Soil carbon budget in different-aged Chinese fir plantations in south China
2014-04-19ShebaoYuDanWangWeiDaiPingLi
Shebao Yu • Dan Wang • Wei Dai • Ping Li
Introduction
Soil carbon cycling is an important component of the carbon balance in ecosystems (Nakane 1995).Small changes in the processes governing soil carbon cycling have potential to release large amounts of CO2(Janssens et al.2001).Soil carbon is strongly influenced by biotic and abiotic factors in ecosystems, such as vegetation, microbial communities and soil condition (Ekschmitt et al.2008; Wang et al.2010; Hoffmann et al.2012).In recent years, major efforts have been made to understand the interactions between environmental variables and soil carbon.Vegetation coverage, microtopography, soil formation, solar radiation and water availability are the dominant controls of soil organic carbon stocks (Hoffmann et al.2012).Lemma et al.(2007) suggested that differences in soil organic carbon storage in different forest stands result more from litter input and the proportion of fine woody litter rather than from litter quality and microclimate.However, study of such interactions still remains a difficult task that is complicated by uncertainties in estimating soil organic carbon in forests.This is especially true where managed forests often consist of a series of plots of different ages (i.e.chronosequences), ranging from young to more mature stands.As forests age, a number of structural and physiological changes occur that are likely to affect key processes that control soil carbon cycling.Reliable estimates of soil carbon cycling are required to estimate the carbon balance and its components in young to mature forest stands (Law et al.2001, 2003; Irvine and Law 2002; Tang et al.2009).
Chinese fir (Cunninghamia lanceolata (Lamb.) Hook), a fast-growing, evergreen coniferous tree with high yield and excellent wood quality (Wu 1984), has been planted widely in China.It accounts for 30% of all plantations in the country in stands of various ages planted on former harvest sites (Wang et al.2009; Anon 2010).Because of their wide distribution in China, Chinese fir plantations could play a significant role in the global carbon balance.Since the early 1980s many studies have quantified biomass production in Chinese fir plantations, and assessed variations in litter, soil respiration, soil organic carbon and soil nutrient cycling in different-aged stands (e.g.Ma et al.2007; Zhao et al.2009; Wang et al.2011a, b).However, it is still unclear how carbon cycling (carbon input and output) varies with stand age.We studied a Chinese fir plantation chronosequence (ages 7, 16, 23, and 29 years) to quantify and compare the variation in soil organic carbon density, soil carbon input (litter decomposition rate, litter production), soil carbon output (soil respiration) and soil microorganisms within a single study period.Our objective was to quantify flows and reservoirs of soil carbon in different-aged plantations to clarify the characteristics of soil carbon budgets in a Chinese fir plantation chronosequence.
Materials and methods
Study site and experimental design
Our study area was at the Dagangshan Forest Ecological Research Station, Dagangshan (27°30′–27°50′ N, 114°30′–114°45′E), Jiangxi Province, south China, which has a typical humid subtropical climate with distinct rainy and dry seasons.Mean annual precipitation is 1590 mm, of which approximately 45% falls between April and June, and 13% between October and December.The highest recorded maximum daily precipitation is 195.7 mm and mean annual potential evaporation is 1503 mm.Mean annual temperature is 16.8°C and monthly mean temperature ranges from 5.2°C in January to 28.8°C in July.The frost-free period is 265 days.The soils are dominated by red or yellow soils and their derivates (American Soil Taxonomy).Chinese fir is widespread at elevations of 300–700 m.Chinese fir was planted in clear-cut sites in natural broad-leaved forests.The plantations were thinned when trees reached middle-age and maturity.Plantations were clear-felled at 25–30 years and then replanted.Vegetation under the trees included Woodwardia japonica, Miseanthus floridulus, Dicranopteris dichotoma, Loropetalum chinense, Rubus trianthus, Erigeron acris, Clerodendrum cyrtophyllum, Parathelyteris glanduligera, Adinandra millettii, Maesa japonica, Smilax glabra, and Schima superba, among others.
In 2009, we studied four Chinese fir plantations aged 7, 16, 23, and 29 years that represented young, middle-aged, near mature, and mature ages, respectively.Prior to planting of Chinese fir all sites had been used for one rotation of primary broadleaved forest.Near mature plantations had been thinned under the standard practice for Chinese fir plantations (removal of alternate rows and cutting of the crowns of thinned trees on site).The characteristics of the four stand age classes are shown in Table 1.Three 20 m×20 m measurement plots for each age class were established in March 2009.Three 1 m×1 m subplots were randomly located in each plot.
Soil carbon input
Litter was collected monthly from March 2009 to March 2010 in 5 litter traps of 1 m × 1 m surface area along the diagonal of each plot (Li et al.2005).Because the plantations were on a slope, the litter traps were supported by four posts to create a level surface area of 1 m2.Litter traps captured all litter from Chinese fir and understorey vegetation, not including some low understorey plants.The litter was oven-dried at 80°C to a constant weight for subsequent measurement of carbon content.
To investigate litter decomposition, 200 g air-dried litter samples (comprising foliage, twigs and cones, mixed according to their fractions in litter) were placed in 0.2 mm mesh nylon litterbags of 15 cm × 20 cm.Litterbags of each stand were randomly placed on the forest floor in March 2009 on sites where existing litter on the forest floor had been removed.To ensure that the litterbags were in good contact with the soil, the bags were attached to the forest floor by wooden pegs.In each plot 24 litterbags were placed on the ground and two litterbags were removed monthly for one year for mass and carbon analyses.Litter decomposition rates were calculated as k-values from equation (Olson 1963).
Soil carbon output
Respiration was measured from March 2009 to March 2010 using a Li-8100 soil CO2flux system (LI-COR Inc., Lincoln, NE, USA).Three PVC collars (10 cm inside diameter and 6 cm high) were installed in each subplot for soil respiration measurements.One day before the first measurements, the collars were inserted 5 cm into the soil.Within each collar, all aboveground parts of living vegetation were removed with minimal soil disturbance.All measurements were done twice per month, every 2 hours during the day and every 3 hours during the night (Wang et al.2011a).To minimize measurement errors and equipment damage, all measurements were taken on sunny days without precipitation and/or high winds.Soil temperature and moisture were measured simultaneously with respiration at a depth of 5 cm in the vicinity of the collars.
Soil properties
In each of 3 plots within the 4 stands (12 plots in total), three soil samples were taken within 1–2 m of the centre of the respiration collars at a depth of 0–20 cm at 3 month intervals from March 2009 to March 2010.After removing visible roots and organic residues, each soil sample was divided into two sub-samples.One sample was immediately sieved through a 2-mm mesh and stored at 4°C until the analysis for microbe numbers (described below).The other sub-sample was passed through a 2-mm sieve and subsequently air-dried for soil organic carbon content.Soil bulk density was determined by collecting three samples with a bulk density corer (diameter 5 cm) at the same depth range at the 0–20 cm depth of each plot and drying to constant weight.Soil organic carbon content was digested in K2Cr2O7–H2SO4solution using an oil-bath heating and then carbon concentration was determined from titration (Ministry of Forestry 2000).Bacterial and fungal populations were enumerated by a conventional dilution-spread plate method (Wollum 1982).Bacteria were enumerated on 1% PTYG (peptone-tryptone-yeast extract-glucoseagar) and fungi were enumerated on RBA (Rose-bengal agar) medium.Bacterial and fungal colonies were counted after 7 d at 21–23°C.β-Glucosidase activity was measured following the method of Tabatabai (1982).The 0.1 M maleate buffer (pH 6.5) of 4 mL and 50 mmol·L-1M ρ-nitrophenyl-β-D-glucopyranoside (1 mL) were added to soil (1 g) and the reaction mixture was incubated at 37°C for 1 h.The ρ-nitrophenol in the fltrate was determined colorimetrically at 410 nm.Cellulase activity was determined with 0.7% carboxymethyl-cellulose as substrate, and the incubation time was 24 h following the method of Schinner and von Mersi (1990).
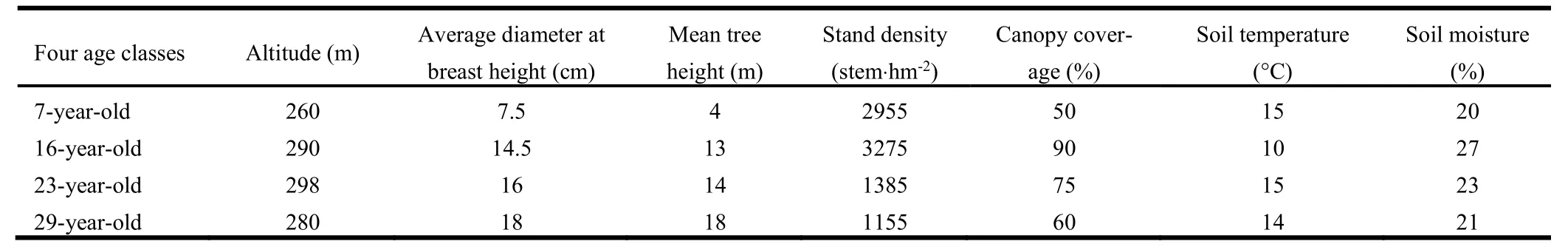
Table 1: General characteristics of different-aged plantations of Chinese fir
Data analysis
Estimation of soil organic carbon density (SOCD)
SOCD (kg·m-2) was calculated by the following formula:
where, Siis the SOCD (kg·m-2) at the 0–20 cm soil depth of the ith Chinese fir stand; Ci, Di, 20 and Piare the soil organic carbon content (g·kg-1), the average soil bulk density (g·cm-3), the thickness (cm) and the volume percentage (%) of the fraction >2 mm of soil horizon in the ith Chinese fir stand.
Statistical analyses were conducted using SPSS Version 11.5 for Windows (SPSS Inc., Chicago, IL, USA).All comparisons were performed using analysis of variance (ANOVA) followed by least significant difference tests between means of different-aged plantations.Significance levels were set at p <0.05 in all statistical analyses.Data were calculated as arithmetic means with standard error.
Results
Litter production and decomposition rate
The litter dry matter and decomposition rate during the development of the trees over 29 years are shown in Fig.1.Litter fell throughout the year, but there were seasonal fluctuations with a peak in autumn.As stand age increased, annual litter dry matter deposition increased (but not significantly), about five-fold from the young to the mature plantations.However, the litter decomposition rates decreased between the 7-year-old and 16-year-old plantations, and then increased significantly from the 16-yearold plantations to the 23-year-old plantation, to a peak in the 29-year-old plantation.
Soil respiration
Respiration ranged from 0.52 to 1.18 t·ha-1·a-1(Fig.1), with the highest values recorded in the 7-year-old plantation.After a marked decrease in the 16-year-old plantation, Respiration peaked again and returned to a rate similar to the 7-year-old for both the 23 and 29-year-old stands.
Soil organic carbon density
SOCD decreased by 16.39% from the 7-year-old to 16-year-old plantations and then increased between the 16-year-old and 29-year-old plantations to exceed the value of 7-year-old plantation (Fig.1), suggesting change of soil carbon cycling with increasing plantation age.
Soil microbe properties
Soil microbe properties of the four Chinese fir plantations are listed in Table 2.Counts of bacteria and fungi decreased signifcantly between the 7-year-old and 16-year- old plantations and then increased with increasing age, particularly in the 29-year-old plantation.The enzyme activities of β-glucosidase and cellulase decreased (but not significantly) from the 7-year-old to 16-year-old plantations and then generally increased significantly in the 29-year-old plantation.

Table 2: Microbial properties (per 1-g soil) in surface soils (0–20 cm) in plantations of Chinese fir of four ages

Fig.1: Carbon budget in different-aged plantations of Chinese fir a.a A, B, C, D in figure 1 are litter production, litter decomposition rate, soil respiration, soil organic carbon density, respectively.
Correlation of soil organic carbon density to soil properties, soil carbon input and output
There was a highly significant correlation between soil organic carbon density and microbe properties, litter and respiration (Table 3).Microbe properties were significantly correlated with litter decomposition rate and respiration in all four age stands.The strongest correlations were between litter decomposition rate and soil organic carbon density in plantations (rx3= 0.741) and the effect was nearly direct.Effects of microbe numbers and enzyme activity on soil organic carbon density were due mostly to the indirect effect of litter and respiration.
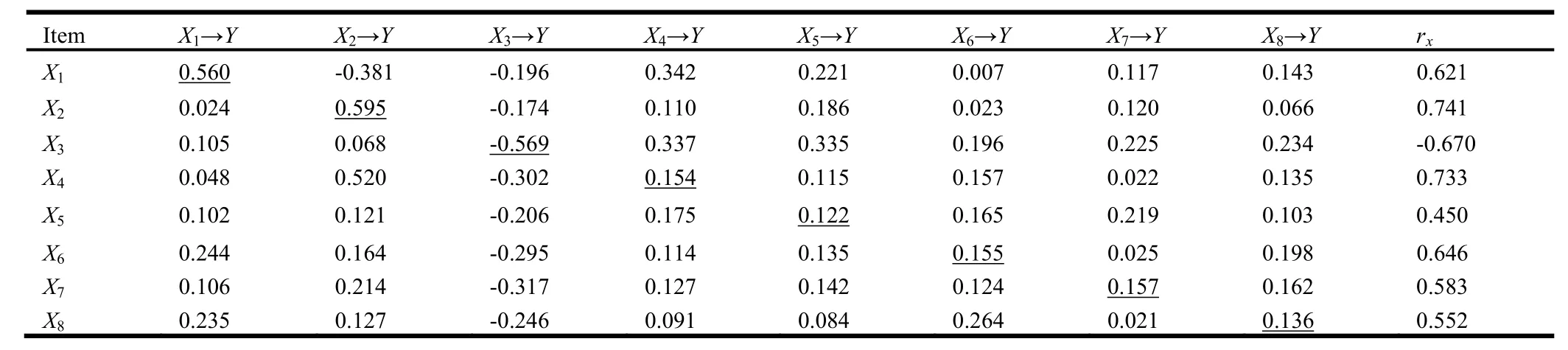
Table 3: Path analysis of the direct and indirect effects of soil microbial properties and carbon budget on organic carbon density in different-aged plantations of Chinese fir a
Discussion
Plant litter input is the major pathway for the return of organic matter to soil (Wang et al.2008).Litter production and decomposition rates are used to estimate carbon input from vegetation to soil (Zhang and Wang 2012).In this study, litter production increased significantly with increasing plantation age (Fig.1), which could be related to the increase in above-ground biomass, in particular the leaf biomass (Nakane 1995; Wang et al.2010).Ma et al.(2007) also found that above-ground biomass of Chinese fir plantations in Fujian Province increased very significantly (p<0.01), from 41.8 t·ha-1·a-1in 8-year-old to 118.0 t·ha-1·a-1in 24-year-old plantations.Chen (1998) reported that total Chinese fir tree biomass increased significantly with increasing plantation age.Thus, litter plays a key role in material cycling in forests (Sheng and Yang 1997).
Litter decomposition rates decreased between the 7-year-old and 16-year-old plantations, and then increased significantly in the 23-year-old plantation.The trend of increasing litter decomposition rate with increasing plantation age is in accordance with increases in abundance of soil microorganisms.Many studies have suggested that the variation of litter decomposition can be well explained by soil microbe properties as described for the rhizosphere and detritusphere soil (Tarafdar and Jungk 1987; Kandeler et al.1999).
Litter production and decomposition rates in Chinese fir plantations were low compared with plantations of other tree species and this resulted in lower soil organic carbon content.These differences could be attributed to chemical and physical differences in the litter of coniferous versus broadleaf plantations, the former containing more components that are difficult to decompose, which results in litter accumulation on the forest floor and less carbon incorporation into the mineral soil (Berg 2000; Wang et al.2009).An additional factor is the more difficult or slower degradation of cellulose resulting from low soil activity in soils of Chinese fir plantations (Yan et al.2004).
Respiration, the major pathway for carbon output from forest ecosystems, accounts for 60−80% of total ecosystem C output.Respiration increased between the 7- and 16-year-old plantations in the current study, a trend reported by others.For example, Tedeschi et al.(2006) showed that respiration in 16-year-old Mediterranean oak stands was lower than in 10-year-old and 1-year-old stands.Saiz et al.(2006) found that respiration of the youngest stands was significant higher than in older stands.Soil respiration rate was closely correlated with the biomass of soil microbes and their activity (Fisk and Fahey 2001; Zhu et al.2009).Similar patterns of microbe numbers in Chinese fir plantations were reported in other studies (Wang et al.2011b).In this study, soil microbe numbers in the 7-year-old plantation were higher than in the 16-year-old plantation, which may partly explain the low respiration in 16-year-old plantation.The changes of microbe numbers could be related to changes in soil temperature and canopy coverage with increasing stand age (Brown 2006; Yan et al.2011).Canopy coverage was greatest in the 16-year-old plantation and soil temperature was lowest.This suggests that conditions for microbes were less favorable in this stand age, resulting in an ecosystem with slower soil carbon cycling.The 7-year-old plantations had the least canopy coverage and more soil microorganisms, which might have facilitated soil carbon cycling.
The variability of soil carbon cycling in forest ecosystems is complex because it is composed of several different components, including environment and carbon input and output (Potter et al.1997; Turner and Lambert 2000).It was evident that 7-year-old plantations had the lowest litter production and decomposition rates and the highest soil respiration rate compared to other plantations (Fig.1).Higher SOCD values in the 7-year-old stand compared with the 16-year-old stand might be attributed to the woody debris left on the soil surface after harvest.In the traditional silvicultural system, Chinese fir was planted on clear-cut sites in natural broad-leaved forests.Wells (2002) recorded younger roots were much more likely to die than older roots.Thus, the decomposition of dead roots soon after harvest would also likely lead to high levels of soil organic matter and respiration.
Litter decomposition was limited by microbe numbers in the 16-year-old plantation where there was high canopy coverage.Although carbon losses by respiration decreased significantly from the 7-year-old to the 16-year-old plantation, it always outstripped carbon return through litter decomposition.Our results suggest that SOCD declined because carbon inputs did not compensate for carbon outputs with increasing plantation age.This showed the greater investment in aboveground carbon by the young plantation to establish and maintain the biomass system supporting the demands for water and nutrients during this phase of rapid growth.Thus, to promote the cycling of carbon in plantations it is important that trees be adequately thinned (Nakane 1995).
The contribution of litter decomposition to carbon return increased significantly, however, from the 16-year-old to the 23-year-old and 29-year-old plantations (Fig.1), thereby providing a source for replenishing soil carbon removed by respiration.The significantly increasing trend of soil organic carbon occurred up to the age of 23 years in plantations that had been thinned.However, the current harvest practice at the age of 20 years is inappropriate for Chinese fir plantations.We found the increase of SOCD was not significant between 23-year-old and 29-year-old plantations.Thus extending the rotation length should help to maintain soil fertility and even enhance productivity.This conclusion is supported by Ma et al.(2007), who reported that prolonging rotation length should be considered for maintaining soil nutrient status in the management of Chinese fir plantations to avert possible reductions in productivity in successive rotations.
Conclusions
Our study showed that soil carbon cycling varied with plantation age in Chinese fir plantations.Litter and respiration directly affected soil carbon content.Soil microbe numbers and enzyme activities had indirect effects on rates of litter decomposition and respiration, and on soil carbon content.When trees were growing under the same climatic conditions, the changes in soil carbon cycling under each plantation were mainly controlled by the levels of carbon input and output, and by environmental conditions.This suggests that plant-microbe-soil interactions in soil are an integral component of the influence of forest age on soil carbon cycling.As stand age increases, carbon cycling in Chinese fir plantations is increasingly dominated by biological processes and becomes less dependent on carbon sources in litter.
Acknowledgement
We gratefully acknowledge the support from Dagangshan Forest Ecological Research Station for field monitoring and sampling.This study was funded by the Special Fund for Forestry Scientific Research in the Public Interest (No.201104009-02).
Anonymous.2010.National Forest Inventory Report of China.Beijing: Chinese Forestry Publishing House, pp.11–20.
Berg B.2000.Litter decomposition and organic matter turnover in northern forest soils.For Ecol Manag, 133: 13–22.
Chen H.1998.Biomass and nutrient distribution in a Chinese fir plantation chronosequence in southwest Hunan, China.For Ecol Manag, 105: 209–216.
Ekschmitt K, Kandeler E, Poll C, Brune A, Buscot F, Friedrich M, Gleixner G, Hartman A, Kastner M, Marhan S, Miltner A, Scheu S, Wolters V.2008.Soil-carbon preservation through habitat constraints and biological limitations on decomposer activity.J Plant Nutr Soil Sci, 171: 27–35.
Fisk MC, Fahey TJ.2001.Microbial biomass and nitrogen cycling responses to fertilization and litter removal in young northern hardwood forests.Biogeochemistry, 53: 201–223.
Hoffmann U, Yair A, Hikel H, Kuhn NJ.2012.Soil organic carbon in the rocky desert of northern Negev (Israel).J Soil Sediment, 12: 811–825.
Irvine J, Law BE.2002.Contrasting soil respiration in young and old-growth ponderosa pine forest.Global Change Biol, 8: 1183–1194.
Janssens IA, Lankreijer H, Matteucci G, Kowalski AS, Buchmann N, Epron D, Pilegaard K, Kutsch W, Longdoz B, Grunwald T, Montagnani L, Dore S, Rebmann C, Moors EJ, Grelle A, Rannik U, Morgenstern K, Oltchev S, Clement R, Gudmundsson J, Minerbi S, Berbigier P, Ibrom A, Moncrieff J, Aubinet M, Bernhofer C, Jensen NO, Vesala T, Granier A, Schulze ED, Lindroth A, Dolman AJ, Jarvis PG, Ceulemans R, Valentini R.2001.Productivity overshadows temperature in determining soil and ecosystem respiration across European forests.Global Change Biol, 7: 269–278.
Kandeler E, Luxhøi J, Tscherkoc D, Magid J.1999.Xylanase, invertase and protease at the soil–litter interface of a loamy sand.Soil Biol Biochem, 31:1171–1179.
Law BE, Sun OJ, Campbell J, Van Tuyl S, Thornton PE.2003.Changes in carbon storage and fluxes in a chronosequence of ponderosa pine.Global Change Biol, 9: 510–524.
Law BE, Thornton PE, Irvine J, Anthoni PM, Van Tuyl S.2001.Carbon storage and fluxes in ponderosa pine forests at different developmental stages.Global Change Biol, 7: 755–777.
Lemma B, Berggren D, Olsson M, Nilsson I.2007.Factors controlling soil organic carbon sequestration under exotic tree plantations: A case study using the CO2Fix model in southwestern Ethiopia.For Ecol Manag, 252: 124–131.
Li YQ, Xu M, Zou XM, Shi PJ, Zhang YQ.2005.Comparing soil organic carbon dynamics in plantations and secondary forest in wet tropics in Puerto Rico.Global Change Biol, 11: 239–248.
Ma XQ, Heal KV, Liu AQ, Jarvis PG.2007.Nutrient cycling and distribution in different-aged plantations of Chinese fir in southern China.For Ecol Manag, 243: 61–74.
Ministry of Forestry.2000.Forest Soil Analysis Methods.Beijing: Chinese Criteria Press, pp.1–60.(in Chinese)
Nakane K.1995.Soil carbon cycling in a Japanese cedar (Cryptomeria japonica) plantation.For Ecol Manag, 72: 185–197.
Olson JS.1963.Energy and the balance of producers and decomposers in ecological systems.Ecology, 44: 322–331.
Potter KN, Jones OR, Torbert HA, Unger PW.1997.Crop Rotation and Tillage Effects on Organic Carbon Sequestration in the Semiarid Southern Great Plains.Soil Sci, 162: 140–147.
Saiz G, Byrnew EA, Butterbach-bahl K, Kiese R, Blujdea V, Farrell EP.2006.Stand age-related effects on soil respiration in a first rotation Sitka spruce chronosequence in central Ireland.Global Change Biol, 12:1–14.
Schinner F, von Mersi W.1990.Xylanase-, CM-cellulase- and invertase activity in soils: An improved method.Soil Biol Biochem, 22: 511–515.
Sheng WT, Yang CD.1997.Effect on ameliorating soil properties by undergrowth vegetation on Chinese fir.Acta Ecol Sin, 17: 377–385.(in Chinese)
Tabatabai MA.1982.Soil enzymes.In: Page AL, Miller RH, Keeney DR (eds.), Methods of Soil Analysis.Madison: Soil Science Society of America, pp.903–947.
Tang J, Bolstad PV, Martin JG.2009.Soil carbon fluxes and stocks in a Great Lakes forest chronosequence.Global Chang Biol, 15: 145–155.
Tarafdar JC, Jungk A.1987.Phosphatase activity in the rhizosphere and its relation to the depletion of soil organic phosphorus.Biol Fertil Soil, 3: 199–204.
Tedeschi V, Rey A, Manca G, Valentini R, Jarvis PG, Borghetti M.2006.Soil respiration in a Mediterranean oak forest at different developmental stages after coppicing.Global Change Biol, 12: 110–121.
Turner J, Lambert M.2000.Change in organic carbon in forest plantation soils in eastern Australia.For Ecol Manag, 133: 231–247.
Wang B, Jiang Y, Wei XH, Zhao GD, Guo H, Bai XL.2011a.Effects of forest type, stand age, and altitude on soil respiration in subtropical forests of China.Scand J For Res, 26: 40–47.
Wang D, Wang B, Dai W, Li P.2011b.Effects of tree growth and soil properties on soil respiration rate in Chinese fir plantations.Acta Ecologica Sinica, 31: 680–688.(in Chinese)
Wang QK, Wang SL, Huang Y.2008.Comparisons of litterfall, litter decomposition and nutrient return in a monoculture Cunninghamia lanceolata and a mixed stand in southern China.For Ecol Manag, 255: 1210–1218.Wang QK, Wang SL, Zhang JW.2009.Assessing the effects of vegetation types on carbon storage fifteen years after reforestation on a Chinese fir site.For Ecol Manag, 258: 1437–1441.
Wang SL, Zhang WD, Sanchez F.2010.Relating net primary productivity to soil organic matter decomposition rates in pure and mixed Chinese fir plantations.Plant Soil, 334: 501–510.
Wells CE, Glenn DM, Eissenstat DM.2002.Changes in the risk of fine-root mortality with age: a case study in peach, Prunus persica (Rosaceae).Amer J Bot, 89: 79–87.
Wollum AG.1982.Cultural methods for soil microorganisms.In:Page AL, Miller RH, Keeney DR (eds.), Methods of Soil Analysis.Part 2.Chemical and Microbiological Properties.Madison: Soil Science Society of America, pp.781–802.
Wu ZL.1984.Chinese Fir.Beijing, China: Chinese Forestry Publishing House, pp.1–10.
Yan M, Zhang X, Zhou G, Gong J, You X.2011.Temporal and spatial variation in soil respiration of poplar plantations at different developmental stages in Xinjiang, China.J Arid Environ, 75: 51–57.
Yan S, Wang S, Yu X, Shen Z, Chen X.2004.Effect of mixtures with alders on soil fauna in plantation forest of Chinese fir (in Chinese).Appl Environ Biol, 10: 462–466
Zhang WD, Wang SL.2012.Effects of NH4+and NO3-on litter and soil organic carbon decomposition in a Chinese fir plantation forest in South China.Soil Biol Biochem, 47: 116–122.
Zhao MF, Xiang WH, Peng CH, Tian Dalun.2009.Simulating age-related changes in carbon storage and allocation in a Chinese fir plantation growing in southern China using the 3-PG model.For Ecol Manag, 257: 1520–1531.
Zhu JJ, Yan QL, Fan AN, Yang K, Hu ZB.2009.The role of environmental, root, and microbial biomass characteristics in soil respiration in temperate secondary forests of Northeast China.Trees-Struct Funct, 23: 189–196
杂志排行
Journal of Forestry Research的其它文章
- Ethno-medicinal plants used by Bengali communities in Tripura, northeast India
- Litter production, decomposition and nutrient mineralization dynamics of Ochlandra setigera: A rare bamboo species of Nilgiri Biosphere Reserve, India
- Temporal changes in nitrogen acquisition of Japanese black pine (Pinus thunbergii) associated with black locust (Robinia pseudoacacia)
- Plant diversity at Chilapatta Reserve Forest of Terai Duars in subhumid tropical foothills of Indian Eastern Himalayas
- Floristic composition and management of cropland agroforest in southwestern Bangladesh
- The changing landscape of mangroves in Bangladesh compared to four other countries in tropical regions
