Purification of four strains of endophytic fungi from Astragalus and their optimized liquid fermentations
2014-04-19WeiMaXiuboLiuJiaoJiaoLeimingZhangWeichaoRenLingMaXiangjunKongNingZhangXiwuZhang
Wei Ma • Xiubo Liu • Jiao Jiao • Leiming Zhang • Weichao Ren Ling Ma • Xiangjun Kong • Ning Zhang • Xiwu Zhang
Introduction
Astragalus, the dried root of Astragalus membranaceus (Fisch.) Bunge or Astragalus mongholicus Bunge (Fabaceae) has been a famous health-promoting herb in China for more than 2000 years.Triterpenoid saponins, flavonoids and polysaccharides are believed to be the principle active constituents of Astragalus (Chu et al.1988; Cho and Leung 2007), and they yield a variety of biological benefits, such as improving immunity and heart/liver functions, enhancing hypoxia tolerance and stress tolerance, promoting metabolism, reducing hypertension, regulating blood glucose and inhibiting fungal and viral infection (Ji 2012).Astragalus is becoming less abundant in the wild as a result of its large-scale harvest for medical uses.
Endophytic fungi are microorganisms that live in the healthy tissues of plants with no obvious effect on the host.These fungi and their host plants have co-evolved, leading the microorganisms to acquire the ability to produce bioactive substances similar to those produced by their hosts (Tan and Zou 2001; Strobel et al.2004; Harper et al.2007).Some of these secreted substances can be used as medicinal components for the treatment of human disease (Aly et al.2011; de Barros et al.2011).In recent years, 171 genera of endophytic fungi have been identified from 47 families, 81 genera and 114 species of plants (Strobel et al.2004).Strains of endophytic fungi have been successfully isolated from A.membranaceus (Sun and Wang 2006; Zhou et al.2012a; Zhou et al.2012b) and A.mongholicus (Ma et al.2012) that provide an alternative way to produce the same active compounds as their host plants.If large-scale fermentation of endophytic fungi isolated from A.membranaceus or A.mongholicus can produce identical secondary metabolites, this will be an easier and more economically feasible source than wild plants, and will enable enhanced conservation of host plant species in the wild.
In this work, four strains of endophytic fungi were successfully isolated and purified from A.mongholicus using the surface disinfection method.The molecular analysis using the ITS-rDNA sequences was applied to construct the phylogenetic tree of these fungi.The main liquid fermentation factors including temperature, volume of potato dextrose agar (PDA) liquid medium, and rotation speed were optimized by Box-Behnken design (BBD) for obtaining the maximum cell dry weight (CDW) yield of these endophytic fungi.Subsequently, the main active ingredients of A.mongholicus including astragalosides I–IV, flavonoids and polysaccharides were identified in the culture liquid and mycelium of these endophytic fungi.
Materials and methods
Isolation and culture of endophytic fungi
Healthy tissues of A.mongholicus were collected from the experimental field of Heilongjiang University of Chinese Medicine, Harbin, China.Fresh clean A.mongholicus taproots were cut into segments of about 3 cm.Segments were rinsed with 75% alcohol for 3 min, washed twice for 2 min in sterile water, immersed in 10% NaClO for 7 min, and again washed twice for 2 min in sterile water.All segments other than the tips of the original taproot were then cut into pieces under aseptic conditions.Four or five pieces of taproot were placed on PDA medium.A.mongholicus taproots were incubated in a homoeothermic incubator at 30°C for 3–7 days.The hyphal tip was removed and placed on new PDA, incubated at 30°C and repeated until a pure culture was obtained.Unbroken taproots, 0.1 mL sterile water from the final wash, and imprints from the cut pieces were cultured as controls under the same conditions used for the experimental samples.
Strain identification
The selected four strains of endophytic fungi were evaluated by the phylogenetic analysis of their ITS-rDNA sequences.Total DNA was extracted and purified using a fungil DNA Mini Kit (Meilian Biotech, Shanghai, China) according to the manufacturer’s instructions.The fungal ITS-rDNA fragments was amplified by polymerase chain reaction (PCR) using a pair of universal primers including 5′-TCCACCAGCTKYGAGAACTC-3′and 5′-ACCTCCTTCATGGAGACCTT-3′.PCR products were purified using a UNIQ-10 Spin Column PCR Products Purification Kit (Gentaur, Kampenhout, Belgium).Sequencing of the PCR products was performed by the service of Sangon Engineering Technology and Service Co.Ltd.(Shanghai, China).The ITS sequence of each isolate was compared with the data available in GenBank using BLAST (http://www.ncbi.nlm.nih.gov/BLAST) to estimate the phylogenetic relationships of the endophytic fungi.The neighbor joining (NJ) phylogenetic tree was constructed from evolutionary distance data by MEGA 4.0 software.The bootstrap was 1000 replications to assess the reliable level to the nodes of the tree.The NJ tree was estimated using pairwise genetic distances based on all substitutions with the Jukes−Cantor distance parameter.
Optimization of fermentation conditions
We selected four strains of A.mongholicus endophytic fungi for the liquid fermentation culture based on the characterization of good growth performance.These four endophytic fungi were grown on PDA at 30 °C for 5 days and then inoculated into 250-ml Erlen-meyer flasks containing PDA liquid medium.The fermentation lasted for 8 days under a range of conditions according to the experimental design.To achieve the optimum fermentation of four strains of fungi, BBD was applied to survey the effects of three key independent variables at three levels (temperature 25–31 °C, volume of PDA liquid medium 60–100 mL, and rotation speed 120–180 rpm) on the dependent variable (CDW yield of each endophytic fungi).A total of 17 randomized experiments including 14 factorial and 3 zero-point tests were designed.Regression analysis was carried out to evaluate the response function as a quadratic polynomial:

where, Y is the predicted response; β0, βj, βjjand βijare the regression coefficients for intercept, linearity, square and interaction, respectively; Xiand Xjare the independent coded variables; and k represents the number of variables.The actual and coded levels of the independent variables used in the experimental design are summarized in Table 1.The experiment data were analyzed statistically with Design-Expert 7.0 (State-Ease, Inc., Minneapolis MN, USA).Analysis of variance (ANOVA) was performed to calculate and simulate the optimal values of the tested parameters.
Determination of active metabolites produced by endophytic fungi
The fermentation process was performed under the conditions of 80 mL PDA liquid medium, 150 rpm, 28°C and 8 days, and the cultures were then separated into culture liquid and mycelium by filtration.The culture liquid was extracted three times with an equal volume of n-butanol.The oven-dried mycelia were resuspended in deionized water, then subjected to ultrasonic processing for 30 min and extracted three times with an equal volume of n-butanol.The dry extracts were obtained by evaporation of the organic solvent and dissolved in methanol for further analysis.A.mongholicus astragalosides I–IV were determined using the LC–MS/MS method reported by Zu et al.(2009).A.mongholicus flavonoids were determined using the Salkowski reaction method.A.mongholicus polysaccharides were determined using the phenol-sulfate acid method.
Statistical analysis
Results were expressed as means ± standard deviations.The data were statistically analyzed using SPSS statistical software, version 17.0 (SPSS Inc, Chicago, Illinois, USA).Differences between means were determined by analysis of variance (ANOVA) with Duncan’s test on the level of significance declared at p < 0.05.
Results
There was no microorganism growth in the control samples, demonstrating that the fungi in the experimental samples had been isolated from A.mongholicus tissue.In this work, a total of twenty-eight strains of endophytic fungi were isolated, but only four strains (strains 16, 17, 23, and 75) showed stable growth, exuberant vitality and good repeatability.Each strain exhibited variation in colony morphology, color and microstructure (Fig.1).Subsequently, the isolated endophytic fungi were identified by sequencing the internal transcribed spacers (ITS) of the rDNA region.The length of the amplified rDNA fragment ranged from 500 to 600 bp.After the BLAST searches, ITS-rDNA sequences of strains 16 and 75 were most closely related to Fusarium oxysporum, and ITS-rDNA sequences of strains 17 and 23 were most closely related to Bionectria ochroleuca (Fig.2).
To obtain the optimum fermentation conditions for the four isolated strains of endophytic fungi, the temperature, volume of PDA liquid medium, and rotation speed were optimized by BBD.The experimental design matrix and all the relevant data are illustrated in Table 1.The ANOVA results of the built quadratic model are presented in Table 2.Significance levels of all models (P < 0.05 in all cases) and desirable determination coefficients (R2≥ 0.9190 for all models) suggested that all the built mathematical models were precise and applicable.The second-order polynomial models were described by the following equations (ignoring insignificant items):

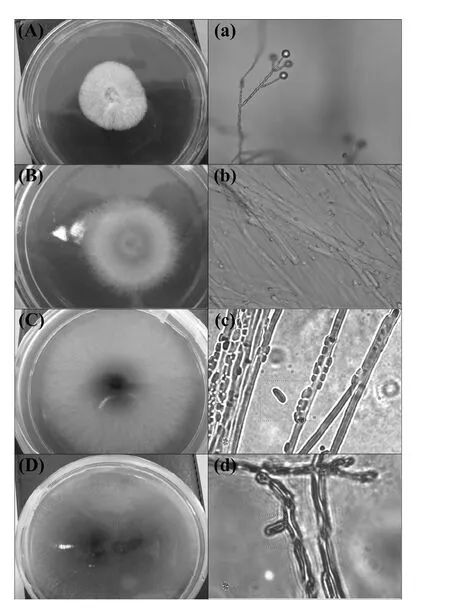
Fig.1: Colony morphology and microscopic characteristics of 16 (A, a), 17 (B, b), 23 (C, c), 75 (D, d) strains of endophytic fungi isolated from A.mongholicus.

Fig.2: Phylogenetic tree constructed by the program neighbour joining (NJ) based on ITS1-5.8S-ITS2 sequences of endophytic fungi isolated from A.mongholicus.Bootstrap values (1000 tree interactions) are indicated at the nodes.

Table 1: Results of CCD for the production of CDW during the liquid fermentation process.

Table 2: ANOVA results of the quadratic model for the production of CDW.
Where Y16, Y17, Y23 and Y75 are the CDW yields of strains 16, 17, 23, and 75 of endophytic fungi, respectively; X1 is the temperature (°C); X2 is the volume of PDA liquid medium (mL); and X3 is the rotation speed (rpm).Based on the above mathematical models and considering the experimental methods, the optimal liquid fermentation parameters were as follows: temperature of 28°C, PDA liquid medium of 80 mL and rotation speed of 150 rpm.Under the optimized fermentation conditions, maximum CDW yields for strains 16, 17, 23, and 75 were 0.2699 kg, 0.3945 kg, 0.2587 kg, and 0.3634 kg, respectively.
As the LC-MS/MS results show in Fig.3, astragalosides I–IV were not identified in the culture liquids or mycelium extracts of the four isolated endophytic fungi.For the detection of flavonoids, hydrochloric acid and magnesium powder reaction indicated that pink was found in the A.mongholicus sample and rutin standard solutions, while no color change was observed in the culture liquids or mycelium extracts of the four isolated endophytic fungi (data not shown).The results of phenol-sulfuric acid tests showed that red precipitate was found in the A.mongholicus sample solution but not in the culture liquids or mycelium extracts of the four isolated endophytic fungi (data not shown).Our results show that neither astragalosides I–IV, flavonoids nor polysaccharides were identified in the culture liquids or mycelium extracts of the four isolated endophytic fungi.
Discussion
Three imortant and innovative outcomes resulted from this study: (1) four strains of endophytic fungi were screened and isolated from A.mongholicus, each showing stable growth, exuberant vitality and good repeatability in the culture medium; (2) molecular phylogenetic analysis of strains 16 and 75 were most closely related to F.oxysporum, and strains 17 and 23 were most closely related to B.ochroleuca; (3) the maximum yields of CDW for strains 16, 17, 23, and 75 were 0.2699 kg, 0.3945 kg, 0.2587 kg and 0.3634 kg, respectively, under the optimal liquid fermentation conditions of temperature (28°C), PDA liquid medium (80 mL) and rotation speed (150 rpm).
The isolated strains 16 and 75 were genetic identical to F.oxysporum, and strains 17 and 23 were genetic identical to B.ochroleuca.No previous reports documented isolation of these two species of endophytic fungi strains from A.mongholicus (growing in northeast China).It is reported that F.oxysporum is a common species of endophytic fungi existing in various plants, such as Cinnamomum kanehirae (Wang et al.2011), Ginkgo biloba (Cui et al.2013), Catharanthus roseus (Kumar et al.2013) and Lilium lancifolium (Liu et al.2012).Recently, B.ochroleuca has been successfully isolated from Nothapodytes foetida for producing antimicrobial and free radical scavenging metabolites (Samaga et al.2013)
In the previous reports, many strains of endophytic fungi isolated from ginseng root and flower produced active compounds, such as ginsenoside Rb1, ginsenoside Rd, ginsenoside Re, xanthatin, isotanshinone II, ginseng falcarinol, 2,4,5- trimethyl-1,3-dihydroxyhenzene, 2,4-dihydroxyl-3,5,6- methyl 3-(trifluoromethyl) benzoate, mannitol, green mycophenolic acid, ergosterol, peroxy ergosterol, hydroxyethyl phenol and brefeldin A (Chen 2007; Sun et al.2008; Park et al.2012).Additionally, a number of endophytic fungi isolated from yew stems and roots produces paclitaxel (Liu et al.2009).In the co-culture of ginsenoside Rbl with three strains of panax endophytic fungi for seven days, the endophytic fungi transformed ginsenoside Rbl into ginsenoside Rd (Chen 2007).In the co-evolution process with plants, endophytic fungi not only produce special chemicals, but also induce the formation and growth of some metabolites in host plants, particularly in medicinal plants.After the endophytic fungi isolated from Dracaena cochinchinensis stem were inoculated into the living D.cochinchinensis, the amount of Sanguis draconis increased by 66−120% (Ou et al.2013).
The above reports show that many endophytic fungi can synthesize the same natural products that occur in host plants.Kusari et al.(2008) hypothesized that the production of these compounds in host plant does not result exclusively from metabolic processes of endophytic fungi but is rather the consequence of concomitant plant and fungal biosynthesis.The gene for biosynthesis of some active compounds might be transferred between host plant cells and endophytic fungi by horizontal gene transfer.Strobel (2002) proposed that endophytic fungi might have developed genetic systems promoting the transfer of information between themselves and the host plant during the long co-evolution process.
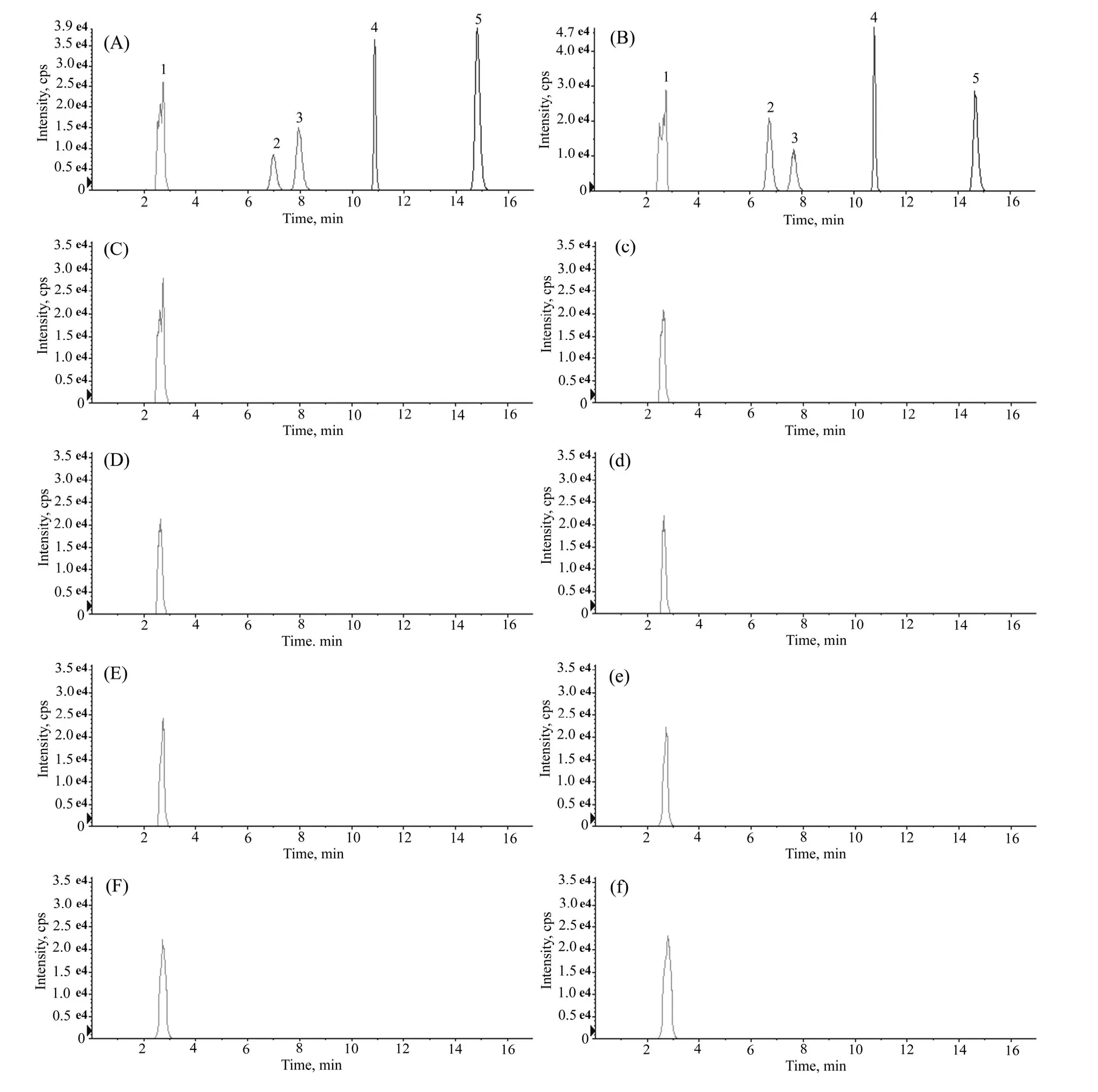
Fig.3: LC-MS/MS chromatograms of standard mixture (A), A.mongholicus sample (B), culture liquid of 16 (C), 17 (D), 23 (E) and 75 (F) strains, and mycelium extracts of 16 (c), 17 (d), 23 (e) and 75 (f) strains.1, Internal standard; 2, astragalosides IV; 3, astragalosides III; 4, astragalosides II; 5, astragalosides I.
To our best knowledge, there are few reports of endophytic fungi from A.mongholicus (growing in northeast China) producing active substances.It is known that the main active ingredients of A.mongholicus are astragalosides I–IV, flavonoids and polysaccharides.In this study, astragalosides I–IV, flavonoids and polysaccharides were not isolated in culture liquids or in mycelium extracts of the four selected endophytic fungi.This work was the first investigation of production of active substances from endophytic fungi of A.mongholicus in northeast China.Other bioactive components from the four selected endophytic fungi will be further clarified in further studies.In addition, fermentation of a mixture of species of endophytic fungi will also be conducted in future based on the presence of multiple species of fungi in the tissues of wild A.mongholicus.
Conclusions
In this study, we successfully isolated and purified four strains of endophytic fungi (strains 16, 17, 23, and 75) from A.mongholicus, and subjected them to phylogenetic analysis.The liquid fermentation parameters of the four isolated fungi were optimized by BBD.Under the optimal conditions of temperature (28°C), PDA liquid medium (80 mL), and rotation speed (150 rpm), the maximum CDW yields for strains 16, 17, 23, and 75 were 0.2699, 0.3945, 0.2587, and 0.3634 kg, respectively.Astragalosides I–IV, flavonoids and polysaccharides were not identified in culture liquids or in mycelium extracts of the four selected endophytic fungi.Moreover, isolation of additional species of endophytic fungi from A.mongholicus or other host plant species in northeast China and determination of their capacity to produce biologically active substances are subjects in need of further research.
Aly AH, Debbab A, Proksch P.2011.Fungal endophytes: unique plant inhabitants with great promises.Applied Microbiology and Biotechnology, 90: 1–17.
Chantasinghab D, Kitikhunb S, Eurwilaichitrb L, Uengwetwanitb T, Pootanakita K.2011.Functional expression in Beauveria bassiana of a chitinase gene from Ophiocordyceps unilateralis, an ant-pathogenic fungus.Biocontrol Science and Technology, 21: 677−686.
Chen XY.2007.Primary studies on correlation of endophyte isolated from panax quinquefolium and gingsenoside.Dissertation, Heilongjiang University of Chinese Medicine.
Cho WCS, Leung KN.2007.In vitro and in vivo immunomodulating and immunorestorative effects of Astragalus membranaceus.Journal of Ethnopharmacology, 113: 132–141.
Chu DT, Lepe-Zuniga J, Wong WL, LaPushin R, Mavligit GM.1988.Fractionated extract of Astragalus membranaceus, a Chinese medicinal herb, potentiates LAK cell cytotoxicity generated by a low dose of recombinant interleukin-2.Journal of Clinical & Laboratory immunology, 26: 183–187.
Cui YN, Yi DW, Bai XF, Sun BS, Zhao YQ, Zhang YX.2012.Ginkgolide B produced endophytic fungus (Fusarium oxysporum) isolated from Ginkgo biloba.Fitoterapia, 83: 913–920.
de Barros BS, da Silva JP, de Souza Ferro JN, Agra IK, de Almeida Brito F, Albuquerque ED, Caetano LC, Barreto E.2011.Methanol extract from mycelium of endophytic fungus Rhizoctonia sp.induces antinociceptive and anti-inflammatory activities in mice.Journal of Natural Medicines, 65: 526–531.
Harper JK, Strohmeier M, Grant DM.2007.Pursuing structure in microcrystalline solids with independent molecules in the unit cell using 1H-13C correlation data.Journal of Magnetic Resonance, 189: 20–31.
Ji RJ.2012.Studies on Astragalus biological characteristics and functions and progress of modern pharmaceutics.Chinese Journal of Clinical Rational Drug Use, 5: 176–177.
Kumar A, Patil D, Rajamohanan PR, Ahmad A.2013.Isolation, purification and characterization of vinblastine and vincristine from endophytic fungus Fusarium oxysporum isolated from Catharanthus roseus.Plos One, 8: e71805.
Kusari S, Lamshöft M, Zühlke S, Spiteller M.2008.An endophytic fungus from Hypericum perforatum that produces hypericin.Journal of Natural Products, 71: 159–162.
Liu K, Ding X, Deng B, Chen W.2009.Isolation and characterization of endophytic taxol-producing fungi from Taxus chinensis.Journal of Industrial Microbiology & Biotechnology, 36: 1171–1177.
Liu XL, Huang KH, Zhou JZ, Meng L, Wang Y, Zhang LX.2012.Identifcation and antibacterial characteristics of an endophytic fungus Fusarium oxysporum from Lilium lancifolium.Letters in Applied Microbiology, 55: 399–406.
Luangsa-ard JJ, Hywel-Jones NL, Samson RA.2004.The polyphyletic nature of Paecilomyces sensu lato based on 18S-generated rDNA phylogeny.Mycologia, 96: 773–780.
Ma W, Sun LY, Zhang XW, Jia YS, Kong XJ, Xie JQ.2012.Preliminary studies on active constituent in endogenous fungal fermented liquid of Radix Astragali.Acta Chinese Medicine and Pharmacology, 3: 041.
Ou LC, Wang XH, Zhang CH.2013.Production and characterization of dragon's blood from leaf blades of Dracaena cambodiana elicited by Fusarium proliferatum.Industrial Crops and Products, 45: 230–235.
Park YH, Lee SG, Ahn DJ, Kwon, TR, Park SU, Lim HS, Bae H.2012.Diversity of fungal endophytes in various tissues of Panax ginseng Meyer cultivated in Korea.Journal of ginseng research, 36: 211.
Samaga PV, Rai VR, Rai KML.2013.Bionectria ochroleuca NOTL33-an endophytic fungus from Nothapodytes foetida producing antimicrobial and free radical scavenging metabolites.Ann Microbiol, 64: 275–285.
Strobel G, Daisy B, Castillo U, Harper J.2004.Natural Products from Endophytic Microorganisms.Journal of Natural Products, 67: 257–268.
Strobel GA.2002.Rainforest endophytes and bioactive products.Critical Reviews in Biotechnology, 22: 315–333.
Sun Y, M XB, Liu JX.2012.Compounds from fraction with cardiovascular activity of Chrysanthemum indicum.Chinese Journal of Chinical Mater Medicine, 37: 61–65.
Sun Y, Wang Q.2006.Identification of secondary metabolites produced by endophytic fungus from Astragalus membranaceus and characterization of their antibacterial activity.Journal of Fungal Research, 4: 47–51.
Tan RX, Zou WX.2001.Endophytes: a rich source of functional metabolites.Natural Product Reports, 18: 448–459.
Wang QX, Li SF, Zhao F , Dai HQ, Bao L, Ding R, Gao H, Zhang LX, Wen HA, Liu HW.2011.Chemical constituents from endophytic fungus Fusarium oxysporum.Fitoterapia, 82: 777–781.
Zhou F, Zhang HC, Liu R, An ZP.2012b.Isolation, identification and antimicrobial activities of endophytic fungi from Astragalus membranaceus.Food Science and Technology, 1: 011.
Zhou F, Zhang HC, Liu R, Zhang YF.2012a.Isolation and biological evaluation of secondary metabolites of endophytic fungus Aspergillus sp.from Astragalus membranaceus.Chinese Journal of Experimental Traditional Medical Formulae, 4: 038.
Zu YG, Yan MM, Fu YJ, Liu W, Zhang L, Gu CB, Efferth T.2009.Determination and quantification of astragalosides in Radix Astragali and its medicinal products using LC–MS.Journal of Separation Science, 32: 517–52.
杂志排行
Journal of Forestry Research的其它文章
- Ethno-medicinal plants used by Bengali communities in Tripura, northeast India
- Litter production, decomposition and nutrient mineralization dynamics of Ochlandra setigera: A rare bamboo species of Nilgiri Biosphere Reserve, India
- Temporal changes in nitrogen acquisition of Japanese black pine (Pinus thunbergii) associated with black locust (Robinia pseudoacacia)
- Plant diversity at Chilapatta Reserve Forest of Terai Duars in subhumid tropical foothills of Indian Eastern Himalayas
- Floristic composition and management of cropland agroforest in southwestern Bangladesh
- The changing landscape of mangroves in Bangladesh compared to four other countries in tropical regions
