甘西鼠尾草化学成分研究
2013-12-23朱路平庄文婷李宝才
朱路平,向 诚,庄文婷,何 静,李 鹏,2,李宝才*
1昆明理工大学生命科学与技术学院,昆明650500;2 澳门大学中华医药研究院中药质量研究国家重点实验室,澳门000856
甘西鼠尾草(Salvia przewalskii Maxim)为唇形科鼠尾草属弧隔鼠尾草亚属植物,又名大紫丹参、紫丹参、甘肃丹参、秦红艽,主要分布于我国甘肃西部、四川西部、云南西北部及西藏等地[1]。甘西鼠尾草在我国是广泛使用的中草药,民间多用其根入药,四川作秦艽的代用品,云南作丹参代用品[2]。其具有与丹参相似的药理活性,具有抗菌、消炎、扩冠、抗脂质过氧化、抗血小板凝聚等作用[3,4]。研究表明甘西鼠尾草中二萜醌类化合物是产生这些药理活性的主要物质基础,所以进一步从甘西鼠尾草中寻找二萜活性成分,对其开发和利用提供理论依据具有重要意义。为此,笔者对云南丽江产甘西鼠尾草的化学成分进行了研究,初步分离出12 个化合物,分别鉴定为:丹参酮ⅡA(1)、丹参酮Ⅰ(2)、丹参内酯(3)、隐丹参酮(4)、丹参酸甲酯(5)、间羟基苯甲醛(6)、迷迭香酚(7)、异迷迭香酚(8)、紫丹参甲素(9)、紫丹参乙素(10)、二氢丹参酮Ⅰ(11)、丹参新醌甲(12)。其中,化合物7、8 为首次从该植物中分离得到,化合物6 为首次从该属植物中分离得到。
1 实验部分
1.1 仪器与材料
Brucker AV-400 和DRX-500 超导核磁共振仪;API QSTAR Pulsar 质谱仪;Agilent 1200 高效液相色谱仪;LC3000 型高效液相色谱仪(北京创新通恒科技有限公司);Sephadex LH-20 (20~100 μm,Pharmacia Fine Chemical Co.,Ltd.);MCI gel CHP20P(75~150 μm,Mitsubishi Chemical Co.,Ltd.);Rp-18(40~50 μm,Merk Co.,Ltd);柱层析硅胶(200~300目)、薄层层析硅胶(GF254)均为青岛海洋化工厂产品;显色剂:5%硫酸-乙醇溶液;柱层析溶剂均为工业级重蒸溶剂。
甘西鼠尾草药材于2010 年8 月采自云南省丽江市,由云南省中医中药研究院郭世民研究员鉴定为唇形科植物甘西鼠尾草(Salvia przewalskii)。样本现存于昆明理工大学生命科学与技术学院天然产物制药实验室。
1.2 提取与分离
干燥甘西鼠尾草(全草)药材7.3 kg,粉碎后过60 目筛,用100%丙酮(每次20 L)室温下超声提取三次,每次2 h,提取液减压浓缩得粗浸膏300 g。所得浸膏经硅胶柱色谱石油醚-乙酸乙酯梯度(1∶0、9∶1、3∶1、1∶1、3∶7)洗脱,TLC 检测合并得5 个部分Fr.1~5。Fr.2(42 g)经硅胶柱层析,石油醚-乙酸乙酯梯度(20∶1、15∶1、10∶1、8∶1、5∶1)洗脱,石油醚-乙酸乙酯(10∶1)洗脱部分反复上硅胶柱得到化合物1(1.8 g)、化合物2(20 mg),石油醚-乙酸乙酯(8∶1)洗脱部分反复上硅胶柱得到化合物3(10 mg);Fr.3(30 g)经MCI 柱层析MeOH-H2O(65%、75%、85%)梯度洗脱,75%MeOH-H2O 洗脱部分反复硅胶柱色谱和Sephadex LH-20 纯化得化合物4(8 g)、化合物5(26 mg);Fr.4(14 g)经MCI 柱层析MeOHH2O(50%、70%、80%、90%)梯度洗脱,50%MeOHH2O 洗脱部分反复硅胶柱色谱得化合物6(10 mg),70%MeOH-H2O 洗脱部分反复硅胶柱色谱和Sephadex LH-20 纯化得化合物7(16 mg)、化合物8(50 mg),80%MeOH-H2O 洗脱部分反复Sephadex LH-20纯化后,利用半制备液相色谱制备得化合物9(200 mg)、化合物10(84 mg);Fr.5(20 g)经Rp-18 反相柱层析MeOH-H2O(40%、70%、80%、90%)梯度洗脱划分为两个组分,组分2 经反复硅胶柱色谱、Rp-18 反相柱层析及Sephadex LH-20 纯化得化合物11(60 mg),化合物12(124 mg)。
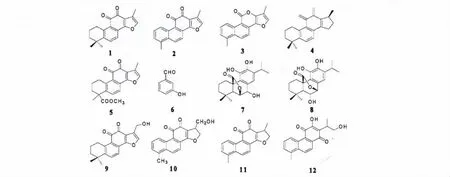
图1 化合物1~12 的结构式Fig.1 Chemical structures of compounds 1-12
2 结构鉴定
化合物1 红色粉末(氯仿),mp:201~202℃,ESI-MS m/z:295[M+H]+;1H NMR (400 MHz,CDCl3)δ:1.80 (2H,m,H-1),1.66 (2H,m,H-2),3.19 (2H,m,H-3),7.64 (1H,d,J =6.4 Hz,H-6),7.55 (1H,d,J=6.4 Hz,H-7),7.26 (1H,s,H-16),2.26 (3H,s,Me-17),1.31 (6H,s,Me-18,Me-19);13C NMR (100 MHz,CDCl3)δ:29.8 (C-1),19.3(C-2),37.8 (C-3),34.9 (C-4),150.1 (C-5),133.5 (C-6),120.2 (C-7),127.4 (C-8),126.4 (C-9),144.6 (C-10),183.6 (C-11),175.7 (C-12),121.1(C-13),161.7 (C-14),120.3 (C-15),141.2(C-16),8.8 (C-17),31.8 (C-18,C-19)。以上波谱数据与文献[5]报道基本一致,故鉴定化合物1 为丹参酮ⅡA。
化合物2 紫红色粉末(氯仿),ESI-MS m/z:277[M +H]+;1H NMR(400 MHz,CDCl3)δ:9.26(1H,d,J = 8.8 Hz,H-1),7.56 (1H,dd,J = 7.0,8.8 Hz,H-2),7.36 (1H,d,J = 7.0 Hz,H-3),8.31(1H,d,J = 8.8 Hz,H-6),7.82 (1H,d,J = 8.8 Hz,H-7),7.28 (1H,s,H-16),2.31 (3H,s,Me-17),2.70 (3H,s,Me-18);13C NMR (100 MHz,CDCl3)δ:124.7(C-1),130.6 (C-2),128.4 (C-3),135.2(C-4),133.7 (C-5),132.8 (C-6),118.7 (C-7),129.7 (C-8),123.2 (C-9),132.9 (C-10),183.5(C-11),175.6 (C-12),121.7 (C-13),161.2 (C-14),120.4 (C-15),142.0 (C-16),8.8 (C-17),19.8 (C-18)。以上波谱数据与文献[6]报道基本一致,故鉴定化合物2 为丹参酮Ⅰ。
化合物3 无色针状结晶(氯仿),EI-MS m/z:264([M]+,100),263 (33),208 (25),184 (24),165 (20);1H NMR (400 MHz,CDCl3)δ:8.34 (1H,d,J = 8.2 Hz,H-1),7.45(1H,dd,J = 8.2,8.2 Hz,H-2),7.35 (1H,d,J = 8.2 Hz,H-3),7.80(1H,d,J = 8.8 Hz,H-6),7.74 (1H,d,J = 8.8 Hz,H-7),7.37 (1H,s,H-16),2.36 (3H,s,Me-17),2.63 (3H,s,Me-18);13C NMR (100 MHz,CDCl3)δ:120.3 (C-1),126.9 (C-2),128.8 (C-3),134.5(C-4),123.5 (C-5),120.7 (C-6),116.6 (C-7),110.2 (C-8),107.9 (C-9),133.1 (C-10),158.6(C-11),158.6 (C-13),149.5 (C-14),141.0 (C-15),120.3 (C-16),8.5 (C-17),19.5 (C-18)。以上波谱数据与文献[6]报道基本一致,故鉴定化合物3 为丹参内酯。
化合物4 红色粉末(氯仿),mp:196~197 ℃,ESI-MS m/z:297 [M + H]+;1H NMR (400 MHz,CDCl3)δ:3.15 (2H,m,H-1),1.73 (2H,m,H-2),1.65 (2H,m,H-3),7.64 (1H,d,J = 8.0 Hz,H-6),7.49 (1H,d,J = 8.0 Hz,H-7),3.52 (1H,m,H-15),4.84 (1H,t,J = 9.2 Hz,H-16α),4.35(1H,dd,J = 6.0,9.2 Hz,H-16β),1.17 (3H,s,Me-17),1.32 (3H,s,Me-18),1.32 (3H,s,Me-19);13C NMR (100 MHz,CDCl3)δ:29.6 (C-1),19.0 (C-2),37.7 (C-3),34.7 (C-4),152.3 (C-5),132.6(C-6),122.5 (C-7),128.3 (C-8),126.8 (C-9),143.7 (C-10),184.2 (C-11),175.6 (C-12),118.4(C-13),170.8 (C-14),34.5 (C-15),81.4 (C-16),18.8 (C-17),31.9 (C-18),31.8 (C-19)。以上波谱数据与文献[6]报道基本一致,故鉴定化合物4 为隐丹参酮。
化合物5 红色粉末(氯仿),mp:174~176 ℃,ESI-MS m/z:339[M + H]+;1H NMR (400 MHz,CDCl3)δ:3.24 (2H,t,J = 7.6 Hz,H-1),1.80(2H,m,H-2),1.74(2H,m,H-3),7.58 (1H,d,J =8.1 Hz,H-6),7.49 (1H,d,J = 8.1 Hz,H-7),7.26(1H,d,J = 8.1 Hz,H-16),2.26 (3H,s,Me-17),1.58 (3H,s,Me-18),3.67 (3H,s,Me-20);13C NMR(100 MHz,CDCl3)δ:29.0 (C-1),19.1 (C-2),33.9(C-3),47.1 (C-4),144.3 (C-5),134.9 (C-6),120.3 (C-7),128.5 (C-8),126.4 (C-9),143.0 (C-10),183.3 (C-11),175.5 (C-12),121.2 (C-13),161.2 (C-14),120.2 (C-15),141.5 (C-16),8.7(C-17),27.5 (C-18),177.0 (C-19),52.5 (C-20)。以上波谱数据与文献[7]报道基本一致,故鉴定化合物5 为丹参酸甲酯。
化合物6 白色粉末(氯仿),mp:103~104 ℃,ESI-MS m/z:107[M + H]+;1H NMR (400 MHz,CDCl3)δ:9.86(1H,s,-CHO),7.33~7.31 (2H,m,H-5,H-6),7.28(1H,d,J = 1.6 Hz,H-2),7.07(1H,dd,J = 8.4,1.6 Hz,H-4);13C NMR (100 MHz,CDCl3)δ:141.5 (C-1),119.0(C-2),149.37(C-3),126.4(C-4,C-6),130.1 (C-5),197.2 (-CHO)。以上波谱数据与文献[8]报道基本一致,故鉴定化合物6 为间羟基苯甲醛。
化合物7 白色粉末(甲醇),mp:240.0~242℃,ESI-MS m/z:347[M+H]+;1H NMR (400 MHz,MeOD)δ:3.15 (1H,br. d,J = 14 Hz,H-1β),1.95(1H,m,H-1α),1.57 (1H,m,H-2β),1.68 (1H,m,H-2α),1.22 (1H,m,H-3β),1.45 (1H,br. d,J =14 Hz,H-3α),2.05 (1H,s,H-5),4.52 (1H,d,J =3.0 Hz,H-6),4.40 (1H,d,J = 3.0 Hz,H-7),6.79(1H,s,H-14),3.00 (1H,sept,J = 7.0 Hz,H-15),1.01 (3H,d,J = 7.0 Hz,Me-16),1.12 (3H,d,J =6.8 Hz,Me-17),0.91 (3H,s,Me-18),0.98 (3H,s,Me-19);13C NMR (100 MHz,MeOD)δ:27.1 (C-1),18.9 (C-2),37.9 (C-3),31.6 (C-4),55.4 (C-5),74.7 (C-6),78.2 (C-7),123.5 (C-8),127.5(C-9),47.6 (C-10),142.8 (C-11),143.1 (C-12),134.5 (C-13),118.5 (C-14),27.4 (C-15),22.3(C-16),22.7 (C-17),21.9 (C-18),31.8 (C-19),178.9 (C-20)。以上波谱数据与文献[9]报道基本一致,故鉴定化合物7 为迷迭香酚。
化合物8 白色粉末(吡啶),1H NMR (400 MHz,C5D5N)δ:3.15 (1H,br. d,J = 14Hz,H-1β),1.97 (1H,m,H-1α),1.55 (1H,m,H-2β),1.69(1H,m,H-2α),1.21 (1H,m,H-3β),1.46 (1H,br. d,J = 14 Hz,H-3α),2.15 (1H,s,H-5),4.48(1H,d,J = 4.5 Hz,H-6),5.12 (1H,d,J = 4.5 Hz,H-7),6.79 (1H,s,H-14),3.00 (1H,sept,J =7.0 Hz,H-15),1.01 (3H,d,J = 7.0 Hz,Me-16),1.13 (3H,d,J = 6.8 Hz,Me-17),0.92 (3H,s,Me-18),0.97 (3H,s,Me-19);13C NMR (100 MHz,C5D5N)δ:28.8 (C-1),20.9 (C-2),39.5 (C-3),33.1 (C-4),56.7 (C-5),72.0 (C-6),81.4 (C-7),132.4 (C-8),126.6 (C-9),49.9 (C-10),146.5 (C-11),145.5 (C-12),138.0 (C-13),120.0 (C-14),29.8 (C-15),24.1 (C-16),24.5 (C-17),23.4 (C-18),32.8 (C-19),180.1 (C-20)。以上波谱数据与文献[10]报道基本一致,故鉴定化合物8 为异迷迭香酚。
化合物9 红色粉末(甲醇),mp:171~173 ℃,ESI-MS m/z:311 [M + H]+;1H NMR (400 MHz,CD3OD)δ:3.30 (2H,d,J = 1.45 Hz,H-1),3.09(2H,t,J = 6.2 Hz,H-2),4.68 (2H,s,H-3),7.73(1H,d,J = 8.1 Hz,H-6),7.57 (1H,s,H-7),7.51(1H,d,J = 8.1 Hz,H-15),1.82 (2H,m,J = 6.0 Hz,Me-17),1.31 (6H,s,Me-18,Me-19);13C NMR(100 MHz,CD3OD)δ:31.1(C-1),20.1 (C-2),38.9 (C-3),35.6 (C-4),151.6 (C-5),134.9 (C-6),121.5 (C-7),128.4 (C-8),127.6 (C-9),145.5(C-10),183.8 (C-11),176.2 (C-12),127.4 (C-13),163.6 (C-14),143.3 (C-15),119.4 (C-16),56.0 (C-17),32.1 (C-18,C-19)。以上波谱数据与文献[11]报道基本一致,故鉴定化合物9 为紫丹参甲素。
化合物10 棕褐色针晶(吡啶),mp:240~242℃,ESI-MS m/z:293[M+H]+;1H NMR (400 MHz,C5D5N)δ:9.57 (1H,d,J = 8.0 Hz,H-1),7.75(1H,d,J = 8.8 Hz,H-2),7.49 (1H,d,J = 6.8 Hz,H-3),8.25 (1H,d,J = 8.4 Hz,H-6),7.99(1H,d,J = 8.8 Hz,H-7),7.25 (1H,s,H-16),5.21(3H,s,Me-17),2.51 (3H,s,Me-18);13C NMR (100 MHz,C5D5N)δ:128.4 (C-1),132.5 (C-2),130.3(C-3),133.7 (C-4),132.8 (C-5),130.3 (C-6),118.9 (C-7),128.8 (C-8),123.7 (C-9),129.4 (C-10),182.9 (C-11),175.3 (C-12),119.6 (C-13),160.9 (C-14),124.8 (C-15),142.6 (C-16),55.7(C-17),19.4 (C-18)。以上波谱数据与文献[7]报道基本一致,故鉴定化合物10 为紫丹参乙素。
化合物11 红色粉末(氯仿),mp:215~216℃,ESI-MS m/z:279[M+H]+;1H NMR (400 MHz,CDCl3)δ:9.26 (1H,d,J = 8.8 Hz,H-1),7.56(1H,t,J = 8.8 Hz,H-2),7.40 (1H,d,J = 8.8 Hz,H-3),8.28 (1H,d,J = 8.8 Hz,H-6),7.75(1H,d,J = 8.8 Hz,H-7),3.63 (1H,m,H-15),4.96 (1H,t,J = 9.6 Hz,H-16α),4.44 (1H,dd,J= 6.4,9.6 Hz,H-16β),1.39 (3H,d,J = 7.0 Hz,Me-17),2.70 (3H,s,Me-18);13C NMR (100 MHz,CDCl3)δ:124.9 (C-1),130.4 (C-2),128.8 (C-3),134.9 (C-4),134.7 (C-5),131.9 (C-6),122.2(C-7),130.6 (C-8),125.9 (C-9),135.4 (C-10),184.2 (C-11),175.9 (C-12),118.7 (C-13),170.8(C-14),34.8 (C-15),81.5 (C-16),18.7 (C-17),19.9 (C-18)。以上波谱数据与文献[6]报道基本一致,故鉴定化合物11 为二氢丹参酮Ⅰ。
化合物12 红色粉末(吡啶),mp:201~203℃,ESI-MS m/z:297[M+H]+;1H NMR (400 MHz,C5D5N)δ:2.55 (3H,s,Me-18),9.72 (1H,d,J =8.8 Hz,H-1),8.45 (1H,d,J = 8.7 Hz,H-6),8.30(1H,d,J = 8.7 Hz,H-7),7.58 (1H,dd,J = 8.8 Hz,H-2),7.37 (1H,d,J = 8.8 Hz,H-3),1.68(3H,d,J = 7.0 Hz,Me-17),4.17 (1H,m,H-15),4.56 (1H,m,H-16α),4.40 (1H,m,H-16β);13C NMR (100 MHz,C5D5N)δ:126.0 (C-1),129.9 (C-2),129.1 (C-3),135.8 (C-4),133.8 (C-5),131.6(C-6),122.5 (C-7),130.8 (C-8),125.4 (C-9),135.4 (C-10),185.1 (C-11),158.5 (C-12),122.7(C-13),185.9 (C-14),34.4 (C-15),65.3 (C-16),15.5 (C-17),19.7 (C-18)。以上波谱数据与文献[5]报道基本一致,故鉴定化合物12 为丹参新醌甲。
致谢:波谱数据由中国科学院昆明植物研究所分析测试中心测定。
1 Delectis Florae Reipublicae Popularis Sinicae Agendae Academiae Sinicae Edita(中国科学院中国植物志编辑委员会).Flora ReipublicaepopularisSinicae(中国植物志). Beijing:Science Press,1977,66:86-89.
2 Yang LX(杨立新),Li XC(李杏翠),Liu C(刘超),et al.Chemical constituents from Salvia przewalskiiMaxim. Acta PharmSin(药学学报),2011,46:818-821.
3 Jiang W(江文),Zhao Y(赵燕),Zhao BL(赵保路),et al.Scavenging effect of tanshinone on the lipids radical during the process ofcardiac sarcoplasmic reticulum lipid peroxidatio.ActaBiophy Sin(生物物理学报),1994,10:685.
4 Yang Y(杨阳),Wu ZJ(吴志军),Yang YB(杨颖博),et al.Three new terpenoids from Salvia przewalskiiMaxim.Chem J ChinUniv(高等学校化学学报),2011,32:1318-1322.
5 Yang LX(杨立新).Studies on the chemical constituents and bioactivities of Salvia Przewalskii Maxim. Beijing:Peking Union Medical College(北京协和医学院),MSc.2011.
6 Xu G(许刚).Study on the chemical constituents of five Salvia species.Kunming:Kunming Institute of Botany,The Chinese Academy of Sciences(中科院昆明植物研究所),PhD.2005.
7 Xue M(薛明),Shi YB(史彦斌),Cui Y(崔颖),et al.Study on the chemical constituents from Salvia Przewalskii Maxim.Nat Prod Res Dev(天然产物研究与开发),1999,12:27-32.
8 Yang W(杨薇),Tang XM(唐长明),Li X(李显),et al.Study on the chemical constituents of Myricaesculenta.J Yunnan Univ(云南大学学报),2011,33:453-457.
9 Luis JG,Quinones W,Grillo TA,et al. Diterpenes from the aerial part of Salvia columbariae. Phytochemistry,1994,35:1373-1374.
10 Gonzalez AG,Andres LS,Aguiar ZE,et al. Diterpenes from Salvia mellifera and their biogenetic significance.Phytochemistry,1992,31:1297-1305.
11 Luo HW,Wu MY,Yong ZG,et al.Pigments from Salvia miltiorrhiza.Phytochemistry,1985,24:815-817.
