ABA 相关基因抗旱基因工程研究进展
2014-01-14田孝威彭守华尉继强姜勇王志强王化琪
田孝威 彭守华 尉继强 姜勇 王志强 王化琪
(1.威海市农业科学院,威海 264200;2.烟台市福山区植保站,烟台 265500;3.中国农业大学,北京 100193)
干旱严重影响着作物的生长发育,造成作物减产,威胁着人类的生存发展。培育抗旱高产作物是全世界生物学家面临的亟待解决的课题。随着分子生物学等相关学科的发展,植物抗旱机理研究已经取得长足的进展,结合植物基因工程可以培育出抗旱转基因作物。脱落酸(Abscisic acid,ABA)合成和信号通路的基因在植物抗旱过程中发挥了巨大作用,目前已经有许多基因工程方面的相关研究(表1)。本文综述了近几年ABA 相关基因抗旱基因工程研究的一些进展。
1 ABA 与植物的抗旱性
植物内源激素之一的脱落酸,在胚胎发育、种子休眠、叶片衰老等过程中起调节作用,同时,在植物干旱等逆境胁迫过程中起着调节因子的作用。在逆境条件下,植物体内合成大量的ABA,可以促进气孔关闭,抑制气孔开放;降低叶片伸展率,调整保卫细胞离子通道;促进植物根吸收水分,增加共质体途径水流,诱导一系列基因的表达,增强植物的抗逆性[1]。逆境期间,植物体内的ABA 来源于束缚ABA 的释放和新的大量合成的ABA。干旱初期,束缚型ABA 为ABA 的主要来源,干旱期间,ABA主要来自新合成的ABA。ABA 主要在根部合成,干旱时,植物的根系感知土壤水分,诱导ABA 合成酶系统,合成大量ABA,然后运输到地上部,促使保卫细胞关闭,降低蒸腾作用,减少水分丢失。干旱胁迫时,外源喷施ABA 可以增强抗氧化酶活性,降低活性氧含量,防止叶绿素降解,增大叶片相对含水量,减小质膜透性及丙二醛含量,增强PSII 的修复功能[2-5]。
2 ABA 合成相关基因基因工程
高等植物体内的ABA 的生物合成主要有两种途径,包括直接途径和间接途径。直接途径,即3 个异戊烯单位聚合成C15 前体法呢基焦磷酸(Farnesyl pyrophospate,FPP), 由FPP 经 环 化和氧化直接形成15 碳的ABA。间接途径,即先由甲羟戊酸(MVA)聚合成C40 前体——类胡萝卜素,再由类胡萝卜素裂解成C15 的化合物,如黄质醛(Xanthoxin,XAN),最后由黄质醛转变成ABA。在高等植物体内主要通过间接途径合成ABA。反应过程中的玉米黄质环氧化酶(Zeaxanthin epoxidase,ZEP)[6]、9-顺式环氧类胡萝卜素双加氧酶(9-cisepoxycarotenoid dioxygenase,NCED)[7]、醛 氧 化 酶(Abscisic aldehyde oxidase,AAO)[8]及钼辅因子硫化 酶(Molybdenum cofactor sulfurase,MCSU)[9,10]可能是关键调控酶。
Park 等[11]在拟南芥中过表达AtZEP 基因,在盐胁迫和旱胁迫条件下转基因植株生长更旺盛,在盐胁迫条件下,转基因植株的RD29A 和Rab18 基因的表达量更高。Iuchi 等[12]在拟南芥中过表达AtNCED3 基因,增加了内源ABA 含量,降低蒸腾速率,增加了转基因植株的抗旱性。Qin 和Zeevaart[13]将菜豆PvNCED 基因转化烟草,增加了烟草植株的抗旱性。徐春英等[14]将35S 启动子、诱导型RD29A 启动子以及气孔特异表达KST1 启动子与AtNCED3 基因转化烟草,得到了抗旱性增强的转基因植株,其中35S 启动子连接AtNCED3 基因转化烟草,转基因植株生长矮小,发育滞后;RD29 转基因植株生长比野生型慢一点,而KST1 转基因植株表型最好,在正常条件下生长旺盛。Aswath 等[15]将豇豆VuNCED1 基因转化匍匐剪股颖,盐和旱胁迫增加了内源ABA 的含量,转基因植株在盐胁迫和旱胁迫处理后具有较高的存活率。Wan 等[16]将花生AhNCED1 基因转化拟南芥增强了转基因植株的ABA含量和耐旱性。贾娟娟等[17]将诱导型启动子rd29A和AtNCED3 基因转化水稻,增强了转基因水稻的抗旱性。Zhang 等[18]将SgNCED1 基因转化烟草,发现降低了转基因植株的气孔导度,蒸腾速率,增强了其抗氧化酶活性,增加了其H2O2和NO 浓度,增强了其耐旱性和耐盐性。Zhang 等[19]将SgNCED1基因转化烟草增加了转基因植株的抗旱性和耐盐性。Hwang[20]将水稻OsNCED3 基因转化拟南芥,增加ABA 的积累,降低水分丢失,推迟种子萌发,增强了抗旱性。Xiao 等[21]将LOS5 基因与Actin1 启动子和HVA22 胁迫诱导启动子连接转化水稻发现,在大田和PVC 管道旱胁迫条件下,Actin1∶LOS5 和HVA22P:LOS5 转基因植株的相对产量和穗育性高于对照。Yue 等[22]将拟南芥LOS5/ABA3 基因转化烟草,在正常条件下,转基因植株离体叶片蒸腾失水率低于对照,在缺水条件下,与对照相比转基因植株萎蔫程度更低,保持更高的水含量,具有更好的细胞膜完整性,积累较多的ABA 和脯氨酸,具有较高的抗氧化物酶(超氧化物歧化酶、过氧化氢酶、过氧化物酶和抗坏血酸过氧化物酶)活性。Yue等[23]在棉花中过表达拟南芥AtLOS5 基因,该基因编码的钼辅因子为醛氧化酶(AAO)所必须,在旱胁迫条件下,增加了转基因植株体内的ABA 和脯氨酸含量,增强了旱反应基因P5CS 和RD22 的表达,提高了抗氧化胁迫酶的活性和膜的完整性,以及生物产量。Li 等[24]将拟南芥LOS5/ABA3 基因转化大豆发现,在干旱条件下增加了AAO 活性和ABA 的积累,降低气孔大小和蒸腾速率,减小叶片萎蔫,维持较高的水含量,通过降低电解质渗出率和MDA含量减小了细胞膜的损害,促进脯氨酸积累,增加了抗氧化酶活性,增强胁迫相关基因表达,增加了大田干旱条件下籽粒产量。Lu 等[25]在玉米中过表达拟南芥LOS5 基因,增加ZmAO 和AO 活性,增加ABA 的积累,减少水分丢失,维持较高的水含量和叶片水势,在干旱条件下,转基因植株表现出较低的叶片萎蔫度,较低的电解质渗出率,较低的MDA和H2O2含量,较高的抗氧化酶活性和脯氨酸含量,同时,也增加了胁迫调控基因的表达,复水后增加根的生物产量(表1)。
3 ABA 信号通路相关基因的基因工程
ABA 通过与受体结合发挥作用。在拟南芥中,PYR/PYL/RCARs,蛋白磷酸酶2C(protein phosphatase type-2c,PP2Cs)和SNF1 相关蛋白激酶2(Suc-rose non-fermenting 1-related protein kinase 2,SnRK2s)3 个蛋白家族组成ABA 的核心信号模式[26]。PYR/PYL/RCARs 是ABA 受体,PP2Cs 是信号通路的负调控者,SnRK2s 是下游信号的正调控者[27,28]。PP2Cs抑 制SnRK2s 活 性,ABA 与PYR/PYL/RCARs 结合抑制PP2Cs 的活性,进而SnRK2s 的活性增强,SnRK2s 活性增强可以磷酸化下游bZIP 转录因子[29]。
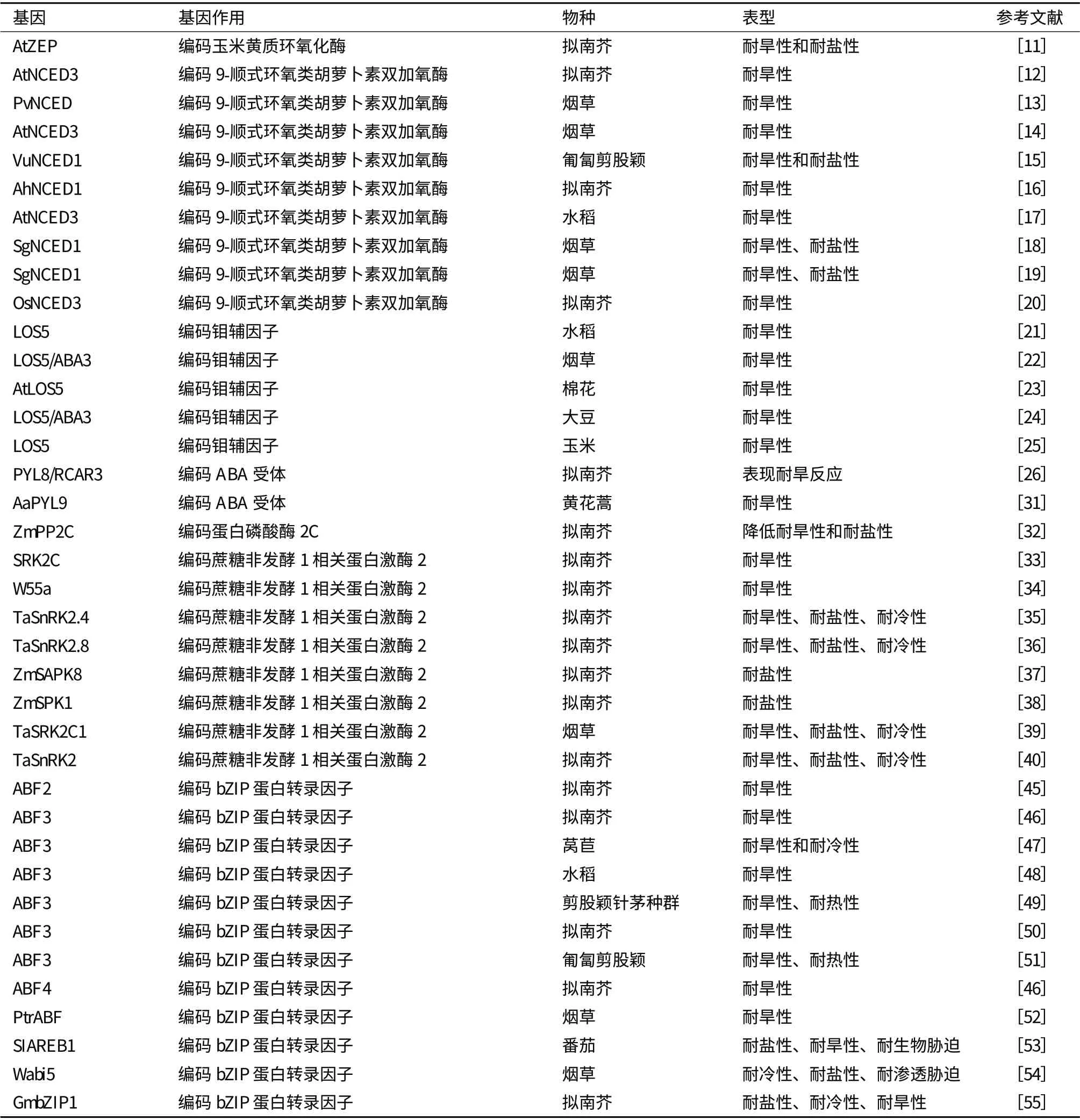
表1 ABA 相关基因与抗旱基因工程
Saavedra 等[30]研究发现过表达PYL8/RCAR3 基因导致拟南芥种子对ABA 超敏感,增强了种子的休眠,在低浓度的甘露醇、NaCl 或多效唑处理下不能正常萌发,在植物组织中,转基因植株表现耐旱反应和早期根生长的抑制,35S∶FsPP2C1 转基因植株中出现相反的表型,说明PYL8/RCAR3 在萌发和非生物胁迫反应中起正调控作用。Zhang 等[31]将黄花蒿AaPYL9 基因转化黄花蒿增强了其抗旱性和ABA处理后青蒿素的含量。Liu 等[32]将玉米ZmPP2C基因转化拟南芥,降低了植物对盐胁迫和旱胁迫的抗性,表现为较低的净光合速率和脯氨酸含量,较高的MDA 水平和相对膜透性,胁迫相关基因转录水平下降,对ABA 不敏感。Umezawa 等[33]将一个SnRK2 基因SRK2C 转化拟南芥发现,诱导了一系列胁迫应答基因的表达,增强了转基因植株的耐旱性。Xu 等[34]将小麦中SnRK2 家族的一个成员W55a 转化拟南芥增强了转基因植株的抗旱性。Mao 等[35]将小麦TaSnRK2.4 基因转化拟南芥,发现转基因植株在正常条件下幼苗推迟发育,有较长的初生根,有较高的产量;具有较强的耐旱性、耐盐性、耐冷性,表现为降低水分丢失,增强相对水含量和膜的稳定性,增强光合势,显著增强渗透势。Zhang 等[36]将小麦的SnRK2 家族的一个成员TaSnRK2.8 转化拟南芥,增强了转基因植株的耐旱性、耐盐性及耐冷性,表现为胁迫下转基因植株具有较长的初生根,较高的相对含水量,更强的细胞膜稳定性,显著更低的渗透势,更多的叶绿素含量,增强的PSII 活性。Ying 等[37]发现玉米中SnRK2 家族的一个成员ZmSAPK8 在玉米植株的很多器官中表达并且在高盐和干旱处理被上调表达,转化拟南芥发现可以显著增强抗盐性,在盐胁迫下,转基因植株具有更高的萌发率和脯氨酸含量,并且未发现生长异常的转基因植株。Zou 等[38]将玉米中SnRK2 家族的一个成员ZmSPK1 转化拟南芥发现,在盐胁迫条件下,转基因植株生长较好,有较高的幼苗鲜重和干重,脯氨酸含量以及SOD 活性,有较低的MDA 含量和较低的相对电导率,增强了其耐盐性。Du 等[39]将小麦SnRK2 基因——TaSRK2C1 转化烟草,发现上调了调控因子RD29a、DREB1A 和DREB2 的表达,增加了自由脯氨酸和可溶性碳水化合物的含量,增强了对盐胁迫、脱水胁迫和低温胁迫的抗性。Tian 等[40]发现将小麦TaSnRK2 基因转化拟南芥促进了转基因植株根系的发育,增强了对干旱胁迫、盐胁迫、冷胁迫的抗性,增加了非生物胁迫相应基因的表达,改善了生理指标,包括降低水分丢失,增加细胞膜的稳定性,提高光合势,显著增强渗透势和游离脯氨酸含量。
目前,已经发现了许多ABA 调节基因表达的顺式作用元件(ABA-responsive element,ABRE),以及与这些顺式作用元件相互作用的转录因子[41,42]。ABFs(ABRE binding factors)是最近发现的一种ABRE 结合bZIP 蛋白[43,44]。过表达ABF 家族的成员——ABF2[45]、ABF3[46-51]、ABF4[52]、PtrABF[53]、SIAREB1[54]、Wabi5[55]和GmbZIP1[56]增强了拟南芥、莴苣、水稻、番茄、匍匐剪股颖和烟草的抗旱性。
4 展望
目前,ABA 相关抗旱基因工程已取得一定的成绩,但同时存在一些问题。具体的问题和主要对策有:(1)目前克隆出的ABA 合成和信号通路的基因有限,今后需要克隆更多的基因用于植物抗旱基因工程;(2)目前植物抗旱基因工程多为单基因转化,而植物的抗旱性是多基因共同作用的数量性状,因此多基因转化能更显著地提高作物的抗旱性;(3)35S 启动子和目的基因转化植物常常产生畸形植株,将胁迫诱导表达启动子、根特异表达启动子等连接抗旱基因转化植物可以避免畸形植株的产生,减少能量消耗;(4)目前基因工程抗旱性鉴定多为温室和实验室条件下的鉴定,而转基因作物应用于生产是在大田自然干旱条件下,因此今后需要进行转基因作物大田自然干旱条件下的抗旱性鉴定,同时植物的抗旱性最终表现在产量上,因此需要对产量指标进行鉴定。
[1] Morillon R, Chrispeels MJ. The role of ABA and the transpiration stream in the regulation of the osmotic water permeability of leaf cells[J]. Proc Natl Acad Sci USA, 2001, 98(24):14138-14143.
[2] 李长宁, Srivastava MK, 农倩, 等. 水分胁迫下外源ABA 提高甘蔗抗旱性的作用机制[J]. 作物学报, 2010, 36(5):863-870.
[3] 木合塔尔·扎热, 齐曼·尤努斯, 山中典和. 干旱胁迫下外源脱落酸和硅对沙枣幼苗叶片水势及保护酶活性的影响[J]. 植物研究, 2010, 30(4):468-472.
[4] 胡秀丽, 杨海荣, 李潮海. ABA 对玉米响应干旱胁迫的调控机制[J]. 西北植物学报, 2009, 29(11):2345-2351.
[5] 汪月霞, 索标, 赵腾飞, 等. 外源ABA 对干旱胁迫下不同品种灌浆期小麦psbA 基因表达的影响[J]. 作物学报, 2011, 37(8):1372-1377.
[6] Marin E, Nussaume L, Quesada A, et al. Molecular identification of zeaxanthin epoxidase of Nicotiana plumbaginifolia, a gene involved in abscisic acid biosynthesis and corresponding to the ABA locus of Arabidopsis thaliana[J]. EMBO J, 1996, 15(10):2331-2342.
[7] Schwartz SH, Tan BC, Gage DA, et al. Specific oxidative cleavage of carotenoids by VP14 of maize[J]. Science, 1997, 276(5320):1872-874.
[8] Seo M, Koiwai H, Akaba S, et al. Abscisic aldehyde oxidase in leaves of Arabidopsis thaliana[J]. Plant J, 2000, 23(4):481-488.
[9] Bittner F, Oreb M, Mendel RR. ABA3 is a molybdenum cofactor sulfurase required for activation of aldehyde oxidase and xanthine dehydrogenase in Arabidopsis thaliana[J]. J Biol Chem, 2001, 276(44):40381-40384.
[10] Xiong LM, Ishitani M, Lee HJ, et al. The Arabidopsis LOS5/ABA3 locus encodes a molybdenum cofactor sulfurase and modulates cold stress-and osmotic stress-responsive gene expression[J]. Plant Cell, 2001, 13(9):2063-2083.
[11] Park HY, Seok HY, Park BK, et al. Overexpression of Arabidopsis ZEP enhances tolerance to osmotic stress[J]. Biochem Biophys Res Commun, 2008, 375(1):80-85.
[12] Iuchi S, Kobayashi M, Taji T, et al. Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis[J]. Plant J, 2001, 27(4):325-333.
[13] Qin XQ, Zeevaart JAD. Overexpression of a 9-cis-epoxycarotenoid dioxygenase gene in Nicotiana plumbaginifolia increases abscisic acid and phaseic acid levels and enhances drought tolerance[J]. Plant Physiol, 2002, 128(2):544-551.
[14] 徐春英. 应用气孔特异性启动子—KST1 改良植物抗旱性的初步探讨[D].北京:中国农业大学, 2004:48-50.
[15] Aswath CR, Kim SH, Mo SY, et al. Transgenic plants of creeping bentgrass harboring the stress inducible gene, 9-cis-epoxycarotenoid dioxygenase, are highly tolerant to drought and NaCl stress[J]. Plant Growth Regul, 2005, 47(2-3):129-139.
[16] Wan XR, Li L. Regulation of ABA level and water-stress tolerance of Arabidopsis by ectopic expression of a peanut 9-cisepoxycarotenoid dioxygenase gene[J].Biochem Biophys Res Commun, 2006, 347(4):1030-1038.
[17] 贾娟娟, 孟秀萍, 刘蕊, 等.拟南芥NCED3 基因在水稻种的过量表达可以提高水稻的干旱胁迫耐受性[J].复旦学报:自然科学版, 2008, 47(3):288-294.
[18] Zhang YM, Yang JF, Lu SY, et al. Overexpressing SgNCED1 in tobacco increases ABA level, antioxidant enzyme activities, and stress tolerance[J]. J Plant Growth Regul, 2008, 27(2):151-158.
[19] Zhang YM, Tan JL, Guo ZF, et al. Increased abscisic acid levels in transgenic tobacco over-expressing 9 cis-epoxycarotenoid dioxygenase influence H2O2 and NO production and antioxidant defences[J]. Plant Cell Environ, 2009, 32(5):509-519.
[20] Hwang SG, Chen HC, Huang WY, et al. Ectopic expression of rice OsNCED3 in Arabidopsis increases ABA level and alters leaf morphology[J]. Plant Sci, 2010, 178(1):12-22.
[21] Xiao BZ, Chen X, Xiang CB, et al. Evaluation of seven functionknown candidate genes for their effects on improving drought resistance of transgenic rice under field conditions[J]. Mol Plant, 2009, 2(1):73-83.
[22] Yue Y, Zhang M, Zhang J, et al. Arabidopsis LOS5/ABA3 overexpression in transgenic tobacco(Nicotiana tabacum cv. Xanthi-nc)results in enhanced drought tolerance[J]. Plant Sci, 2011, 181(4):405-411.
[23] Yue YS, Zhang MC, Zhang JC, et al. Overexpression of the AtLOS5 gene increased abscisic acid level and drought tolerance in transgenic cotton[J]. J Exp Bot, 2012, 63(10):3741-3748.
[24] Li YJ, Zhang JC, Zhang J, et al. Expression of an Arabidopsis molybdenum cofactor sulphurase gene in soybean enhances drought tolerance and increases yield under field conditions[J]. Plant Biotechnology J, 2013, 11(6):747-758.
[25] Lu Y, Li YJ, Zhang JC, et al. Overexpression of Arabidopsis molybdenum cofactor sulfurase gene confers drought tolerance in maize(Zea mays L.)[J]. Plos One, 2013, 8(1):e52126.
[26] Agarwal PK, Jha B. Transcription factors in plants and ABA dependent and independent abiotic stress signalling[J]. Biologia Plantarum, 2010, 54(2):201-212.
[27] Phang TH, Shao GH, Lam HM. Salt tolerance in soybean[J]. Joumal of Integrative Plant Biology, 2008, 50(10):1196-1212.
[28] Katharine EH, Noriyuki N, Kenichi H, et al. Early abscisic acid signal transduction mechanisms:newly discovered components and newly emerging questions[J]. Genes Dev, 2010, 24(16):1695-1708.
[29] Raghavendra AS, Gonugunta VK, Christmann A, et al. ABA perception and signalling[J]. Trends Plant Sci, 2011(15):395-401.
[30] Saavedra X, Modrego A, Rodriguez D, et al. The nuclear interactor PYL8/RCAR3 of Fagus sylvatica FsPP2C1 is a positive regulator of abscisic acid signaling in seeds and stress[J]. Plant Physiol, 2010, 152(1):133-150.
[31] Zhang FY, Lu X, Lv ZY, et al. Overexpression of the Artemisia orthologue of ABA receptor, AaPYL9, enhances ABA sensitivity and improves artemisinin content in Artemisia annua L.[J]. Plos One, 2013, 8(2):e56697.
[32] Liu, LX, Hu, XL, Song, JA, et al. Over-expression of a Zea mays L. protein phosphatase 2C gene(ZmPP2C)in Arabidopsis thaliana decreases tolerance to salt and drought[J]. J Plant Physiol, 2009, 166(5):531-542.
[33] Umezawa T, Yoshida R, Maruyama K, et al. SRK2C, a SNF1-related protein kinase 2, improves drought tolerance by controlling stress-responsive gene expression in Arabidopsis thaliana[J]. Proc Natl Acad Sci USA, 2004, 101(49):17306-17311.
[34] Xu ZS, Liu L, Ni ZY, et al. W55a Encodes a novel protein kinase that is involved in multiple stress responses[J]. J Integr Plant Biol, 2009, 51(1):58-66.
[35] Mao XG, Zhang HY, Tian SJ, et al. TaSnRK2.4, an SNF1-type serine/threonine protein kinase of wheat(Triticum aestivum L.), confers enhanced multistress tolerance in Arabidopsis[J]. J Exp Bot, 2010, 61(3):683-696.
[36] Zhang HY, Mao XG, Wang CS, et al. Overexpression of a common wheat gene TaSnRK2.8 enhances tolerance to drought, salt and low temperature in Arabidopsis[J]. Plos One, 2010, 5(12):e16041.
[37] Ying S, Zhang DF, Li HY, et al. Cloning and characterization of a maize SnRK2 protein kinase gene confers enhanced salt tolerance in transgenic Arabidopsis[J]. Plant Cell Rep, 2011, 30(9):1683-1699.
[38] Zou HW, Li CH, Liu HF, et al. Zmspk1, a member of plant snrk2 subfamily in maize enhances tolerance to salt in transgenic Arabidopsis[J]. Aust J Crop Sci, 2011, 5(10):1179-1184.
[39] Du XM, Zhao XL, Li XJ, et al. Overexpression of TaSRK2C1, a wheat SNF1-related protein kinase 2 gene, increases tolerance to dehydration, salt, and low temperature in transgenic tobacco[J]. Plant Mol Biol Rep, 2013, 31(4):810-821.
[40] Tian SJ, Mao XG, Zhang HY, et al. Cloning and characterization of TaSnRK2.3, a novel SnRK2 gene in common wheat[J]. J Exp Bot, 2013, 64(7):2063-2080.
[41] Busk PK, Pages M. Regulation of abscisic acid-induced transcription[J]. Plant Mol Biol, 1998, 37(3):425-435.
[42] Rock C. Pathways to abscisic acid-regulated gene expression[J]. New Phytol, 2000, 148(3):357-396.
[43] Choi HI, Hong JH, Ha JO, et al. ABFs, a family of ABA-responsive element binding factors[J]. Biol Chem, 2000, 275(3):1723-1730.
[44] Uno Y, Furihata T, Abe H, et al. Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions[J]. Proc Natl Acad Sci USA, 2000, 97(21):11632-11637.
[45] Kim S, Kang SY, Cho DI, et al. ABF2, an ABRE-binding bZIP factor, is an essential component of glucose signaling and its overexpression affects multiple stress tolerance[J]. Plant J, 2004, 40(1):75-87.
[46] Kang JY, Choi HI, Im MY, et al. Arabidopsis basic leucine zipper proteins that mediate stress-responsive abscisic acid signaling[J]. Plant Cell, 2002, 14(2):343-357.
[47] Vanjildorj E, Bae TW, Riu KZ, et al. Overexpression of Arabidopsis ABF3 gene enhances tolerance to drought and cold in transgenic lettuce(Lactuca sativa)[J]. Plant Cell Tiss Org Cult, 2005, 83(1):41-50.
[48] Oh SJ, Song SI, Kim YS, et al. Arabidopsis CBF3/DREB1A and ABF3 in transgenic rice increased tolerance to abiotic stress without stunting growth[J]. Plant Physiol, 2005, 138(1):341-351.
[49] Vanjildorj E, Bae TW, Riu KZ, et al. Transgenic Agrostis mongolica Roshev. with enhanced tolerance to drought and heat stresses obtained from Agrobacterium-mediated transformation[J]. Plant Cell Tiss Org, 2006, 87(2):109-120.
[50] Abdeen A, Schnell J, Miki B. Transcriptome analysis reveals absence of unintended effects in drought-tolerant transgenic plants overexpressing the transcription factor ABF3[J]. BMC Genomics, 2010, 11:69.
[51] Choi YS, Kim YM, Hwang OJ, et al. Overexpression of Arabidopsis ABF3 gene confers enhanced tolerance to drought and heat stress in creeping bentgrass[J]. Plant Biotechnol Rep, 2013, 7(2):165-173.
[52] Huang XS, Liu JH, Chen XJ. Overexpression of PtrABF gene, a bZIP transcription factor isolated from Poncirus trifoliata, enhances dehydration and drought tolerance in tobacco via scavenging ROS and modulating expression of stress-responsive genes[J]. BMC Plant Biol, 2010, 10:230.
[53] Orellana S, Yanez M, Espinoza A, et al. The transcription factor SIAREB1 confers drought, salt stress tolerance and regulate biotic and abiotic stress-related genes in tomato[J]. Plant, Cell and Environ, 2010, 33(12):2191-2208.
[54] Kobayashi F, Maeta E, Terashima A, et al. Positive role of a wheat HvABI5 ortholog in abiotic stress response of seedlings[J]. Physiol Plant, 2008, 134(1):74-86.
[55] Gao SQ, Chen M, Xu ZS, et al. The soybean GmbZIP1 transcription factor enhances multiple abiotic stress tolerances in transgenic plants[J]. Plant Mol Biol, 2011, 75(6):537-553.
