Litter production, decomposition and nutrient mineralization dynamics of Ochlandra setigera: A rare bamboo species of Nilgiri Biosphere Reserve, India
2014-04-19KuruvillaThomasJijeeshSeethalakshmi
Kuruvilla Thomas • C.M.Jijeesh • K.K.Seethalakshmi
Introduction
Litter production and decomposition dominate biogeochemical nutrient cycling in natural and plantation forests (Singh et al.1999; Weltzin et al.2005).Litter deposited on the forest floor acts as an input-output system of nutrients and the rates at which litter falls and decays are the main regulators of primary productivity, energy flow and nutrient cycling in forest ecosystems (Bray and Gorham 1964).Plant litter acts as a temporary sink for nutrients and functions as ‘a slow release nutrient source’ (White 1988), thereby guaranteeing a permanent contribution of nutrients to the soil (Cuevas and Medina 1988).Even though litter constitutes only a portion of total biomass, it accounts for the major share of nutrients stored in organic matter (Enright 1979).Moreover, litterfall as a major component of net primary productivity may provide important information as a phenological indicator of climate change effects on forests (Hansen et al.2009).
Litter dynamics studies are reported to be very important in the nutrition budgeting on tropical ecosystems where vegetation depends on the recycling of the nutrients contained in plant debris (Prichett and Fisher 1987).In recent years, there has been a striking increase in the number litter dynamics studies.Most bamboo plantations are on nutrient-poor marginal lands where bamboo litter could play a role in recuperation of soil fertility.Bamboo plantations are reported to be attractive options for marginal or degraded lands (Hunter 2003; Yang 2004).They improved the degraded sites by accumulating high amounts of soil organic matter through their decomposing leaf and abundant fine root litter (Das and Chaturvedi 2006).Ochlandra setigera Gamble is a bamboo species endemic to Nilgiri Biosphere Reserve which has to be conserved with higher priority due to restricted habitat and over-exploitation.Our study was designed to understand the production and decomposition of litter of O.setigera with the following objectives: (1) to investigate litter production dynamics (2) to investigate the pattern of litter mass loss; (3) to investigate litter chemistry and decomposition dynamics of leaf litter; and (4) to investigate the pattern of nutrient release from leaf litter.
Materials and methods
Study species
Ochlandra setigera Gamble is a perennial, gregarious small straggling bamboo species endemic to Nilgiri Biosphere Reserve of India with culms reaching a height of 5−8 m, diameter of 1.5−2.2 cm and an internodal length of 23−35 cm.Its distribution is restricted to Malappuram and Palakkad districts of Kerala state and in Gudallur of Tamil Nadu at elevations of 600−1000 m (Kumar 2011).
Study area
The present investigation was conducted from June 2011 to May 2012 at Pothukal station of Vazhikadavu range in Nilambur Forest Division, Kerala, India.The area has a warm humid climate, receiving rain from the southwest and northeast monsoons.Monthly averages of daily maximum temperatures during the study ranged from 29.4 to 35.6ºC with hottest period in March and the minimum temperature ranged from 20.0 to 25.5ºC with lowest temperature in January.Average monthly rainfall ranged from 0.0−459.7 mm with the highest rainfall occurring in June.Relative humidity ranged from 58%−85% with higher values during June and July.
Methods
Monthly litter production of O.setigera was captured using specially designed litter traps made of bamboo baskets having diameter of 1 m and depth of 10 cm.Each bamboo culm were surrounded by five litter-traps and were fixed 25 cm above ground using wooden pegs.
To study litter decomposition dynamics, we collected freshly abscised leaves during the February−March from the forest floor under the O.setigera canopy.Samples of air dried litter weighing 20 g were placed in 15 cm × 15 cm nylon litter bags (2-mm mesh size) and 80 such bags were prepared.Litter bags were placed under the closed canopy of O.setigera on June 1, 2011.Five litter bags were retrieved at monthly intervals until 95% decomposition of the litter was observed.The residual material from the monthly retrieved litter bags was separated carefully from the adhering soil particles using a small brush.Litter samples from each bag were oven dried at 70ºC to constant weight.
In order to estimate initial litter chemistry and chemistry of litter retrieved at each sampling period, litter samples were ground in a Wiley mill for chemical analysis.Total carbon was estimated using Euro vector (EA 3000) CHNS Elementar analyser and nitrogen and phosphorus using continuous flow analyzer (Skalar San++).Potassium was estimated using a flame photometer (ELICO) and calcium and magnesium were estimated using an Atomic Absorption Spectrophotometer (VARIAN) (Jackson 1973).Mass loss over time was computed using the negative exponential decay model (Olson 1963).The time required for 50% (t50) and 99% (t99) decay was calculated as t50= 0.693/k and t99= 5/k.Nutrient content of the litter was calculated using the formula.

where, N is percentage of nutrient remaining in the litter.C is the concentration of the element in litter at the time of sampling; C0is the concentration of element in the initial litter kept for decomposition; DMis the mass of dry matter at the time of sampling and DM0is the mass of initial dry matter kept for decomposition (Bockheim et al.1991).The percentage of nutrient released from the litter mass was calculated as 100-N.
Statistical analysis
The data were subjected to one-way analysis of variance in SPSS 16 and treatment means were compared using least significant difference (lsd) as necessary.Correlation and regression analysis also were carried out in SPSS 16.Litter decay constant was calculated using MS-Excel 2007.
Results
Litter deposition
Average annual litter deposition of O.setigera from June 2011 to May 2012 was 1.981 t·ha-1.In general, litter deposition was spread throughout the study period with obvious monthly variations in quantity (Fig.1).Litter deposition followed a triphasic pattern with two major peaks in November 2011 and January 2012 and a minor peak in July 2011.The litter production varied with season and during June to September 2011 (rainy season), October 2011 to January 2012 (winter) and February to May 2012 (summer) it was 0.447, 0.765 and 0.769 t·ha-1, respectively.Litter deposition during rainy season was low, accounting for only 23% of total fall but during winter and summer seasons was at par.The highest monthly litter deposition occurred in February 2012 (0.368 t·ha-1) followed by December 2011 (0.341 t·ha-1).Analysis of variance revealed significant differences in litter deposition by month at p <0.01 indicating the significant monthly variation litter production.
Litter decomposition
Mass loss (dry weight basis) during litter decomposition of O.setigera litter (Fig.2) showed an initial rapid loss followed by a slower rate of loss towards the later part of decomposition.A negative exponential model 2 was fitted to the mass loss data.

where, X is the litter mass at time t, X0the initial litter weight, e is the base of natural logarithm and k is the decomposition rate constant.The exponential regression equation used to describe mass loss trough time was significant at one percent level (p = 0.01).The rate of decomposition was a good fit to exponential decay model of Olson (1963).The regression model that depicted the progression of litter decomposition was y=33.845e-0.0078t(R²=0.94).The decomposition rate constant was 0.008 day-1, consequently t50and t99were 89 and 641 days, respectively.

Fig.1: Litter production dynamics of Ochlandra setigera
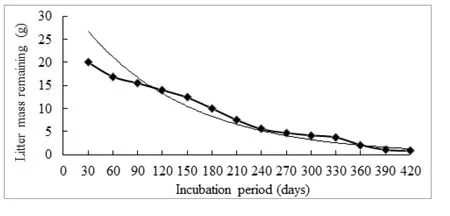
Fig.2: Pattern of litter decomposition of Ochlandra setigera (coloured line shows the actual weight loss and other the predicted mass loss).
Nutrient release
The concentrations of N, P, K, Ca and Mg in the monthly retrieved litter samples of O.setigera are shown in Fig.3.Of the five nutrients, N was in the highest concentration in litter.The N concentration of the litter mass declined until 210 days after incubation which indicated the release of nutrients.An increase in nitrogen concentration was observed from 210 to 300 days indicating accumulation phase.Thereafter, N concentration declined steadily.
Phosphorous showed an initial accumulation phase of up to 60 days followed by a decline of up to 240 days and subsequent increase to reach the highest P concentration at 360 days from which P concentration declined thereafter.
With some exceptions, K content of the litter declined until 300 days indicating continuous release of K.After 300 days a slight increase was recorded.
Calcium concentration of litter mass declined until 120 days followed by a sudden accumulation at 150 days after incubation.Thereafter, the concentration fluctuated.
Mg concentration in litter mass declined until 210 days and it was followed by an accumulation phase and subsequent decline.Analysis of variance revealed significant differences in monthly nutrient concentration at one per cent level indicating significant variation in N, P, K, Ca and Mg concentration of the monthly retrieved litter samples.

Fig.3: Nutrient concentrations in the monthly retrieved litter samples of Ochlandra setigera
The percentage nutrient in the remaining litter mass was computed from residual nutrient concentrations and litter mass.There was faster release of nutrients in the initial stages of decomposition corresponding to the onset of the southwest monsoon and slowing later (Fig.4).The percentage release of nutrients from the decomposing litter mass during incubation is given in Fig.5.Nutrient release rates were all rapid in the early stages of decomposition but slowed later.Hence, a negative exponential model was fitted (y=e-kt).The relation between time and the rate of nutrient release was analyzed using regression analysis.The exponential regressions equation used to describe mass loss through time were significant (p = 0.01).The equations were
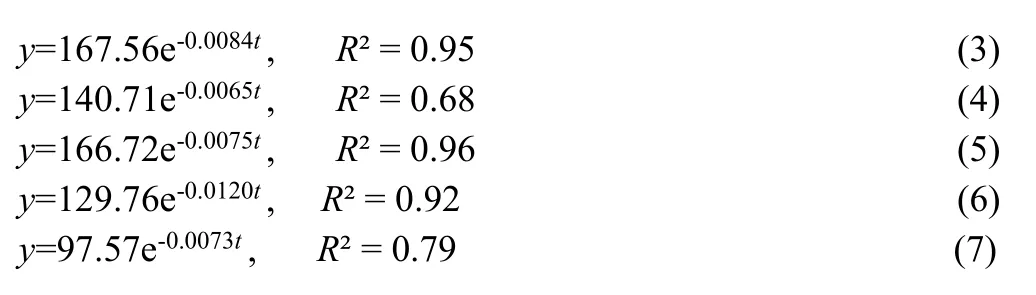
where, y is the fraction of nutrient release at time t and initial nutrient release.The equations 3, 4, 5, 6, 7, are for N, P, K, Ca and Mg.The decomposition rate constants, half-life and times for 99% decomposition are listed in Table 1.Phosphorous recorded the lowest k value and consequently longest half-life.Nutrients were released in the order Ca> N> K> Mg> P.

Fig.4: The percentage nutrient remaining in the litter mass during decomposition of Ochlandra setigera

Fig.5: The percentage release of nutrients from the decomposing litter mass of Ochlandra setigera during the incubation

Table 1: The litter decay constant and time required for decomposition of Ochlandra setigera
Discussion
Litter deposition and decomposition
Litterfall as the first phase of the biogeochemical cycle returns nutrients to the soil (Lebret et al.2001).It is a fundamental process in nutrient cycling and is the main means of transfer of organic matter and mineral elements from vegetation to the soil surface (Regina et al.1999; Robertson and Paul 1999).Much of the annual gain of energy and dry matter of plants is shed as litter, which, through decomposition, plays a major role in ecosystem structure and function (Christensen 1975).
Evaluation of litterfall production is important for understanding nutrient cycling of ecosystems.Litter production varies with climate, season, substrate quality and type of vegetation (Vitousek et al.1994).Knowledge of litter production is important when estimating nutrient turnover, C and N fluxes, and C and N pools in ecosystems.Proctor (1987) reported that the litter accumulation of forest floor mass is usually low in moist topical forests and in many ecosystems amounts to only 2 to 11 t·ha-1.Litter deposition in our O.setigera stand was near to the range reported by Proctor (1987).
Litter production by Ochlandra setigera was low compared to that of most tropical and sub-tropical bamboo species (Tripathi and Singh 1994; Christanty et al.1996; Shanmughavel and Francis 1996; Isgai et al.1997; Upadhyaya et al.2008).However, litter deposition in the present study was greater than that reported for Dendrocalamus strictus (Joshi et al.1991) and Arundinaria racemosa (Upadhyaya et al.2008).Although litter production and climatic factors were not significantly correlated, the correlation was negative with minimum temperature, rainfall and humidity and positive with maximum temperature.Similar to our study, marked seasonal variation in litter accumulation was reported by many scientists (Austin and Vitousek 2000; Pragasan and Parthasarathy 2005; Bhat et al.2009; Ndakara 2011).They reported an increase in monthly litter production during the dry season and lower litter production in the wet season.We observed a similar trend in the present investigation.One of the direct effects of dry season on bamboos is water stress, when moisture availability is limited and air temperatures also rise.Thus the periodicity of litterfall in general could be viewed as an effect of water stress.The lower litter production in the present study also might be due to smaller clump size and biotic disturbances by humans and animals.Frequent attack by elephants was common during the study period and led to destruction of bamboo clumps.
O.setigera was grown as an understorey species in semi-evergreen area near a stream.The annual inputs of nutrients to the soil via litter deposition (kg·ha-1) were in the order N (33.182) > K (11.609) > Ca (7.488) > P (4.338) >Mg (1.941).
Mass loss rates during litter decomposition were higher during the rainy season, due to the rapid multiplication and intense activity of microbes.As a result, most easily decomposable substances are lost from the system (Palm and Rowland 1997; Berg and Matzner 1997).With the release of easily decomposable materials, relatively more decay-resistant materials remained in the litterbags and this caused a decrease of mass loss during subsequent months.
The decomposition rate constant for O.setigera (0.24) was slightly higher than that for O.travancorica (0.23) in Vazhachal of Thrissur district in the southern Western Ghats of India (Sujatha et al.2003).Decay rate constants in tropical plantations are reported to range between 0.11−2.00 (O’connell and Sankaran 1997).The k values calculated at the end of decomposition in the present study corroborate this range.Initial N (Meentemeyer and Berg 1986) and lower C:N ratio (Swift et al.1979) have been well correlated with weight loss during decomposition.Initial litter chemistry of O.setigera is depicted in Fig.6.Among the nutrients, carbon (29.07%) was the major component, followed by nitrogen.The nutrient in lowest concentration was Mg.

Fig.6: Initial chemistry of the of Ochlandra setigera
The ratios of carbon to N, P and K also varied: the C:N ratio was 22.62; C:P ratio was 183.09; and C:K ratio was 63.Litter decomposition was negatively correlated with N concentration (r = -0.903), Ca (r = -0.857) and C (r = -0.979).The C:N and C:P ratios were also negatively correlated and r values were -0.922 and -0.602, respectively.Seneviratne (2000) suggested that N proportions lower than 2% limit the decomposition of tropical litter.The lower decomposition rates in this species may be attributed to lower nitrogen content and lower C:N ratio.Relationships between decay rates and litter P concentrations have been reported for sites where P availability is low due to either edaphic factors or N deposition (Vitousek et al.1994).Indices that incorporate both C chemistry and nutrient content, such as C:N or lignin:N or C:P ratios, are often negatively correlated with early decay rates (Moore et al.2006).
The C: N ratio is a good indicator of whether net mineralization or immobilization will occur during decomposition.Since the litter mass had C: N ratios less than 30:1, it can be assumed that mineralization took place during litter decomposition.Significant correlation between litter decomposition and climatic factors (p <=0.05 in all cases) was recorded in the present study.Litter decomposition was negatively correlated with maximum temperature (r =-0.564) and positively correlated with rainfall (r =0.647) and relative humidity (r =0.518).The relatively slow decomposition of bamboo leaf litter should lead to accumulation of soil organic matter over time and is expected to provide benefits of mulching.Because of the accumulation of leaf mulch bamboo serves as an efficient agent in preventing soil erosion and conserving soil moisture (Yadav 1963)
Nutrient release from decomposing litter
Declining concentrations of N, K, Ca and Mg were observed in the initial stages of decomposition, indicating nutrient release, whereas P showed an initial accumulation phase.The decline in N is associated with loss of easily leachable components of litter mass.The increase in N after initial release is associated with microbial fixation of atmospheric N2inputs from external sources like throughfall and microbial immobilization (Laskowski et al.1995).There are contrasting reports on concentration of P in decomposing litter mass.In some cases, the concentration of P has been reported to decrease, while others report that P remains constant or increases during decomposition.This is a characteristic of the leaf litter quality and the site, namely whether P is limited (Moore et al.2006).Our results are inconsistent with those of Sujatha et al.(2003) for O.travancorica where an increase in P concentration was recorded during decomposition.Attiwill (1968) found K was the most mobile element and this explains the rapid release of this nutrient.In contrast to N and P, K is not bound as a structural component in plants and is highly water soluble.Attiwill (1968) reported that the loss of calcium from decomposing litter was slow due to its importance as a structural component.Many authors reported Mg dynamics in decomposing litter similar to the two-phase pattern recorded in our study (initial leaching phase and late immobilization phase (Blair 1988; Laskowski and Berg 1993; Hasegawam and Takeda 1997).Magnesium is not a structural material and exists mainly in solution in plant cells and thus leached out from litter in the initial phase of decomposition.
The rank order of nutrient release rates in our study was Ca> N> K> Mg> P.Release of nitrogen from the litter mass was continuous and did not include an accumulation phase.More than 70% of nitrogen was released within 210 days corresponding to rainy and winter seasons (Fig.4).The release of P was continuous until 210 days, followed by a rapid accumulation phase at 240 days and minor subsequent accumulations.More than 80% of P was released within 210 days.K release was highest during the first month of incubation when more than 90% release occurred.Later, two slight accumulation phases were recorded.More than 60% of Ca was released within three months of incubation corresponding to the southwest monsoon and followed by an immobilization phase where 14% of Ca accumulated in litter mass.More than 80% of Mg released within 210 days.Four slight accumulation phases were recorded later.The release of nutrients from the litter mass was in synchrony with the growth of the new culms which were typically produced at the onset of monsoon season.
Conclusions
Litterfall density in O.setigera, a small clump-forming bamboo, was lower than for other bamboo species.Litter production was continuous but the quantity of litter produced varied by season and month.Nutrient concentrations in monthly litter samples varied by nutrient.Nutrient release from litter was continuous and it was in synchrony with the growth of the new culms.Since, this species has only limited distribution, it has to be conserved as a high priority and to be brought under cultivation.We recommend that litter dynamics studies be conducted before introduction of a bamboo species into degraded or marginal lands.Further studies of other bamboo species are needed to improve understanding of nutrient requirements prior to bringing them into cultivation.
Acknowledgment
Authors acknowledge Dr.K.V.Sankaran, Former Director, Kerala Forest Research Institute for providing facilities and Kerala Forest Department for financial support.
Attiwill PM.1968.The loss of elements from decomposing litter.Ecology, 49: 142−45.
Austin AT, Vitousek PM.2000.Precipitation, decomposition and litter decomposability of Meterosideros polymorpha in native forests on Hawai.Jornnal of Ecology, 88: 129−138.
Berg B, Matzner E.1997.Effect of N deposition on decomposition of plant litter and soil organic matter in forest systems.Environmental Review, 5: 1–25.
Bhat N, Verma R, Reshi Z.2009.Seasonal variation of litter Production in Fraxinus excelsior Linn.And Ulmus villosa Brandis in forests of Dachigam national park (J&K).Indian Forester, 135: 825−830.
Blair JM.1988.Nitrogen, sulphur and phosphorus dynamics in decomposing deciduous leaf litter in the southern Appalachians.Soil Biology & Biochemistry, 17: 827−830.
Bockheim JG, Jepson EA, Helsey DM.1991.Nutrient dynamics in decomposing leaf litter of four tree species in northern Wisconsin.Canadian Journal of Forest Research, 21: 267–286.
Bray JR, Gorham E.1964.Litter production in the forests of the world.Advamces in Ecological Research, 2: 101−157.
Christanty L, Mailly D, Kimmins JP.1996.Without bamboo, the land dies: biomass, litterfall, and soil organic matter dynamics of a Javanese bamboo talun-kebun system.Forest Ecology and Management, 87: 75−88.
Christensen O.1975.Wood litterfall in relation to abscission, environmental factors, and the decomposition cycle in a Danish oak forest.Oikos, 26: 187−195.
Cuevas E, Medina, E.1988.Nutrient dynamics within Amazonian forests II.Fine root growth, nutrient availability and leaf litter decomposition.Oecologia, 76: 222−235.
Das DK, Chaturvedi OP.2006.Bambusa bambos (L.) Voss plantation in eastern India: II.Nutrient dynamics.Journal of Bamboo and Rattan, 5: 105−116.
Enright NJ.1979.Litter production and nutrient partitioning in rain forest near Bulolo, Papua New Guinea.Malaysian Forester, 42: 202–207.
Hansen K, Vesterdal L, Schmidt IK, Gunderson P, Sevel L, Bastrup-Birk A, Pedersen LB, Bille-Hansen J.2009.Litterfall and nutrient return in five tree species in a common garden experiment.Forest Ecology and Management, 257: 2133–2144.
Hasegawam M, Takeda H.1996.Carbon and nutrient dynamics in decomposing pine needle litter in relation to fungal and faunal abundances.Pedobiologia, 40: 171–184.
Hunter IR.2003 Bamboo resources, uses and trade: the future? Journal of Bamboo and Rattan, 2: 319.
Isagi Y, Kawahara T, Ito H.1997.A computer-aided management system of Phyllostachys stands based on the ecological characteristics of carbon cycling.In: GP Chapman (ed.), The bamboos.London: Academic Press, pp.125−134.
Jackson JK 1973.Soil chemical analysis.New York: Printice hall, p.498.
Joshi AP, Sundrial RC, Baluni DC.1991.Nutrient dynamics of Lower Siwalik bamboo forest in the Garhwal Himalaya.Journal of Tropical Forest Science, 3: 338−350.
Kumar MS.2011.All India coordinated project on taxonomy (aicoptax) grasses & bamboos (Part II) Bamboos of Peninsular India.KFRI Research Report, p.399.
Laskowski R, Berg B.1993.Dynamics of some mineral nutrients and heavy metals in decomposing forest litter.Scandinavian Journal of Forest Research, 8: 446−456.
Laskowski R, Niklinska M, Maryanski M.1995.The dynamics of chemical elements in forest litter.Ecology, 76: 1393−1406.
Lebret M, Nys C.Forgeard F.2001.Litter production in an Atlantic beech (Fagus sylvatica L.) time sequence.Annals of Forest Science, 58: 755–768.
Meentemeyer V, Berg B.1986.Regional variation in mass-loss of Pinus sylvestris needle litter in Swedish pine forests as influenced by climate and litter quality.Scandinavian Journal of Forest Research, 1: 167–180.
Moore TR, Trofymow JA, Prescott C.E, Fyles J, Titus BD.2006.Patterns of carbon, nitrogen and phosphorus dynamics in decomposing foliar litter in Canadian forests.Ecosystems, 9: 46–62.
Ndakara.2011.Litterfall and Nutrient Returns in Isolated Stands of Persea gratissima (Avocado Pear) in the Rainforest Zone of Southern Nigeria.Ethiopian Journal of Environmental Studies and Management, 4: 42−50.
O'Connell AM, Sankaran KV.1997.Organic matter accretion, decomposition and mineralisation.In: Nambiar EKS & Brown AG (eds.), Management of Soil, Nutrients and Water in Tropical plantation forests (ACIAR Monograph).Canbera, Australia: Australian Centre for International Agriculture Research, p.443−480, p.571.
Olson JS.1963.Energy storage and the balance of producers and decomposers in ecological systems.Ecology, 44: 322−331.
Palm CA, Rowland AP.1997.A minimum dataset for characterization of plant quality for decomposition.In: G.Cadish, and K.E.Giller (eds.), Driven by Nature: Plant Litter Quality and Decomposition.London: CAB International, pp.379–392.
Pragasan A, Parthasarathy N.2005.Litter production in tropical dry evergreen forests of south India in relation to season, plant life-forms and physiognomic groups.Current Science, 88: 1255−1263.
Pritchett WL, Fisher RF.1987.Properties and Management of Forest Soils.New York: John Wiley and Sons, p.494.
Proctor J.1987.Nutrient cycling in primary and old secondary rain forests.Applied Geography, 7: 135−152.
Regina M, Wetrington BD, Vana stru FF aldi-De Vuono.1999.Litter and nutrient content in two Brazilian tropical Forest.Revta brasil Bot, 22: 1999.
Robertson GP, Paul EA.1999.Decomposition and soil organic matter dynamics.In: O.E.Sala, R.B.Jackson, H.A.Mooney, and R.W.Howarth, (eds).Methods of Ecosystem Science.New York: Springer- Verlag, pp.104−116.
Seneviratne G.2000.Litter quality and nitrogen release in tropical agriculture: a synthesis.Biology and Fertility of Soils, 31(1): 60–64.
Shanmughavel P, Francis K.1996.Biomass and nutrient cycling in bamboo (Bambusa bambos) plantations of tropical areas.Biology and Fertility of Soils, 23(4): 431−434.
Singh KP, Singh PK, Tripathi SK.1999.Litterfall, litter decomposition and nutrient release patterns in four native tree species raised on coal mine spoil at Singrauli, India.Biology and Fertility of Soils, 29: 371–378.
Sujatha MP, Jose AI, Sankar S.2003.Leaf litter decomposition and nutrient release in reed bamboo (Ochlandra travancorica).Journal of Bamboo and Rattan, 2:65−78.
Swift MJ, Heal OW, Anderson JM., 1979.Decomposition in Terrestrial Ecosystems.Oxford: Blackwell Scientific Publications, pp.372.
Tripathi SK, Singh KP.1992.Abiotic and litter quality control during the decomposition of different plant parts in dry tropical bamboo savanna in India.Pedobiologia, 36: 241–56.
Upadhyaya K, Arunachalam A, Arunachalam K, Das AK.2008.Above ground biomass and productivity appraisal of four important bamboo species growing along different altitudinal regimes in Arunachal Prasdesh.Journal of Bamboo and Rattan, 7: 219−234.
Vitousek PM, Turner DR, Parton WJ, Sanford RL.1994.Litter decomposition on the Mauna Loa environmental matrix, Hawaii: Patterns, mechanisms and Models.Ecology, 75: 418−429.
Weltzin JF, Keller, JK, Bridgham SD, Pastor J, Allen PB, Chen J.2005.Litter controls plant community composition in a northern fen.Oikos, 110: 537–546.
White DL.1988.Litter decomposition in southern Appalachian black locust and pine-hardwood stands: litter quality and nitrogen dynamics.Canadian Journal of Forest Research, 18: 54–63.
Yadav JSP.1963.Site and soil characteristics of bamboo forests.Indian Forester, 89: 177.
Yang E.2004.A gender assessment study on bamboo-based rural development and utilization activities -a case study in Yunnan, China.Peking: International Network for Bamboo and Rattan (INBAR), p.29.
杂志排行
Journal of Forestry Research的其它文章
- Ethno-medicinal plants used by Bengali communities in Tripura, northeast India
- Temporal changes in nitrogen acquisition of Japanese black pine (Pinus thunbergii) associated with black locust (Robinia pseudoacacia)
- Plant diversity at Chilapatta Reserve Forest of Terai Duars in subhumid tropical foothills of Indian Eastern Himalayas
- Floristic composition and management of cropland agroforest in southwestern Bangladesh
- The changing landscape of mangroves in Bangladesh compared to four other countries in tropical regions
- The effect of fire disturbance on short-term soil respiration in typical forest of Greater Xing’an Range, China
