A three-dimensional tetrahedral-shaped conjugated small molecule for organic solar cells
2014-03-20QINYangYANGJianzhong
QIN Yang,YANG Jianzhong
(Department of Chemistry & Chemical Biology,University of New Mexico,Albuquerque NM 87131-001,U.S.A.)
1 Introduction
Organic solar cells have attracted tremendous attention from both academia and industry due to the promises as low-cost alternative energy sources[1-2].Research efforts in these areas have mostly been devoted to materials based on conjugated polymers[3-5],and power conversion efficiencies(PCEs) of these devices have steadily increased to approach 10% in laboratory settings[6-8].However,the intrinsic polydisperse and amorphous nature of conjugated polymers often leads to batch-to-batch variations and low materials conductivity,respectively,which can potentially impede device mass production and further improvement.On the other hand,conjugated small molecules can be highly crystalline,while having discrete and reproducible molecular structures[7-11].Bulk heterojunction(BHJ) devices employing conjugated small molecules and fullerene derivatives have been constantly improved to rival their conjugated polymer counterparts and thus shown great promises in solar cell research[12-14].
Most small molecules applied in solar cells have linear structures containing multiple aromatic groups connected in series.Such molecules are typically highly crystalline and conductive along the π-stacking direction.However,charge migration along both long and short axes are relatively limited due to the one-dimensional nature of these molecules.Unfavorable film forming ability and grain boundaries originated from high crystallinity of linear molecules can also have detrimental effects on device performances.Thus,small molecules having conjugation extended in three-dimensions(3-D) can offer both good film forming ability and isotropic charge mobility.Tetra-substituted silanes have been a common starting core for building 3-D molecules due to the tetrahedral geometry and easy preparation.Roncali etc.,reported the synthesis of two silane-cored terthiophene armed 3-D small molecules bearing respective alkyl and thioalkyl side chains[15].Although overall solar cell performances were significantly limited by narrow absorption due to the large bandgaps,both star-shaped molecules out-performed their corresponding single-arm counterparts,proving the effectiveness of 3-D strategy.Köse etc.,recently reported low bandgap small molecules based on tetrathienyl core and benzothiadiazole containing arms,and found favorable impact of high dimensionality on charge mobility in disordered media[16].On the other hand,silicon thiophene bonds are quite unstable due to the electron richness of thienyl moieties,which can potentially complicate synthesis and reduce device lifetimes.Herein,we report the synthesis and characterization of a stable low bandgap 3-D conjugated molecule based on tetraphenylsilane core.Initial investigation of this molecule in BHJ solar cells indicates that lack of phase separation with fullerene molecules is responsible for relatively low device performance.
2 Results and discussions
Synthesis of the 3-D conjugated small molecule,SO,is shown in Figure 1 and synthetic details can be found in the experimental section.The compound1was prepared from commercial 1,4-dibromobenzene through lithium halogen exchange followed by reaction with SiCl4,which can be conveniently used as a common core for grafting with different arms toward 3-D molecules.After Stille coupling reaction with10followed by acetal deprotection,the tetra-aldehyde compound11was obtained.The aldehyde groups can be transformed to several strongly electron withdrawing substituents,e.g. dicyanovinyl and cyanoester groups.However,11was found to have very limited solubility in common organic solar cell processing solvents such as chlorobenzene and dichlorobenzene.We thus chose n-octyl cyanoacetate(12) to install the functionality.Indeed,after a simple condensation reaction,compoundSOwas obtained in high yields and has good solubility in a wide range of organic solvents including CHCl3,THF and chlorobenzene.All compounds are fully characterized by1H and13C NMR spectroscopy,which agree well with proposed structures.High resolution mass spectrometry(HR-MS) was applied to confirm the tetra-arm nature of our target compounds.Unfortunately,SOcould not be ionized under experimental conditions and no meaningful mass signals could be observed.Instead,HR-MS was performed on the precursor11,in which the measured molar mass(1 768.341 9[M+]) matches very well with the calculated value(1 768.341 4[M+]),confirming the tetra-arm structure.Since NMR showed full conversion of the aldehyde groups to cyanoester moieties,the compoundSOunambiguously has the proposed tetra-arm 3-D structure.
The electronic properties ofSOwere first investigated by UV-Vis absorption spectroscopy in both diluted solutions and as thin films as shown in Figure 2.A structureless absorption profile with aλmax=478 nm was observed in solution,originated from intra-molecular charge transfer interactions.From the absorption onset at ca.560 nm,a solution optical bandgap of ca.2.2 eV is calculated.Significant red-shift ofλmaxto 575 nm was observed in the thin film ofSOand the absorption profile became more structured,indicating aggregation and enhanced crystallinity in the solid state.From the absorption onset at ca.650 nm,the solid-state bandgap ofSOis estimated to be ca.1.9 eV that is comparable to that of the most commonly applied conjugated polymer,poly(3-hexylthiophene)(P3HT).In order to further quantify the frontier energy levels,cyclic voltammetry(CV) measurements were performed onSOthin films drop cast onto the glassy carbon working electrode.A Ag/AgCl reference and a Pt wire counter electrodes were used,and 0.1 mol/L tetrabutylammonium hexafluorophosphate in acetonitrile was used as the supporting electrolytes.Two quasi-reversible oxidation peaks at 0.9 V and 1.3 V and one irreversible reduction peak at -1.0 V(all values are onsets) were observed.When calibrated externally with ferrocene redox couple(-4.8 eV below vacuum,0.4 V vs.Ag/AgCl),a HOMO energy level of -5.3 eV and a LUMO energy level of -3.4 eV were calculated.This leads to an electrochemical bandgap of 1.9 eV,agreeing very well with the film′s optical bandgap.
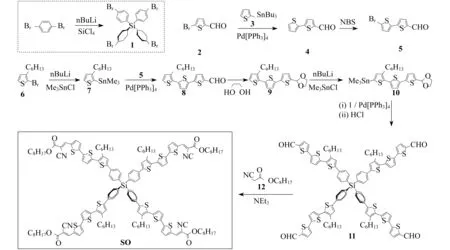
Figure 1 Synthesis of the compound SO
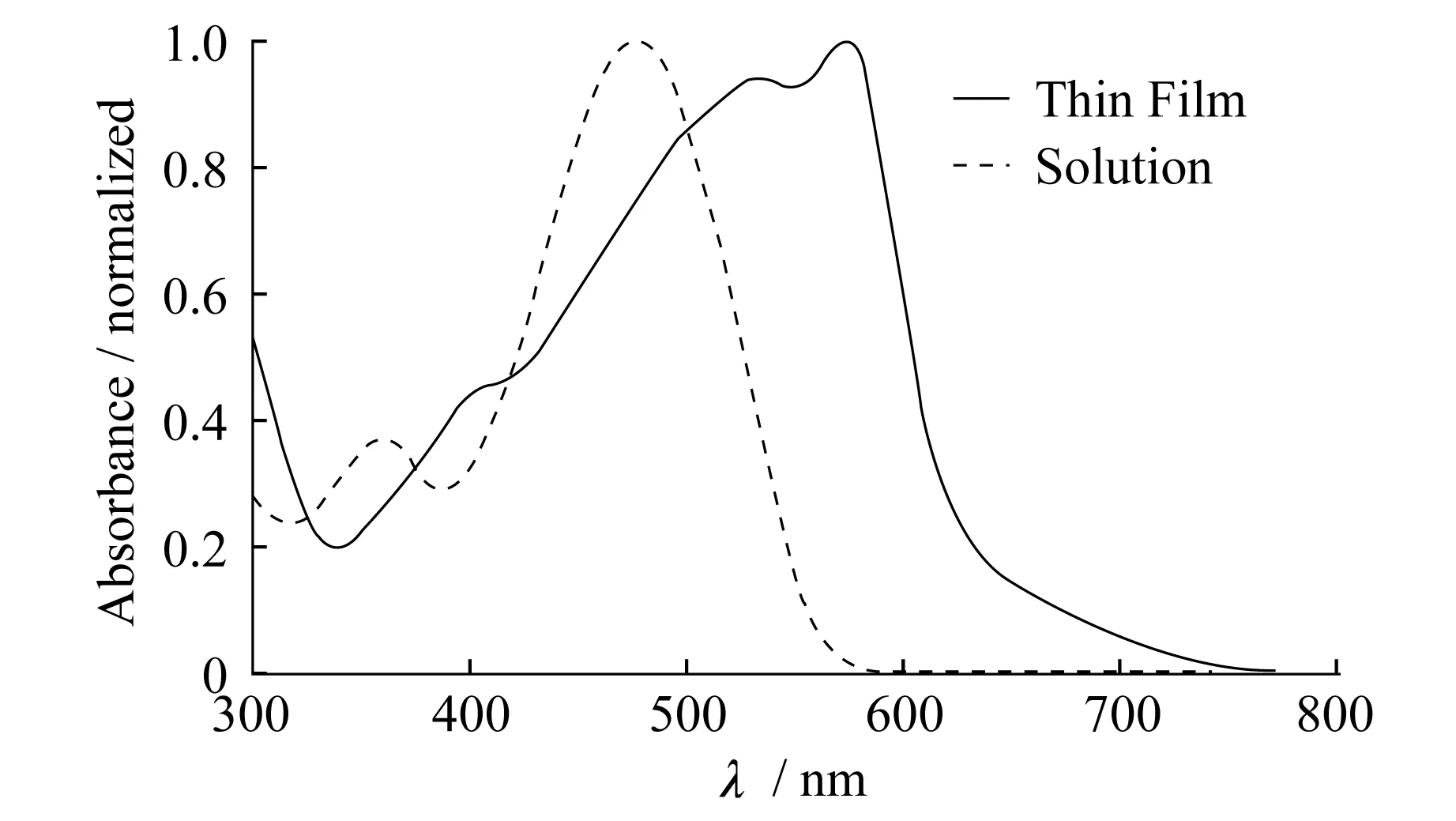
Figure 2 UV-Vis absorption spectra of SO in diluted chlorobenzene solution(10-5 mol/L,dashed line) and as thin film drop cast onto glass substrate
The absorption spectra indicate certain crystallinity ofSO,which was further studied by thin film X-ray scattering and differential scanning calorimetry(DSC) measurements.As shown in Figure 3A,the out-of-plane scattering profile clearly contains four distinct peaks at 2θvalues of 3.9°,5.6°,8.1° and 12.2°,corresponding to d-spacings of 2.3,1.6,1.1 and 0.7 nm,respectively.Due to the film′s small thickness,the signal-to-noise ratio of scattering profiles is not high enough to unambiguously assign the nature of each of these peaks.More detailed studies will be performed on high-power synchrotron sources and the results will be reported in due courses.Crystallinity ofSOwas further confirmed by DSC studies(Figure 3B).The 2ndheating curve showed a glass transition with an onset of 43 ℃ and a crystallization event peaked at 114 ℃.Two closely spaced melting transitions peaked at 180 ℃ and 187 ℃ were detected.These observations confirm crystallinity of theSOcompound.
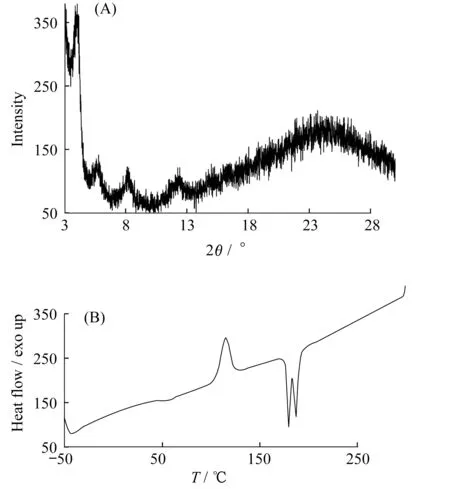
Figure 3 Out-of-plane X-ray scattering profiles of SO thin film drop cast on glass substrate(A) and differential scanning calorimetry(DSC) histogram of SO powder(2nd heating,10 ℃/min)(B)
Solar cell devices were fabricated using conventional structures: ITO glass/MoO3(10 nm)/active layer(100 nm)/Al(100 nm).Mixtures ofSOand phenyl-C61-butyric acid methyl ester(PCBM) at various weight ratios in chlorobenzene were spun cast to form the active layer.Thermal annealing at various temperatures was found to deteriorate device performance and the best power conversion efficiencies(PCEs) were found in as cast devices employingSO/PCBM at a weight ratio of 1/3.Figure 4 shows the current density-voltage(I-V) curves of the best performing device both in dark and under simulated sunlight(100 mW/cm2).From the graph,an open circuit voltage(VOC) of 0.85 V and a short circuit current(JSC) of 1.1 mA/cm2were found and the fill factor(FF) is calculated to be 25%.Device performances suffer greatly from the low FFs and JSC′s.Large current at negative bias was observed in all devices,indicating severe geminate charge recombination commonly caused by lack of proper phase separation.We have thus performed optical microscopy measurements on thin films ofSOand its corresponding blends with PCBM at a 1/3 weight ratio.As shown in Figure 5,optical micrograph ofSOthin film shows weak crystalline domains(darker areas,A) which becomes significantly enhanced after thermal annealing at 150 ℃ for 10 min(B).This observation is consistent with high crystallinity of the compound.On the other hand,both as cast and thermally annealed thin films ofSO/PCBM blends(1/3 by weight) showed no macroscopic phase separation,despite the large excess of PCBM molecules.Certain type of inter-molecular forces betweenSOand PCBM molecules are strong enough to prevent phase segregation,the nature of which is currently under investigation.
3 Conclusions
We have successfully prepared a tetraphenylsilane cored 3-D small molecule possessing high crystallinity and low bandgap.Solar cell devices employing such molecule showed lackluster performance due to absence of appreciable phase separation.We are currently optimizing solar cell devices through solvent additives and molecular structural variation in order to gain further insight on the nature of strong interactions of such 3-D molecules with fullerene acceptors.
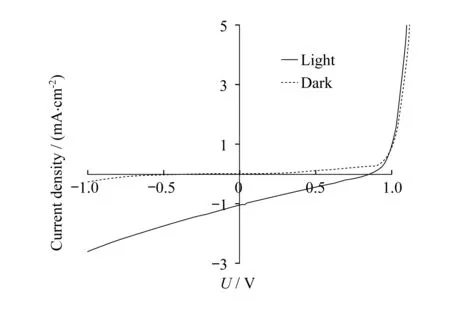
Figure 4 I-V curves of solar cell device employing SO and PCBM at a 1/3 weight ratio as the active layer in dark(dashed line) and under simulated white light(100 mW/cm2)
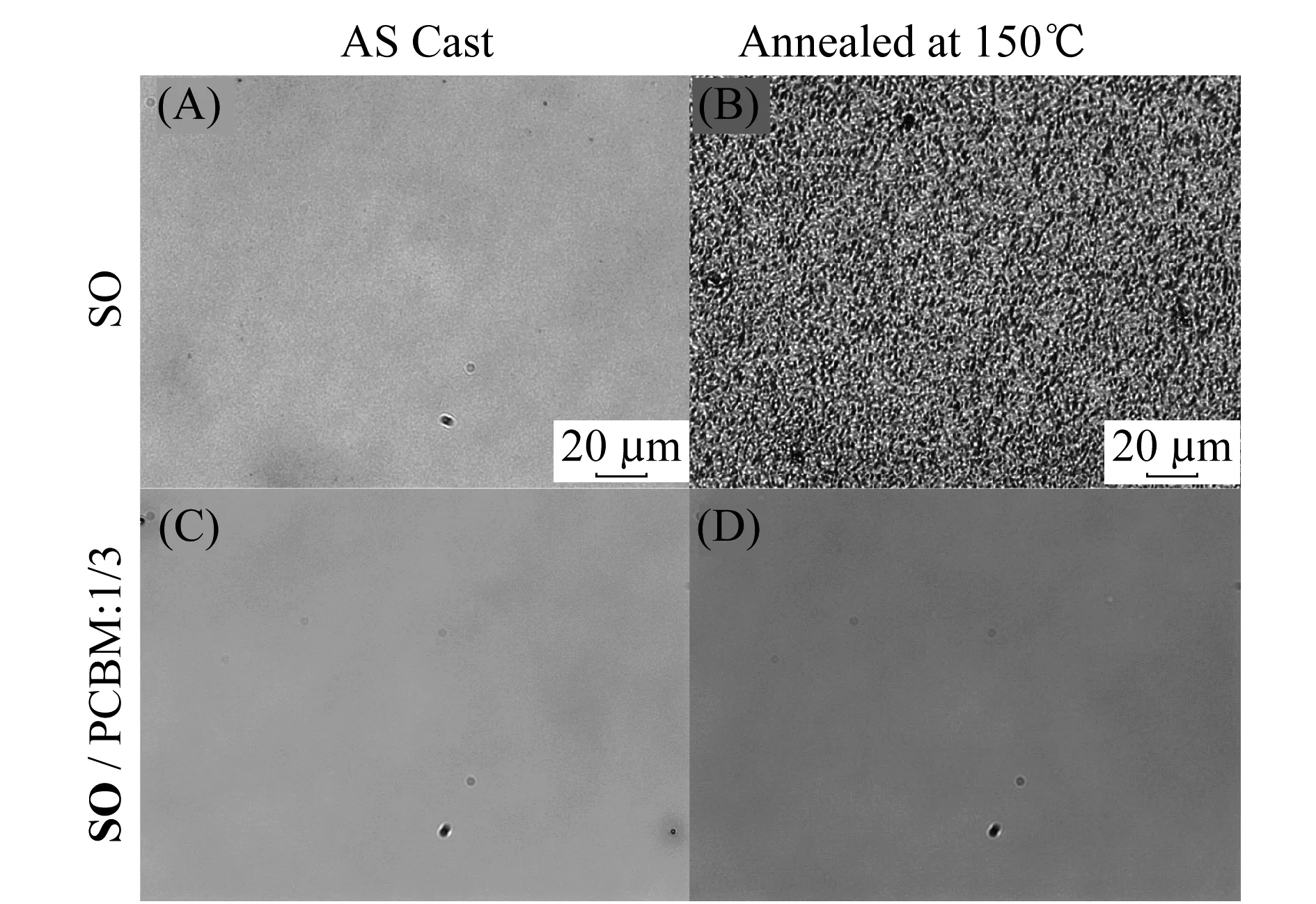
Figure 5 Optical microscopy images(40X zoom) of as cast thin film of SO(A) and SO/PCBM 1/3 blends(C),and thermally annealed(150 °C,10 min) thin films of SO(B) and SO/PCBM 1/3 blends(D)(Scale bar: 20 μm)
4 Experimental section
MaterialsandGeneralMethodsAll reagents and solvents were used as received from Sigma-Aldrich or Alfa Aesar unless otherwise noted.THF was distilled from Na/benzophenone prior to use.300.13 MHz1H and 75.48 MHz13C NMR spectra were recorded on a Bruker Avance III Solution 300 spectrometer.2-Tributylstannylthiophene(3)[17], 2,2′-bithiophene-5-carbaldehyde(4)[18],5′-bromo-(2,2′-bithiophene)-5-carbaldehyde(5)[18],2-bromo-3-hexylthiophene(6)[19], 3-hexyl-2-trimethylstannylthiophene(7)[20], were prepared to literature procedures.All solution1H and13C NMR spectra were referenced internally to solvent signals.Ultraviolet-Visible(UV-Vis) absorption spectra were recorded on a Shimadzu UV-2401 PC spectrometer over a wavelength range of 240-1 100 nm.Fluorescence emission spectra were obtained using a Varian Cary Eclipse fluorimeter.Time-of-flight mass spectrometry(TOF MS) was performed on a Waters/Micromass LCT Premier system operating under atmospheric pressure photoionization(APPI+) mode.Cyclic voltammetry was performed at 25 ℃ on a CH Instrument CHI604xD electrochemical analyzer using a glassy carbon working electrode,a platinum wire counter electrode,and a Ag/AgCl reference electrode calibrated using ferrocene redox couple(4.8 eV below vacuum).
SolarCellFabricationandTestingSolar cell devices adopt a structure of ITO/MoO3/active layer/Al.Thin films of active layers were spun-cast from blend solutions prepared by dissolvingSOand PCBM(American Dye Source,Inc.) at predetermined weight ratios in chlorobenzene(CB) and stirred at 80 ℃ for 10 h in a nitrogen glove box(Innovative Technology,model PL-He-2 GB,O2<10-7,H2O<10-7) before device fabrication.Solar cell devices were fabricated according to the following procedure:ITO-coated glass substrates(China Shenzhen Southern Glass Display.Ltd) were cleaned by ultrasonication sequentially in detergent,DI water,acetone and isopropyl alcohol,each for 15 min.These ITO-coated glass substrates were further treated by UV-ozone(PSD Series,Novascan) for 45 min before transferred into a nitrogen glove box(Innovative Technology,model PL-He-4GB-1800,O2<10-7,H2O<10-7) for MoO3deposition.MoO3(10 nm) was deposited using an Angstrom Engineering Åmod deposition system at a base vacuum level<7×10-8Torr.The blend solution was first filtered through a 0.45 μm PTFE filter and spin-coated on top of the MoO3layer at preset speeds for 30 s.Typical thickness of organic layers was ca.100 nm.Al(100 nm) was finally thermally evaporated through patterned shadow masks as anodes.Current-voltage(I-V) characteristics were measured by a Keithley 2 400 source-measuring unit under simulated AM1.5 G irradiation(100 mW/cm2) generated by a Xe arc-lamp based Newport 67005 150-W solar stimulator equipped with an AM1.5 G filter.The light intensity was calibrated by a Newport thermopile detector(model 818P-010-12) equipped with a Newport 1916-C Optical Power Meter.
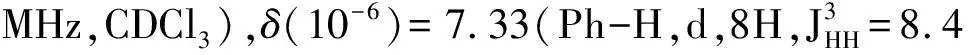
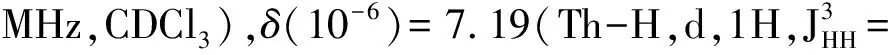
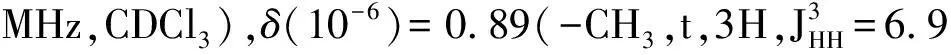
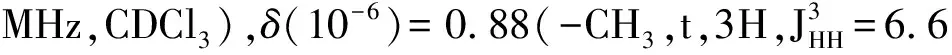
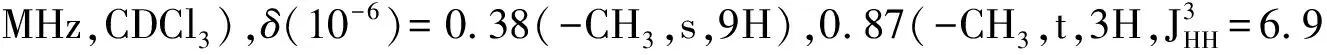
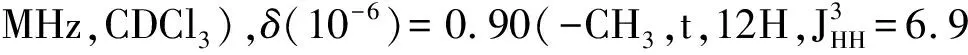
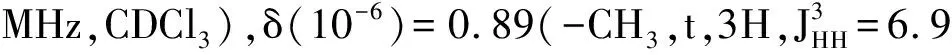
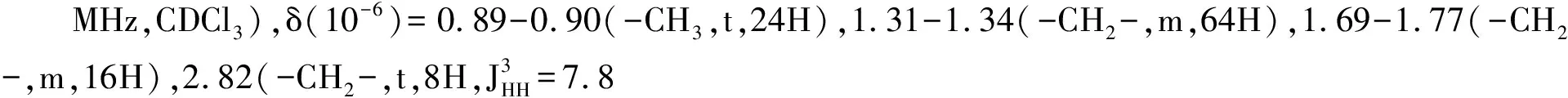
5 Acknowledgment
The authors would like to acknowledge University of New Mexico for financial support for this research.National Science Foundation(NSF) is acknowledged for supporting the NMR facility at UNM through grants CHE-0840523 and 0946690.
:
[1] HOPPE H,SARICIFTCI N S.Organic solar cells:An overview[J].J Mater Res,2004,19(7):1924-1945.
[2] SPANGGAARD H,KREBS F C.A brief history of the development of organic and polymeric photovoltaics[J].Sol Energy Mater Sol Cells,2004,83(2-3):125-146.
[3] THOMPSON B C,FRéCHET J M.Polymer-fullerene composite solar cells[J].Angew Chem Int Ed,2008,47(1):58-77.
[4] DENNLER G,SCHARBER M C,BRABEC C.Polymer-fullerene bulk-heterojunction solar cells[J].Adv Mater,2009,21(13):1323-1338.
[5] CHENGY J,YANG S H,HSU C S.Synthesis of conjugated polymers for organic solar cell applications[J].Chem Rev,2009,109(11):5868-5923.
[6] HE Z,ZHONG C,SU S,et al.Enhanced power-conversion efficiency in polymer solar cells using an inverted device structure[J].Nat Photon,2012,6:591-595.
[7] YOU J,DOU L,YOSHIMURA K,et al.A polymer tandem solar cell with 10.6% power conversion efficiency[J].Nat Commun,2013,4,doi:10.1036/n Comms 2411.
[8] YOU J,CHEN C C,HONG Z,et al.10.2% power conversion efficiency polymer tandem solar cells consisting of two identical sub-cells[J].Adv Mater,2013,25(29):3973-3978.
[10] WALKER B,KIM C,NGUYEN T Q.Small molecule solution-processed bulk heterojunction solar cells[J].Chem Mater,2011,23(3):470-482.
[11] CHEN Y,WAN X,LONG G.High performance photovoltaic applications using solution-processed small molecules[J].Acc Chem Res,2013,46(11):2645-2655.
[12] STEINMANN V,KRONENBERG N M,LENZE M R,et al.Simple,highly efficient vacuum-processed bulk heterojunction solar cells based on merocyanine dyes[J].Adv Energy Mater,2011,1(5):888-893.
[13] SUN Y,WELCH G C,LEONG W,et al.Solution-processed small-molecule solar cells with 6.7% efficiency[J].Nat Mater,2012,11:44-48.
[14] LIU X,SUN Y,PEREZ L A,et al.Narrow-band-gap conjugated Chromophores with Extended Molecular Lengths[J].J Am Chem Soc,2012,134(51):20609-20612.
[15] ROQUET S,DE B R,LERICHE P,et al.Three-dimensional tetra(oligothienyl)silanes as donor material for organic solar cells[J].J Mater Chem,2006,16:3040-3045.
[16] LIN Z,BJORGAARD J,YAVUZ A G,et al.Low band gap star-shaped molecules based on benzothia(oxa)diazole for organic photovoltaics[J].J Phys Chem C,2011,115:15097-15108.
[17] PU S,ZHENG C,SUN Q,et al.Enhancement of cyclization quantum yields of perfluorodiarylethenes via weak intramolecular interactions[J].Chem Commun,2013,49:8036-8038.
[18] GRISORIO R,DE M L,ALLEGRETTA G,et al.Anchoring stability and photovoltaic properties of new D(-π-A)2dyes for dye-sensitized solar cell applications[J].Dyes Pigments,2013,98(2):221-231.
[19] AMIR E,SIVANANDAN K,COCHRAN J E,et al.Synthesis and characterization of soluble low-bandgap oligothiophene-[all]-S,S-dioxides-based conjugated oligomers and polymers[J].J Polym Sci A Polym Chem,2011,49(9):1933-1941.
[20] CROUCH D J,SPARROWE D,HEENEY M,et al.Polyterthiophenes Incorporating 3,4-Difluorothiophene Units: Application in Organic Field-Effect Transistors[J].J Macromol Chem Phys,2010,211(24):2642-2648.
