Potency of a novel multi-epitope vaccine againstfoot-and-mouth disease type Asia 1 in guinea pigs
2014-04-02SHAOJun-jun,WANGJing-feng,GAOShan-dian等
Foot-and-mouth disease virus (FMDV) is a member of the genusAphthovirusof thePicornaviridaefamily that causes the highly contagious vesicular disease of the cloven-hoofed animals including cattle, pig and sheep[1]. The current inactivated FMD vaccines play a key role in control and prevention of this disease in some parts of the world. However, several disadvantages of the inactivated FMD vaccines limit its widely using[1]. In the year of 2001, outbreaks of FMD in the UK and other counties have resulted in the severe economic losses[2], which highlighted the needing for development of new FMD vaccines to control FMD.
In recent years, the new FMD vaccines including the recombinant FMDV multi-epitope vaccines have been extensively explored[3-4]. The vaccines, however, often render weak immune responses in the natural hosts. To improve potency of FMD epitope vaccines, T cell epitopes located in the structural proteins of FMDV has been extensively explored[5-6]. Unfortunately, the epitopes located in the structural proteins of FMDV can not be widely recognized by the different species lymphocytes. Inspiringly, as a highly conserved non-structural protein in 7 serotypes of FMDV, several universal T cell epitopes in FMDV 3D protein have been verified that can be recognized by the immune lymphocytes from the different infected species[5-7]. More importantly, a recombinant 3D protein of FMDV conferred partial protection to pigs against challenge[5]. The 3D protein of FMDV as a potent stimulator enhanced cellular and humoral immune responses in the natural host[7].
To develop a novel high-efficiency vaccine against FMDV type Asia 1, in present study, the recombinant FMD multi-epitope antigen (RE-oIgG) combined with FMDV 3D protein as a novel FMD multi-epitope vaccine formula was developed and its immune potency was evaluated in guinea pigs.
Materials and methods
Construction of the recombinant expression plasmid
A multi-epitopes gene recombinant plasmid based on FMDV type Asia 1 was constructed as description[8]and was slightly modified. Briefly, A tandem repeated multi-epitope gene (RE) consisted of residues amino acid 135-160 and 198-211 of VP1 of FMDV type Asia 1 (GenBank accession no. EF149009) was synthesized by sequential linking of the tandem repeats. To minimize interference between adjacent epitopes and to avoid development of a new epitope, the linker sequence, GGSSGG, was used to separate adjacent epitopes. An ovine immunoglobulin G heavy constant region gene (oIgG) was obtained by digesting of a recombinant plasmid withEcoR I/xho I which constructed previously in our laboratory. RE was cloned into an expression vector pET-30a (+) pre-linea-rized withBamH I/EcoR I (NEB) termed as pRE. Subsequently, the fragment of oIgG was inserted into the C-terminus of RE in the pRE pre-linearized vector with the same enzymes namely pRE-oIgG (Figure 1). Additionally, the recombinant expression plasmid with the complete FMDV 3D gene termed as pPRO-3D was constructed previously and stored in our laboratory.

Fig.1Schematicrepresentaionoftandemrepeatedmultiple-epitopesgenecoupledwithovineIgGheavyconstantregiongene(oIgG)andconstructuonoftherecombinantplasmidpRE-oIgG
The multi-epitope gene RE was excised from a recombinant plasmid p18RE byBamHI andEcoRI, and then cloned into the expression vector pET-30a (+) pre-linearized withBamHI andEcoRI (NEB) termed as pRE. The fragment of oIgG was digested byEcoRI andxhoI and then was inserted into the C-terminus of RE in the pRE pre-linearized with the same enzymes.
Expression and purification of the recombinant proteins
Expression and purification of the recombinant proteins, RE-oIgG and FMDV 3D inE.coliBL21 (DE3) cells (Novagen) was performed as described previously[8].
Immune potency of the vaccine candidates
All the proteins were formulated with ISA 206 adjuvant (Seppic, France) as described previously[8]. Twenty-five female guinea pigs were equally divided into five groups and then were vaccinated intramuscularly at two sites in the rear legs. Groups 1 to 4 were separately vaccinated with RE-oIgG alone (20 μg/mL), a mixture of RE-oIgG (20 μg/mL) and 3D (10 μg/mL), 3D alone (20 μg/mL), and a commercial inactivated FMDV vaccine (type Asia 1). Group 5 as a negative control was injected only PBS in oil adjuvant. All the animals were boosted with the same dosage on day 28 after first vaccination. Blood samples were collected for detection of the anti-FMDV specific antibodies and the neutralizing antibodies at 0, 28 and 42 days post-vaccination (dpv).
All the guinea pigs were challenged with 103GPID50of virus at 42 dpv, and the typical FMD signs were monitored for 10 days. The protection was determined according to the criteria as below:briefly, the lesions appeared only at the site of virus injection were referred to as an indicator of partial infection and showed at two back sole as an indicator of whole infection. No lesion showed at all four pads including virus injection site was an indicator of complete protection.
Detection of FMDV specific antibodies
Ninety-six well flat-bottomed plates (Coster) were coated with the purified inactivated FMDV (type Asia 1) in 0.05 M of carbonate buffer (pH 9.6) at 4 ℃ overnight. Sera were diluted at 1:100 in the blocking buffer and was added into the micro-reaction plates, and then incubated for 1 hour at 37 ℃. The plates were washed for three times with PBST and incubated with rabbit anti-guinea pig IgG-peroxidase (Sigma) diluted at 1:5 000 in the blocking buffer for 1 hour at 37 ℃. After tho-roughly washing, the chromogenic substrate was added and color was developed at 37 ℃ for about 10-15 minutes, and the reaction was then stopped by 2.0 M H2SO4. The optical density (OD) values were read at 450 nm by a microplate reader (Bio-Rad).
Lymphoproliferation assay
Proliferation assays with guinea pig lymphocytes were performed as described elsewhere[8].
Virus neutralization test (VNT)
The micro-neutralization assay was performed according to the manual of Diagnostic Tests and Vaccines for Terrestrial Animals published by World Organization for Animal Health (http://www.oie.int/fileadmin/Home/eng/Health_stan-dards/tahm /2.01.05_FMD.pdf).
Statistical analysis
Where appropriate, data are expressed as the mean values SD. For comparison of the mean of two experimental groups, statistical significance was assessed by the two-tailed Student'st-test and χ2-test (chi-square test).
Results
Characterization of the recombinant proteins
The target proteins, 3D and RE-oIgG, were expressed as a formation of inclusion bodies in theE.colicells, and a specific band was clearly visua-lized on 12% SDS-PAGE, whereas no band was found in the cellular lysate from the recombinantE.colicells with the negative express plasmid pET-30a (+) (data not shown). Additionally, the purity of the target protein was greater than 95%. Western blot showed that both 3D and RE-oIgG reacted well with FMDV infection positive sera (type Asia 1) gifted from the National Foot-and-Mouth Disease Reference Laboratory of the P.R. China (data not shown).
FMDV specific antibodies
As shown in Figure 2, the RE-oIgG alone or combined with 3D elicited high levels of anti-FMDV specific antibodies in the vaccinated animals. The levels of anti-FMDV specific antibodies were significantly increased after boosting. As expected, RE-oIgG combined with 3D elicited the signifi-cantly high levels of anti-FMDV specific antibodies when compared to RE-oIgG alone (t-test,P<0.05). There was no statistical difference in eliciting anti-FMDV specific antibodies between a mixture of RE-oIgG and 3D and the inactivated vaccine (t-test,P>0.05). The vaccinated animals with 3D alone produced negligible anti-FMDV specific antibodies.
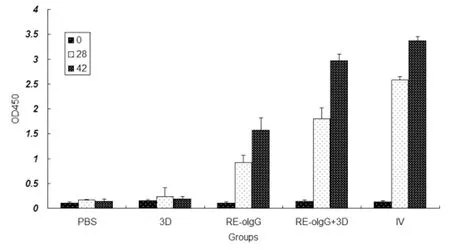
Fig.2Profileofanti-FMDVspecificantibodiesinserumsamplesfromvaccinatedguineapigswithvaccinecandidates
The data represent the means ± standard deviation from 5 animals in each group. Significant differences compared with RE-oIgG alone or PBS control group were denoted byP<0.05.
Neutralizing antibodies
As shown in Figure 3, all of the vaccinated animals produced the anti-FMDV specific neutralizing antibodies on 28 days after the primary vaccination except the animals vaccinated with 3D alone. Inspiringly, the neutralizing antibody titers were dramatically increased after boosting. Unexpectedly, RE-oIgG combined with 3D did not elicit significantly higher level of neutralizing antibodies than that of RE-oIgG after boosting (t-test,P>0.05). The RE-oIgG alone or in combination with 3D and an available commercial inactivated vaccine elicited the similar titers of the anti-FMDV specific neutralizing antibodies (t-test,P>0.05). Both 3D alone and the placebo vaccine produced the negligible neutralizing antibodies.
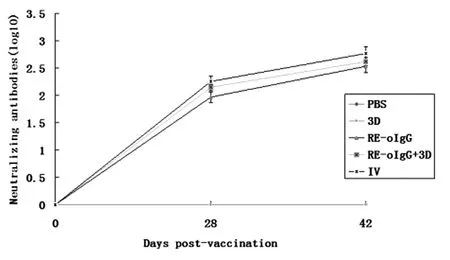
Fig.3Profileofanti-FMDVneutralizingantibodytitersinserumsamplesfromthevaccinatedguineapigs
The data represent the means ± standard deviation from 5 animals each group. No significant difference in anti-FMDV neutralizing antibody titers between RE-oIgG alone or the inactivated vaccine is denoted byP>0.05.
Challenge test
Table 1 showed that RE-oIgG alone or combined with 3D and an available commercial inactivated vaccine conferred complete protection to guinea pigs against virus challenge. Compared to the group PBS, there were the significant diffe-rences in protection ratio amongst the test groups except the 3D group (χ2-test,P<0.01). Two out of five vaccinated animals with 3D alone were completely protected against challenge with a virulent virus. Additionally, the other three guinea pigs vaccinated with 3D alone showed a delay of 2-3 days in the onset of clinical signs. Whereas, all the animals vaccinated with placebo vaccine showed the typically clinical signs including the vesicle was in four feet within 3 days after challenge.
Tab.1 Recombinant FMD multi-epitopes vaccines in guinea pigs after challenge
Note:N--negative neutralizing antibodies; * A delay in the onset of clinical signs after challenge;IV:inactivated vaccine (type Asia 1).Pvalue is a statistical difference amongst all test groups compared to the negative control group PBS.P<0.01 represent a statistically significant difference, and >0.05 is not difference in protection ratio between 3D and PBS.
Lymphoproliferation responses
As summarized in Figure 4, PBMC from all the vaccinated animals elicited significantly strong lymphocyte proliferative responses as compared to the placebo vaccine after stimulationinvitroby the inactivated FMDV (type Asia 1) (t-test,P<0.05). RE-oIgG combined with 3D elicited stronger lymphoproliferative responses than that of RE-oIgG alone (t-test,P<0.05). Interestingly, the mixture of RE-oIgG and 3D elicited the similar lymphoproliferation responses to an inactivated vaccine (t-test,P>0.05). The lymphoproliferation responses to BSAinvitrostimulation maintained negligible in all the samples. As a nonspecific stimulator, Con A stimulated all the prolymphoctyes to produce strong lymphoproliferation responsesinvitro.
Discussion
The previously reported recombinant FMD N represents negative control; BSA is a non-correlated antigen control; Antigen is the purified FMDV (type Asia 1) whole-virus antigen; ConA is a concanavalin A, a nonspecific stimulator of lymphoproliferation used as a positive control. The significant differences between RE-oIgG plus 3D and REoIgG alone or 3D alone are denoted byP>0.05.
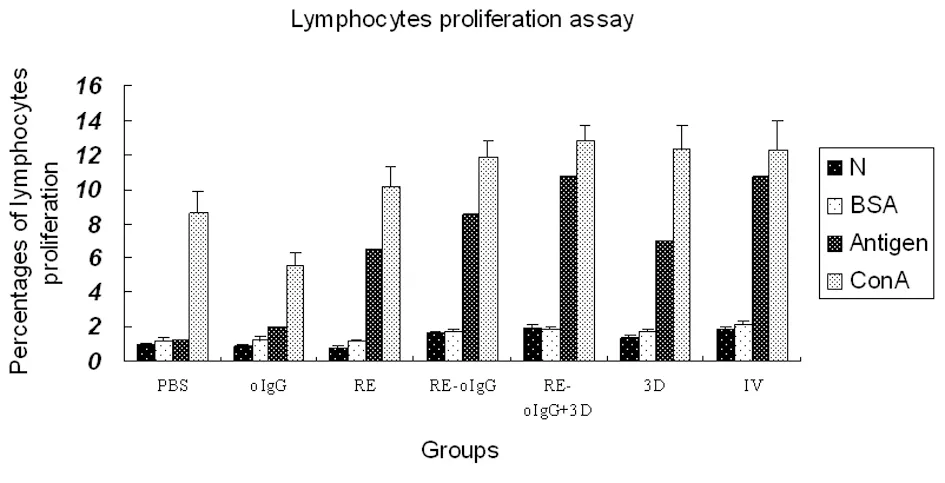
Fig.4ProfileoflymphocyteproliferationfromPBMCpriortoorafterstimulation
multi-epitope vaccines offered fully protection in small animals and pig against challenge[8-10]. However, the neutralizing antibody titers in other host animals were lower than that of an inactivated FMD vaccine[11-12]. Previous reports indicated that induction of anti-FMDV antibodies was T cell dependent[13], and the cellular immune response plays an important role in eradicating FMDV infectioninvivo[5, 14]. Therefore, the most likely limi-ted factors for low immunogenicity of the recombinant FMDV multi-epitope vaccines may be the absence of T cell epitopes that are capable of inducing T cell in cooperation with immune B lymphocytes for the production of anti-FMDV specific neutralizing antibodies. Development of the recombinant FMD multi-epitope vaccines to prevent and eradicate FMD requires either increase the number of antigenic epitopes or some appropriate T cell epitope to enhance the neutralizing antibodies induction and the cellular immune responses.
In this study, we developed the novel recombinant FMD multi-epitope vaccine formula that showed good immune potency in guinea pigs. The novel vaccine formula could not only elicit high le-vels of the neutralizing antibodies and significant lymphocyte proliferation response, but also offer complete protection to guinea pigs against virus challenge. The most likely explanation for this is that the synergy of humoral immune responses and cellular immune responses effectively limit virus dissemination and propagationinvivo, and then immune system timely clears all the invasion viruses. Additionally, the increase of the immunodomi-nant epitope copy is also a very important reason to improve immunogenicity of this vaccine, compared to previous design strategy. Strong lymphocyte proliferation responses were obtained in PBMC from the vaccinated guinea pigs with FMDV 3D after stimulation of the killed FMDVinvitro. Three vaccinated animals with 3D alone showed a delay of 2-3 days in the onset of clinical signs. This phenomenon supported by previous reports is that the cellular immunity also plays an important role in elimination of virusinvivo. The significant difference in terms of their ability in eliciting anti-FMDV specific antibodies was obtained between RE-oIgG combined with 3D and RE-oIgG alone. Our previous study showed that the recombinant FMDV 3D protein can improve immunogenicity of FMDV multi-epitope vaccine (RE) that elicited higher levels of the neutralizing antibodies than that of FMD multi-epitope vaccine alone. How-ever, RE-oIgG combined with 3D elicited the similar neutralizing antibody titers to RE-oIgG alone. The most possible explanations for this are that an indirect ELISA is more sensitive than a virus neutrali-zation assay. In addition, IgG as a protein carrier prolongs the recombinant antigen half-lifeinvivoand enhances the immunogenicity of the recombinant antigen, which most likely make 3D protein to further improve humoral immune responses is not obvious shown in this study. Fortunately, 3D protein enhances cellular immune responses to FMDV. We shall further investigate this phenomenon in the future. Previous reports indicated that immunization of domestic pigs with a vaccinia virus (VV) recombinant expressing FMDV 3D protein conferred partial protection against challenge with infectious virus[5]. To our knowledge, this is the first report that theE.coliexpress FMDV 3D protein can improve immunogenicity of a FMD multiple-epitope vaccine.
Taken together, the novel formula, RE-oIgG combined with 3D, elicited humoral immune responses and induced cellular immune responses in guinea pigs. The potency of this vaccine formula will be further evaluated and confirmed in nature host species in the future. This strategy may be used to develop a novel generation subunit vaccines to other FMDV serotypes.
[1]Doel TR. FMD vaccines[J]. Virus Res, 2003, 91(1):81-99. DOI:10.1016/S0168-1702(02)00261-7
[2]Thompson D, Muriel P, Russell D, et al. Economic coasts of the foot-and-mouth disease outbreak in the United Kingdom in 2001[J]. Rev Sci Tech, 2002, 21(3):675-687.
[3]Chan EW, Wong HT, Cheng SC, et al. An immunoglobulin G based chimeric protein induced foot-and-mouth disease specific immune response in swine[J]. Vaccine, 2000, 19(4-5):538-546. DOI:10.1016/S0264-410X(00)00186-9
[4]Zhang YL, Guo YJ, Wang KY, et al. Enhanced immunogenicity of modified hepatitis B virus core particle fused with multiepitopes of foot-and-mouth disease virus[J]. Scand J Immunol, 2007, 65(4):320-328. DOI:10.1111/j.1365-3083.2007.01900.x
[5]Garcia-Briones MM, Blanco E, Chiva C, et al. Immunogenicity and T cell recognition in swine of foot-and-mouth disease virus polymerase 3D[J]. Virology, 2004, 322(2):264-275. DOI:10.1016/j.virol.2004.01.027
[6]Blanco E, Garcia-Briones M, Sanz-Parra A, et al. Identification of T-cell epitopes in nonstructural proteins of foot-and-mouth di-sease virus[J]. J Virol, 2001, 75(7):3164-3174. DOI:10.1128/JVI.75.7.3164-3174.2001
[7]Gerner W, Denyer MS, Takamatsu HH, et al. Identification of novel foot-and-mouth disease virus specific T-cell epitopes in c/c and d/d haplotype miniature swine[J]. Virus Res, 2006, 121(2):223-228. DOI:10.1016/j.virusres.2006.05.006
[8]Shao JJ, Wong CK, Lin T, et al. Promising multiple-epitope recombinant vaccine against foot-and-mouth disease virus type O in swine[J]. Clin Vaccine Immunol, 2011, 18(1):143-149. DOI:10.1128/CVI.00236-10
[9]Yi JZ, Liu MQ, Zhu CZ, et al. Recombinant bivalent vaccine against foot-and-mouth disease virus serotype O/A infection in guinea pig[J]. Acta Biochim Biophys Sin (Shanghai), 2004, 36(9):589-596. DOI:10.1093/abbs/36.9.589
[10]Su C, Duan X, Wang X, et al. Heterologous expression of FMDV immunodominant epitopes and HSP70 inP.pastorisand the subsequent immune response in mice[J]. Vet Microbiol, 2007, 124(3-4):256-263. DOI:10.1016/j.vetmic.2007.04.030
[11]Rodriguez LL, Grubman MJ. Foot and mouth disease virus vaccines[J]. Vaccine, 2009, 27(4):D90-94. DOI:10.1016/j.vaccine.2009.08.039
[12]Rodriguez LL, Gay CG. Development of vaccines toward the global control and eradication of foot-and-mouth disease[J]. Expert Rev Vaccines, 2011, 10(3):377-387. DOI:10.1586/erv.11.4
[13]Collen T, Pullen L, Doel TR. T cell-dependent induction of antibody against foot-and-mouth disease virus in a mouse model[J]. J Gen Virol, 1989, 70(Pt2):395-403. DOI:10.1099/0022-1317-70-2-395
[14]Patch JR, Pedersen LE, Toka FN, et al. Induction of foot-and-mouth disease virus-specific cytotoxic T cell killing by vaccination[J]. Clin Vaccine Immunol, 2011, 18(2):280-288. DOI:10.1128/CVI.00417-10
