普通干扰素-α2b联合阿德福韦酯治疗HBeAg阳性慢性乙型肝炎患者多中心随机开放对照临床观察*
2014-03-11王兵贾红宇梁柱石陆春燕盛海宋礼华
王兵,贾红宇,梁柱石,陆春燕,盛海,宋礼华
·乙型肝炎·
普通干扰素-α2b联合阿德福韦酯治疗HBeAg阳性慢性乙型肝炎患者多中心随机开放对照临床观察*
王兵,贾红宇,梁柱石,陆春燕,盛海,宋礼华
目的观察普通干扰素α-2b联合阿德福韦酯治疗HBeAg阳性慢性乙型肝炎患者的疗效和安全性。方法采用随机、开放、多中心对照临床试验研究,纳入87例HBeAg阳性慢性乙型肝炎患者,将其随机分为普通干扰素α-2b治疗组32例,给予注射600万单位普通干扰素α-2b,1次/隔日,疗程48w;阿德福韦酯治疗组27例,给予阿德福韦酯10 mg口服,1次/d,疗程72w;和联合治疗组28例,同时给予普通干扰素α-2b,48w和阿德福韦酯72w。每隔12w检测各组患者ALT、血清HBV标志物和HBV DNA水平。结果干扰素单药治疗、阿德福韦酯单药治疗和联合治疗组患者平均年龄分别为(31.8±6.6)岁、(34.2±6.4)岁和(30.5±7.2)岁,基线HBV DNA水平分别为(7.68±1.56)log10IU/ml、(7.61±2.00)log10IU/ml和(7.80±1.79)log10IU/ml,三组患者间两指标无统计学差异(P>0.05);三组间性别构成和基线ALT水平亦无显著统计学差异(P>0.05);在治疗72w时,干扰素单药治疗和阿德福韦酯单药治疗患者HBeAg转阴率分别为41%和19%,HBV DNA转阴率分别为53%和63%,ALT复常率分别为63%和67%,HBsAg血清学转换率为0.0%,均显著低于联合治疗组患者(57%、89%、93%和14%,P<0.05)。结论在病毒抑制、转氨酶复常和血清学转换率方面,普通干扰素α-2b与阿德福韦酯联合治疗HBeAg阳性慢性乙型肝炎患者72w的疗效明显优于两药单独应用的效果。
慢性乙型肝炎;普通干扰素;阿德福韦酯;血清学转换;疗效
随着核苷(酸)类似物和干扰素两种抗病毒药物在临床上的广泛使用,慢性乙型肝炎的治疗在过去的十年内获得了很大发展[1~4],但两种药物的单独治疗尚不能实现病毒清除的理想终点,即HBsAg血清学转换和HBV DNA持续阴转。目前认为,阻止病毒耐药和增强血清学转换应该最大限度地快速实现病毒学抑制[5],而抑制病毒复制和免疫控制是实现病毒清除的两种有效途径,据此建立的联合治疗策略对于病毒清除是非常重要的。根据两大类抗病毒药物的作用原理,采用干扰素和阿德福韦酯联合治疗或许可以改变病毒和宿主的免疫平衡,并相互为另一种药物增强疗效创造条件。本文主要观察了普通干扰素α2b与阿德福韦酯联合治疗的效果,现报道如下。
1 资料与方法
1.1 病例来源自2010年10月至2012年12月我们开展了一项多中心临床研究。病例来自浙江大学医学院附属第一医院、重庆医科大学附属第二医院、梧州市东湖医院、广东江门市人民医院、湖南湘雅医院、太原传染病医院、安徽中医药大学附属医院和昆明市第三医院。本研究共收集180例慢性乙型肝炎患者,经过仔细对照发现部分病例因为不符合纳入标准而被排除(表1),其中联合组排除24例,干扰素治疗组排除17例,ADV治疗组排除32例。为了保证3组治疗前病毒载量和转氨酶水平无显著性统计学差异,将联合治疗组再去除8例,干扰素组也再去除5例。在纳入的87例慢性乙型肝炎患者中,男性56例,女性31例;年龄18~55岁,平均年龄(32.1±8.2)岁。诊断符合我国2010年版“慢性乙型肝炎防治指南”的临床诊断标准,入选病例入选时血清HBeAg均为阳性,ALT处于正常值上限的2~10倍之间,HBVDNA≥1× 105copies/ml,血清总胆红素在正常值上限2倍以内。排除孕妇和哺乳期妇女、有严重心、肺和中枢神经系统疾病、自身免疫性疾病、糖尿病和合并其它肝脏疾病者。
1.2 治疗方法所有慢性乙型肝炎患者均自愿签署知情同意书。采用多中心、随机、开放、对照临床试验设计,并按照药物临床试验质量管理规范(GCP)的原则组织实施,其中联合治疗组(A组)28例,给予预充式普通干扰素α-2b[安达芬,安徽安科生物工程(集团)股份有限公司]600万单位,肌肉注射,1次/2d,阿德福韦酯(久乐,浙江福韦药业有限公司)10 mg口服,1次/d,在疗程48 w时停用干扰素,继续口服阿德福韦酯至72 w;在干扰素单药组(B组)32例患者,给予预充式普通干扰素α-2b 600万单位,肌肉注射,1次/2d,疗程48周;在阿德福韦酯单药组(C组)27例患者,仅给予阿德福韦酯口服,疗程72周。
1.3 检测方法采用ELISA法检测血清乙型肝炎病毒标志物(上海科华生物工程股份有限公司);采用日立7600020全自动生化仪检测血清ALT;采用COBAS Amplicor PCR-ELISA法定量检测血清HBV DNA水平(罗氏诊断公司,最低检测下线为500IU/ml)。各中心保留血清2.5 ml,统一送浙江大学医学院第一附属医院检测HBV DNA。
1.4 疗效评价指标比较治疗72周时三组患者在
病毒学应答率、血清学应答率和ALT复常率方面的差异。病毒学应答指血清HBV DNA检测不到(PCR法)或低于检测下限;血清学应答指血清HBeAg转阴或HBsAg转阴;生化学应答指血清ALT恢复正常。
1.5 统计学处理采用SPSS 17.0统计分析软件及Cochran’s&Mantel-Haenszel检验,比较各组之间应答率差异的显著性,定量和定性参数分别采用t检验和x2检验,在单元格期望计数小于5时采用Fisher确切概率法,P<0.05为统计学上具有显著性差异。
2 结果
2.1 治疗前入选病例基线特征比较入选的三组慢性乙型肝炎患者治疗前在年龄、性别比、基线ALT水平和病毒载量方面比较,差异无统计学意义(P>0.05,表2)。
2.2 疗效情况在治疗72周时,两药联合治疗组疗效明显高于其它两个单药治疗组,联合治疗组除HBeAg转阴率与干扰素治疗组无显著性差异外,在HBV DNA阴转和ALT复常方面均显著高于其他治疗组,有统计学意义(P<0.05,表3)。
2.3 血清HBV DNA和ALT水平动态变化情况在治疗和随访过程中,三组患者血清HBV DNA水平均呈下降趋势(图1),自24周开始,两药联合治疗患者血清HBV DNA水平明显低于单用干扰素或单用阿德福韦酯治疗组。血清ALT水平也呈下降趋势(图2),但是干扰素单药治疗患者在治疗24周时ALT水平显著高于另两个治疗组(P<0.05)。
表2 三组入选病例基线特征()比较
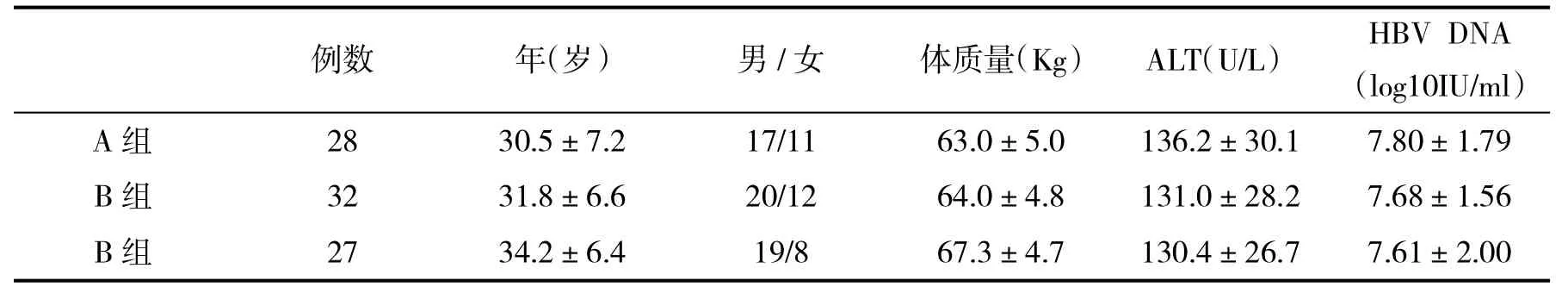
表2 三组入选病例基线特征()比较
例数年(岁)男/女体质量(Kg)ALT(U/L)HBV DNA(log10IU/ml)A组2830.5±7.217/1163.0±5.0136.2±30.17.80±1.79 B组3231.8±6.620/1264.0±4.8131.0±28.27.68±1.56 B组2734.2±6.419/867.3±4.7130.4±26.77.61±2.00

表3 三组患者疗效(%)的比较
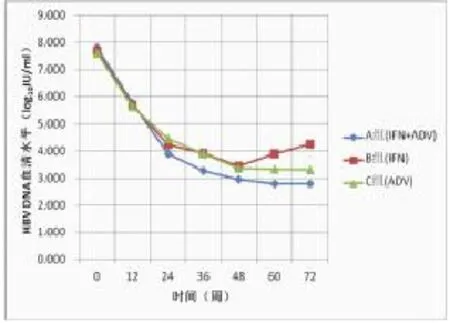
图1 三组血清HBV DNA水平下降情况

图2 三组患者血清ALT水平下降情况
3 讨论
HBeAg血清学转换通常被作为治疗应答的有效指标[6]。George et al[7~11]曾报道PEGIFNα2a联合拉米夫定治疗慢性乙型肝炎患者48周时HBeAg血清学转换率为27%,而单用PEGIFNα2a治疗的患者HBeAg血清学转换率为30%,两者相比并无显著性差异。在另一项研究中,Yalcin et al[8,12~15]比较了IFNα联合拉米夫定治疗HBeAg阳性的慢性乙型肝炎患者的疗效,对照组为单用IFNα治疗,在随访结束时联合治疗组HBeAg血清学转换率为55%,而单用IFNα组HBeAg血清学转换率仅为19%(P<0.05)。以上两项研究的区别在于前者使用长效干扰素,而后者使用普通干扰素。我们研究的结果支持后一种情况,而且,我们使用普通干扰素和ADV联合治疗48周,也显示出非常好的疗效。
在本研究,我们应用低于检测下限作为HBV DNA阴转的标志,发现联合治疗组血HBV DNA检测不出率显著低于干扰素或ADV单药治疗组,这种阴转状态在48周停用干扰素后一直维持。我们研究的结果支持其他研究的结论,例如Schalm et al[9,16~19]发现应用干扰素和核苷类联合治疗的结果都比单一用药的疗效好。约14%接受联合治疗的患者获得了HBsAg清除,这个数据稍高于单用干扰素组,也高于其它单用干扰素的研究[10,20~24],例如何艳等人[11,25~27]采用干扰素和核苷类似物联合治疗慢性乙型肝炎患者52周,结果发现HBsAg阴转高达30.1%,显著高于单用干扰素治疗患者的16.4%。陈新月等人[12]通过延长两大类药物联合治疗的时间获得26.7%HBsAg血清学转换率,这些结果支持干扰素和核苷类似物联合治疗HBeAg阳性的慢性乙型肝炎患者,以便获得较高的HBsAg血清学转换率。
[1]Lee W.Hepatitis B virus infection.N Engl J Med,1997,337: 1733-1745.
[2]Ganem D,Prince AM.Hepatitis B virus infection-natural history and clinical consequences.N Engl J Med,2004,350(11): 1118-1129.
[3]McMahon BJ.Selecting appropriate management strategies for chronic hepatitis B:who to treat.Am J Gastroenterol,2006,101: S7-S12.
[4]Liang X,Bi S,Yang W,et al.Epidemiological serosurvey of hepatitisBinChina-decliningHBVprevalencedueto hepatitis B vaccination.Vaccine,2009,27(47):6550-6657.
[5]vanNunenAB,JanssenHL.Iscombinationtherapywith lamivudine and interferon-alpha superior to monotherapy with either drug Antiviral Res,2001,52(2):139-146.
[6]Bhattacharya D,Thio CL.Review of hepatitis B therapeutics. Clin Infect Dis,2010,51(10):1201–1208.
[7]Lau GKK,Piratvisuth T.Peginterferon alfa-2a,lamivudine,and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med,2005,352:2682-2695.
[8]Yalcin K,Degertekin H,Yildiz F et al.Comparison of 12-month courses of interferon-alpha-2b-lamivudine combination therapy and interferon-alpha-2b monotherapy among patients with untreated chronic hepatitis B.Clin Infect Dis,2003,36: 1516-1522.
[9]Schalm SW,Heathcote J,Cianciara J,et al.Lamivudine and alpha interferon combination treatment of patients with chronic hepatitis B infection:a randomized trial.Gut,2000,46:562-568.
[10]Craxi A,Di Bona D,Camma C.Interferon alpha for HBeAg positive chronic hepatitis B:systemic review.J Hepatogastroenterol,2003,50:2040-2042.
[11]何艳,唐晓鹏,郑宣鹤,等.干扰素联合核苷(酸)类似物治疗慢性乙型肝炎的疗效观察.临床肝胆杂志,2013,29(2):114-119.
[12]Chen X,Cao Z,Liu Y,et al.Potent hepatitis B surface antigen response to treatment of hepatitis-B-e-antigen-positive chronic hepatitis B with α-interferon plus a nucleos(t)ide analog.J Gastroenterol Hepatol,2012,27(3):481-486.
[13]Yao GB,Cui ZY,Xu DZ,et al.A 3-year clinical trial of lamivudine in treatment of patients with chronic hepatitis B. Hepatobiliary Pancreat Dis Int,2004,3(2):188-193.
[14]彭晓谋.慢性H B V感染抗病毒治疗中转氨酶变化的规律、机理及临床意义.国外医学.流行病学传染病学分册,1992,4:156-158.
[15]Naumov NV,Mario Mondellil.Relationship between expression ofhepatitisBvirusantigensinisolatedhepatocytesand autologouslymphocytecytotoxicityinpatientswithchronic hepatitis B virus infection.Hepatology,1984,4(1):63-68.
[16]Kim HJ,Chung JH,Shin HP,et al.Polymorphisms of interferon gamma gene and risk of hepatocellular carcinoma in korean patientswithchronichepatitisBviralinfection. Hepatogastroenterology,2013,60(125):1117-1120.
[17]Caroleo B,StaltariO,GallelliL,etal.Pegylatedinterferon/ telbivudine sequential therapy in hepatitis Be antigen negative severe chronic hepatitis B patient.J Res Med Sci,2013,18(4):368-369.
[18]Lu X,Qin B,Ma Q,et al.Differential expression of ISG20 in chronic hepatitis B patients and relation to interferon-alpha therapy response.J Med Virol,2013,85(9):1506-1512.
[19]Viganò M,Mangia G,Lampertico P.Results of treatment of chronic hepatitis B with pegylated interferon.Clin Liver Dis,2013,17(3):425-443.
[20]Suh DJ,Lee HC,Byun KS,et al.Efficacy and safety of pegylated interferon-α2a in patients with lamivudine-resistant HBeAgpositive chronic hepatitis B.Antivir Ther,2013,18(6):765-773.
[21]Mahdavi M,Amirrasouli H,Alavian SM,et al.Impact of pegylated interferon-alfa-2aonperforinlevelinpatientswithchronichepatitisB: Preliminarystudy.HepatMon,2013,13(11):e11903.
[22]Huang YW,Lin SC,Wei SC,et al.Reduced Toll-like receptor 3 expression in chronic hepatitis B patients and its restoration by interferon therapy.Antivir Ther,2013,18(7):877-884.
[23]Zhao J,Fan YC,Sun FK,et al.Peripheral type I interferon receptor correlated with oxidative stress in chronic hepatitis B virus infection.J Interferon Cytokine Res,2013,33(8):405-414.
[24]Dogan UB,Golge N,Akin MS.The comparison of the efficacy of pegylated interferon α-2a and α-2b in chronic hepatitis B patients.Eur J Gastroenterol Hepatol,2013,25(11):1312-1316.
[25]Yu FX,Zhang XL,Wang YP,et al.Gene polymorphisms of interleukin-28,p21-activated protein kinases 4,and response to interferon-α based therapy in Chinese patients with chronic hepatitis B.Chin Med J(Engl),2013,126(9):1726-1731.
[26]Papatheodoridis G,Goulis J,Manolakopoulos S,et al.Changes of HBsAg and interferon-inducible protein 10 serum levels in naive HBeAg-negative chronic hepatitis B patients under 4-year entecavir therapy.J Hepatol,2014,60(1):62-68.
[27]Vlachogiannakos J,Papatheodoridis GV.HBeAg-negative chronic hepatitisB:whydoItreatmypatientswithpegylated interferon-alfa Liver Int,2014,34 Suppl 1:127-132.
[28]Kao JH.HBeAg-positive chronic hepatitis B:why do I treat my patients with pegylatedinterferon Liver Int,2014,34 Suppl 1:112-119.
[29]ZhangQ,LapalusM,AsselahT,etal.IFNL3(IL28B)polymorphism does not predict long-term response to interferon therapy in HBeAg-positive chronic hepatitis B patients.J Viral Hepat,2013Oct10.doi:10.1111/jvh.12177.[Epubaheadof print]PubMed PMID:24118626.
(收稿:2014-02-18)
(校对:陈从新)
Multi-centered randomized controlled trial of clinical observation of interferon-α2b andadefovir dip-ivoxil in the treatment of patients with HBeAg positive chronic hepatitis B
Wang Bing,Jia Hongyu,Liang Zhushi,et al.Institute of Biology,Hefei 230088,Anhui Province,China
ObjectiveTo investigate the therapeutic effect of standard interferon plus adefovir dipivoxil for patients with HBeAg-positive chronic hepatitis B.MethodsIn this randomized multi-centered controlled clinical trial,87 patients with HBeAg-positive chronic hepatitis B were randomly divided into interferon α-2b monotherapy group(32 cases,with injection of 6 million units of interferon α-2b every other days for 48 weeks),adefovir dipivoxil monotherapy group(27 cases,with oral 10 mg per day for 72 weeks)and combinational treatment group(28 cases,with interferon α-2b 6 MU every other day for 48 weeks and 10 mg of adefovir dipivoxil per day for 72 weeks).Serum ALT,serum HBV markers and HBV DNA levels in these patients were tested every 12 weeks.ResultsThe average ages of patients receiving interferon α-2b alone,adefovir dipivoxil alone or the combinational treatment were(31.8±6.6),(34.2±6.4)and(30.5±7.2)years,respectively,and the baseline serum HBV DNA levels in the three groups were(7.68±1.56)log10IU/ml,(7.61±2.00)log10IU/ml and(7.80±1.79)log10IU/ml,respectively,and there were no significant difference in average age and baseline HBV DNA level among three groups(P>0.05),either gender composition or the baseline ALT level(P>0.05);At the end of week 72,the HBeAg seroconversion rates were 41%and 19%,the rates of HBV DNA loss were 53%and 63%,the ALT normalization rates were 63%and 67%,and HBsAg seroclearance rates were 0%in interferon or adefovir dipivoxil monotherapy patients,all of which were significantly lower than those in patients receiving combinational treatment(57%,89%,93% and 14,respectively,P<0.05).ConclusionIn terms of viral suppression,ALT normalization,andHBeAg or HBsAg seroconversion rates,the therapy of standard interferon α-2b plus adefovir dipivoxil for 48 weeks is significantly better than using them alone.
ChronichepatitisB;Standard interferon;Adefovir dipivoxil;Seroconversion;Efficacy
10.3969/j.issn.1672-5069.2014.04.010
安徽省科技厅长三角联合攻关项目(编号:10140702027)
230088合肥市安徽省生物研究所(王兵,陆春燕,盛海,宋礼华);浙江大学医学院第一附属医院感染病科(贾红宇);梧州东湖人民医院(梁柱石)
王兵,男,42岁,大学本科,助理研究员。主要从事基因工程药物的研发与推广。E-mail:wancin@163.com
