).In this study we documented the diversity of culturable endophytic fungi and their seasonal distribution patterns in herbs of Malnad region, southern India.
Material and methods
Sample collection and isolation of endophytes
Apparently healthy leaf samples of 7 medicinal plants (Table 1) growing at various sites in the Malnad region were collected, brought in sterile polythene bags to the laboratory, and processed within 24 h of collection.From each host 200 segments were randomly selected from the leaves of two individuals/season that were located within 1 km of each other.Surface sterilization of samples was done by cleaning leaves under running tap water and cutting them into 1 cm segments followed by stepwise washing with 95% ethanol (10 s), 10% chlorine bleach (0.525% Naocl) for 2 min and 70% ethanol for 2 min followed by two rinses in sterile distilled water.Leaf segments were then allowed to surface dry under sterile conditions.This method of surface sterilization has been shown to effectively eliminate contaminants from endophyte cultures (Arnold et al.2000).Leaf segments were placed on 9 cm Petri plates containing potato dextrose agar medium (PDA, Hi Media Laboratories, Mumbai, India) amended with streptomycin 250 (mg·L-1) to suppress growth of bacteria.The efficacy of surface sterilization was confirmed by pressing the sterilized leaf segments on to the surface of PDA medium.The absence of growth of any fungi on the medium confirmed that the surface sterilization procedure was effective (Schulz et al.1993).Petri plates were incubated at (28±1) ºC with a 12 h photoperiod, and sporulation was induced by incubation in a light chamber under near UV light for 1 to 12 d.Fungi growing out from the leaf segments were subsequently transferred onto fresh PDA plates.Pure cultures were spread on fresh PDA slants.Endophytic fungal species were identified on the basis of cultural characteristics and morphology of fruiting bodies and spores by using standard keys (Barnett and Hunter 1998; Subramanian 1983; Sutton 1980).Cultures that failed to sporulate were recorded as sterile forms.All the isolates were numbered and maintained in Culture Collection Centre of Department of Applied Botany, Kuvempu University, Shankaraghatta, India.
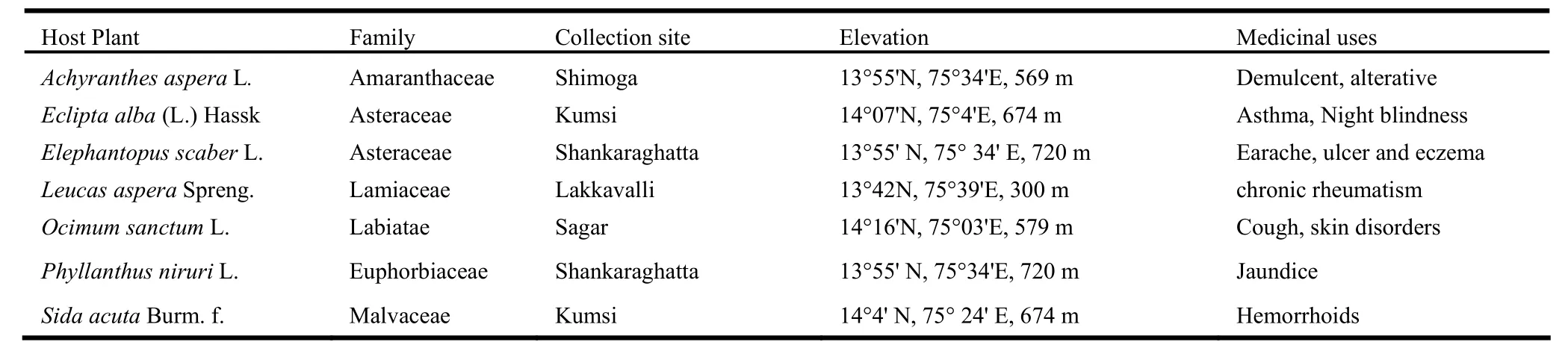
Table 1: Herbaceous plants studied for the isolation of endophytic fungi from Malnad region of Karnataka and their medicinal uses
Data analysis
The colonization rate of endophytic fungi was determined as the total number of segments yielding ≥ 1 isolate in a host sample divided by total number of segments incubated in that sample x 100.Frequency of colonization by individual taxa was calculated similarly.Significance of differences in the frequency of colonization among the host plants was determined using the Kruskal Wallis method (Gibbons 1976).Differences between winter, monsoon and summer seasons were tested by ANOVA and Similarity among the hosts and between the study sites were estimated.Shannon diversity index (H'), Shannon evenness index (J') and Simpson diversity index (1/D) were used for evaluation of fungal species richness (Zar 2004).
Results
A total of 3611 fungal isolates were recovered from 4200 leaf segments that were cut from 7 species of medicinal herbs and incubated during monsoon, winter and summer seasons.These fungal isolates belonged to teleomorphic Ascomycota (23.5%), anamorphic Ascomycota producing conidiomata (17.4%), anamorphic Ascomycota without conidiomata (46.9%), Zygomycota (1.42%) and sterile forms (10.6%) (Table 2).Chaetomium globosum Kunze & Schm (14%), Aspergillus niger Tiegh.(6.5%), Aureobasidium pullulans (de Bary) Arnaud.(5.7%), Curvularia lunata Boed.(2.7%), Fusarium spp.(1.30%), Penicillium chrysogenum Thom.(3.04%), Pestalotiopsis sp.(2.9%), Trichoderma viridae Per.Ex Fr.(1.8%), Cladosporium cladosporioides (Fr.) de vries.(3.54%) and sterile forms (3.02%), were isolated from more than one host plant.The total colonization rate was higher in winter than during monsoon and summer seasons (Fig 1).The colonization frequency (%) of endophytic fungi differed significantly between monsoon, winter and summer seasons (F=18.30).Maximum colonization was observed in winter followed by monsoon and summer.
The total colonization frequency ranged from 35.5% on Lecas aspera Spreng.to 62.3 % on Eclipta alba (L.) Hassk.Maximum colonization frequency was observed on E.alba (155%) and minimum on A.aspera (54.5%) during monsoon season, whilehigh colonization frequency was recorded on E.alba (150%) and low on L.aspera (66%) during the winter season.Similarly, maximum frequency of colonization was observed on E.alba (96%) and minimum on E.scaber (47.5%) during summer. Chaetomium was isolated as a dominant genus from E.alba and Elephantopus scaber L.Similarly Aspergillus, Aureobasidium and Phyllosticta were recovered as dominant endophytes from L.aspera, P.niruri and S.acuta, respectively.The sterile forms dominated in A.aspera and O.sanctum.The maximum fourteen endophytic fungal species were recovered from E.alba whereas only six species were recovered from Achyranthes aspera L.(Table 3).Colonization frequency (%) did not differ significantly between herbs.
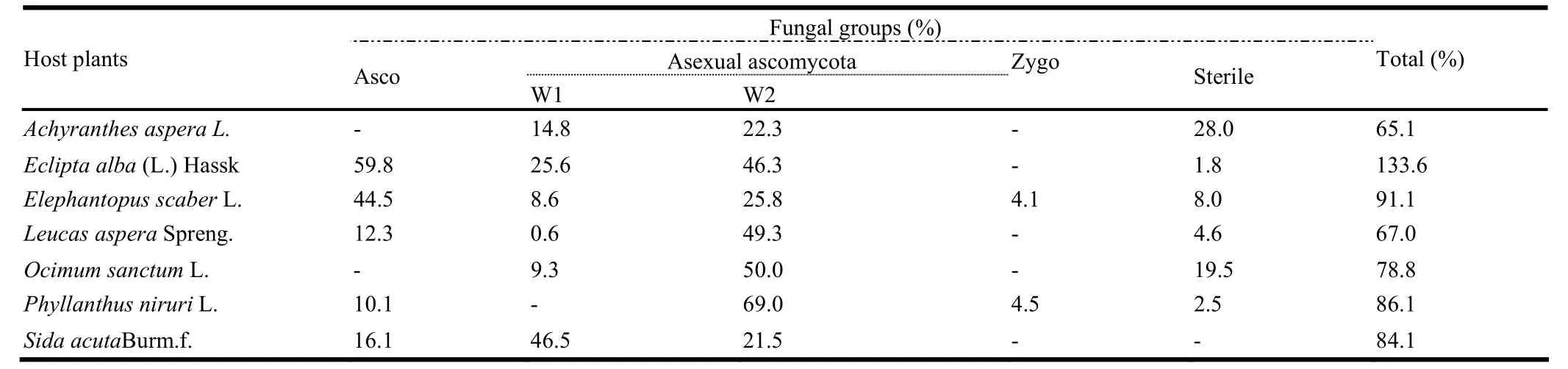
Table 2: Percentage of different fungal classes encountered in some medicinal herbs
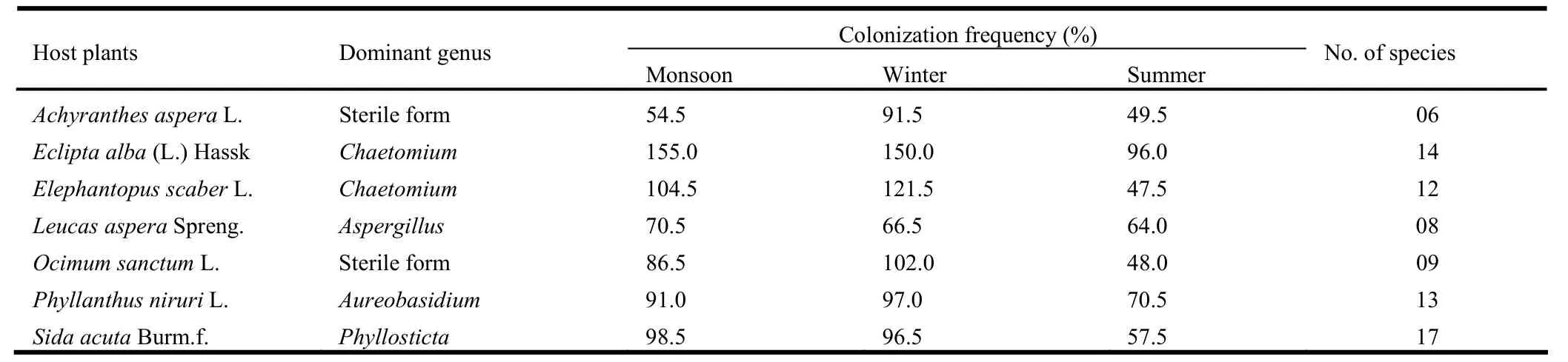
Table 3: Percent colonization frequency, dominant genus and total number of species encountered in different medicinal herbs
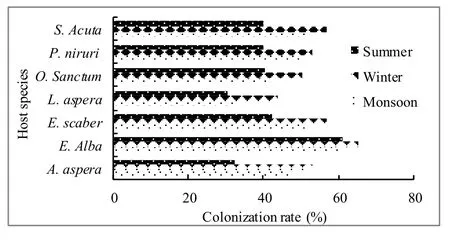
Fig.1: Colonization rate (CR) % of endophytic fungi in different medicinal plants collected from Malnad region of Western Ghats, Karnataka
Sida acuta Burm.f.had high endophytic diversity with H¹ at1.06.Low diversity was recorded on A.aspera with H' at 0.65.Shannon Evenness was high on O.sanctum, with J’ at 0.90 and lowest on L.aspera with J’ at 0.78.Species abundance was high on S.acuta with a 1/D value of 10.Low species abundance was observed on A.aspera (1/D = 3.84) (Table 4).Alternariaalternata, A.pullulans, C.globosum, C.lunata, Fusarium chlamydosporum, Pestalotiopsis spp., Sordaria fimicola and T.viridae isolates recovered more during monsoon and winter, while A.flavus, C.cladosporiodes, Fusarium spp.recovered more during summer.

Table 4: Diversity indices of endophytic fungi in some medicinal herbs of Malnad region, Karnataka
Discussion
The colonization frequency (%) of fungal endophytes in this study was within the range of the large number of host plants reported on by Suryanarayanan et al.(2003).The dominant taxa isolated in this study, including A.pullulans, C.globosum, Pestalotiospsis spp., Penicillium spp., and Phyllosticta, have repeatedly been reported as endophytes from large numbers of plants surveyed from the tropics (Rodrigues et al.2005; Krishnamurthy et al.2009).
Many of these endophytes colonize broad ranges of hosts and grow rapidly and competitively on non-selective, plant-based media frequently used in survey work (eg: PDA, MEA, CMA) (Lodge et al.1996; Frohlich and Hyde 1999).Many of the strains like Aspergillus, Penicillium and Cladosporium isolated in this study are considered as saprobic, soil or air borne fungi but these were regularly recovered from surface sterilized healthy tissues of host plants studied by Krishnamurthy et al.(2008) and Zamora et al.(2008).Maximum colonization during winter season suggests that infection levels are positively correlated with environmental factors (Bills 1996; Wilson 2000).
In many instances, leaves sampled during the wet season harbor more endophytes than those screened during the dry season (Wilson and Carroll 1994).The spores of fungi like Colletotrichum, Pestalotiopsis produce slimy conidia that are not forcibly released but dispersed by water in various ways andmightbe responsible for isolation of greater numbers of isolates during wet months (Wilson and Carroll 1994; Schulthess and Faeth1998).In winter season, humidity and moderate temperatures might enable fungal propagules to germinate successfully (Gore and Bucak 2007).The fungi such as Aspergillus, Cladosporium, and Penicillium have been frequently isolated during the dry season (Shankar Naik et al.2008).Spores of these fungi can survive and even grow in a low water environment (Hyde et al.2007).
The differences between host plants in terms of colonization by endophytes might be due to prevailing microhabitats, environment, stress, senescence of the hosts, virulence of the endophytes and host defense responses (Schulz and Boyle 2005).Inoculum volume plays an important role in determining the infection success of plant-associated fungi (Agrios 1997; Arnold 2008).The frequency of isolation of endophytic fungi also correlates with leaf fragment size, type of growth medium, culturing conditions, and surface sterilization protocols (Gamboa et al.2002).The major challenge that remains to be addressed for clear understanding of endophyte diversity is sterile fungi that do not sporulate in media.For this reason, endophytic fungi are often grouped as morphospecies based on mycelial characteristics (Guo et al.2003).Molecular techniques like ITS sequences and DGGE have now been effectively used for identification and classification of morphospecies of endophytic fungi (Guo et al.2003; Jeewon and Hyde 2006).Some of the fungi isolated in this way might be sources of novel compounds and we are currently pursuing fermentation of these endophytes to obtain wide array of secondary metabolites to facilitate screening against therapeutic targets.
We acknowledge financing from the University Grant Commission, India to B.Shankar Naik and Kuvempu University for providing the laboratory facilities in Dept.of Applied Botany.
Agrios GN.1997.Plant Pathology, 4thedition.London: Academic Press, p.635.
Aly AH, Debbab A, Kjer J, Proksch P.2010.Fungal endophytes from higher plants: a prolific source of phytochemicals and other bioactive nature products.Fungal Diversity, 41: 1–16.
Arnold AE.2008.Endophytic Fungi: Hidden Components of Tropical Community Ecology.In: Schnitzer S, Carson W.(eds), Tropical Forest Community Ecology.London: Blackwell Scientific, pp.254–271.
Arnold AE, Lutzoni F.2007.Diversity and host range of foliar fungal endophytes: are tropical leaves biodiversity hotspots? Ecology, 88: 541–549.
Arnold AE, Maynard Z, Gilbert GS, Coley PD, Kursar TA.2000.Are tropical fungal endophytes hyper diverse? Ecological Letters, 3: 267–274.
Bacon CW, White JF.2000.Microbial Endophytes.New York: Marcel Decker INC, pp.237–261.
Barnett HL, Hunter BB.1998.Illustrated Genera of Imperfect Fungi, .4th edn.New York: Prentice-Hall, Inc., p.218.
Bills GF.1996.Isolation and analysis of endophytic fungal communities from woody plants.In: Redlin S, Carris LM.(eds), Systematics, Ecology and Evolution of Endophytic Fungi in Grasses and Woody Plants.St Paul, MN: APS Press, pp.31–65.
Clay K.1988.Fungal endophytes of grasses: a defensive mutualism between plants and fungi.Ecology, 69: 10–16.
Clay K, Holah J.1999.Fungal endophyte symbiosis and plant diversity in successional fields.Science, 285: 1742–1744.
Ernst M, Mendgen KW, Wirsel SGR.2003.Endophytic fungal mutualists: seed-borne Stagonospora spp.enhances reed biomass production in axenic microcosms.Molecular Plant Microbe Interactions, 16: 580–587.
Fisher PJ, Anson AE, Petrini O.1986.Fungal endophytes in Ulexeuropaeus and Ulexgallii.Transactions of the British Mycological Society, 86: 153–156.
Fröhlich J, Hyde KD.1999.Biodiversity of palm fungi in the tropics: are global fungal diversity estimates realistic? Biodiversity and Conservation, 8: 1742–1744.
Gamboa MA, Laureano P, Bayman P.2002.Measuring diversity of endophytic fungi in leaf fragments: Does size matter? Mycopathologia, 156: 41–45.
Gibbons JD.1976.Non-parametric methods for quantitative analysis.New York: Holt, Rine hart and Winston, p.463.
Göre ME, Bucak C.2007.Geographical and seasonal influence on the distribution of fungal endophytes in Laurusnobilis.Forest Pathology, 37: 281–288.
Gunatilaka AAL.2006.Natural products from plant-associated microorganisms: distribution, structural diversity, bioactivity, and implications of their occurrence.Journal of Natural Products, 69: 509–526.
Guo LD, Huang GR, Wang Y.2003.Molecular identification of white morphotype strains of endophytic fungi from Pinustabulaeformis.Mycological Research, 107: 680–688.
Hawksworth DL.2001.The magnitude of fungal diversity: the 1.5 million species estimate revisited.Mycological Research, 105: 1422–1432.
Huang WY, Cai YZ, Hyde KD, Corke H, Sun M.2008.Biodiversity of endophytic fungi associated with 29 traditional Chinese medicinal plants.Fungal Diversity, 33: 61–75.
Hyde KD, Bussaban B, Paulus B, Crous PW, Lee S, Mckenzie EHC, Photita W, Lumyong S.2007.Biodiversity of saprobic fungi.Biodiversity and Conservation, 16: 17–35.
Jeewon R, Hyde KD.2006.Diversity and detection of fungi from environmental samples: traditional versus molecular approaches.In: Varma A, Oelmuller R.(eds), advanced techniques in Soil microbiology, Soil biology series.BerlinHeidelberg: Springer-Verlag press, pp.1–11.
Koide K, Osono T, Takeda H.2005.Colonization and lignin decomposition of Camellia japonica leaf litter by endophytic fungi.Mycoscience, 46: 280–286.
Krishnamurthy YL, Shankar Naik B, Shashikala J.2008.Fungal communities in herbaceous medicinal plants, Malnad region, Southern India.Microbes and Environment, 23(1): 24–28.
Krishnamurthy YL, Shashikala J, Shankar Naik B.2009.Diversity and seasonal variation of endophytic fungal communities associated with some medicinal trees of Western Ghats, Southern India.Sydowia, 61(2): 255–266.
Kusari S, Lamshöft M, Zühlke S, Spiteller M.2008.An endophytic fungus from Hypericumperforatum that produces hypericin.J Nat Prod, 71: 159–162.
Lodge DJ, Fisher PJ, Sutton BC.1996.Endophytic fungi of Manilkarabidentataleave in Puerto Rico.Mycologia, 88: 733–738.
Márquez LM, Redman RS, Rodriguez RJ, Roossinck MJ.2007.A virus in a fungus in a plant: three-way symbiosis required for thermal tolerance.Science, 315: 513–515.
Pelaez F.2005.Biological activities of fungal metabolites.In: An Z.(ed).Handbook of industrial mycology.New York: Marcel Dekker, p.49–92.Petrini O.1986.Taxonomy of endophytic fungi of aerial plant tissues.Microbiology of Plant Microbe Interactions, 16: 580–587.
Redman RS, Sheehan KB, Stout RG, Rodrigues RJ, Henson JM.2002.Thermo tolerance conferred to plant host and fungal endophyte during mutualistic symbiosis.Science, 298: 1581.
Rodrigues KF, Costa GL, Carvalho MP, Epifanio RDA.2005.Evaluation of extracts produced by some tropical fungi as potential cholinesterase inhibitors.World Journal of Microbiology and Biotechnology, 21: 1617–1621.
Rodriguez RJ, White JF, Arnold AE, Redman RS.2009.Fungal endophytes: diversity and functional roles.New Phytologist, 182: 314–330.
Rudgers JA, Clay K.2007.Endophyte symbiosis with tall fescue: how strong are the impacts on communities and ecosystems? Fungal Biology Reviews, 21: 107–124.
Schulthess FM, Faeth SH.1998.Distribution, abundances and association of the endophytic fungal community of Arizona fescue (Festucaarizonica Vasey).Mycology, 90: 569–578.
Schulz B, Boyle C.2005.The endophytic continuum.Mycological Research, 109: 661–686.
Schulz B, Wanke U, Draenger S, Aust HJ.1993.Endophytes from herbaceous plants and shrubs: effectiveness of surface sterilization methods.Mycological research, 97: 1447–1450.
Shankar Naik B, Shashikala J, Krishnamurthy YL.2008.Diversity of endophytic fungal communities in shrubby medicinal plants of Western Ghat region, Southern India.Fungal Ecology, 1: 89–93.
Shankar Naik B, Shashikala J, Krishnamurthy YL.2006.Study on diversity of endophytic communities from rice (Oryza sativa L.) and their antagonistic activities in vitro.Microbiological Research, 3: 290–296.
Shentu XP, Chen LZ, Yu XP.2007.Anti-fungi activities and cultural characteristics of gingko endophytic fungus No.1028.Acta Phytophyl Sin, 34: 147–152.
StrobelGA, Daisy B.2003.Bioprospecting for microbial endophytes and their natural products.Microbiology and Molecular Biology Reviews, 67: 491–502.
Strobel GB, Daisy U, Castillo Harper JJ.2004.Natural products from endophytic microorganisms.Natural Products, 67: 257–268.
Subramanian CV.1983.Hyphomycetes, Taxonomy and Biology.New York: Academic Press, p.450.
Suryanarayanan TS, Murali TS, Venkatesan G.2003.Endophytic fungal communities in leaves of tropical forest trees: diversity and distribution patterns.Current Science, 85: 489–493.
Sutton BC.1980.The Coelomycetes.London, England: Kew, Commonwealth Mycological Institute, p.696.
White JF Jr, Col GT.1985.Endophyte host associations in forage grasses.I.Distribution of Fungal endophytes in some species of Lolium and Festuca.Mycologia, 77: 323–327.
Wilson D.2000.Ecology of woody plant endophytes.In: Bacon CW, White JF.(eds), Microbial endophytes.New York, Basel: Marcel Dekker, pp 389–420.
Wilson D, Carroll GC.1994.Infection studies of Disculaquercina and endophyte of Quercusgarryana.Mycologia, 86: 635–647.
YuW, Dai CC.2011.Endophytes: a potential resource for biosynthesis, biotransformation, and biodegradation.Annals of Microbiology, 61: 207–215.
Zamora P, Martínez-Ruiz C, Diez JJ.2008.Fungi in needles and twigs of pine plantations from northern Spain.Fungal Diversity, 30: 171–184.
Zar HJ.2004.Biostatistical analysis, 4thedn.Delhi: Pearson Education Pvt.Ltd, p.663.
Zhou L, Zhao J, Xu L, Huang Y, Ma Z, Wang J, Jiang W.2009.Antimicrobial compounds produced by plant endophytic fungi.In: De Costa P, Bezerra P.(eds), Fungicides: Chemistry, Environmental Impact and Health Effects.New York: Nova Science Publishers, 91:116–119.
杂志排行
Journal of Forestry Research的其它文章
- Ethno-medicinal plants used by Bengali communities in Tripura, northeast India
- Litter production, decomposition and nutrient mineralization dynamics of Ochlandra setigera: A rare bamboo species of Nilgiri Biosphere Reserve, India
- Temporal changes in nitrogen acquisition of Japanese black pine (Pinus thunbergii) associated with black locust (Robinia pseudoacacia)
- Plant diversity at Chilapatta Reserve Forest of Terai Duars in subhumid tropical foothills of Indian Eastern Himalayas
- Floristic composition and management of cropland agroforest in southwestern Bangladesh
- The changing landscape of mangroves in Bangladesh compared to four other countries in tropical regions
