Refining scientific procedures involving animals to reduce suffering and improve scientific validity
2013-11-27ElliotLilleyPennyHwkinsMggyJenningsPulLittlefirNikkiOsorneBrneyReed
Elliot Lilley ,Penny Hwkins,Mggy Jennings,Pul Littlefir,Nikki Osorne,Brney Reed.
(a.Research Animals Department,RSPCA,Wilberforce Way,Southwater,Horsham,RH13 9RS;b.RSPCA International,RSPCA,Wilberforce Way,Southwater,Horsham,RH13 9RS)
1 Introduction
Russell and Burch published their influential book“The Principles of Humane Experimental Technique”in 1959 and introduced the concept that scientific research involving animals should only be conducted following the implementation of the Three Rs(Replacement, Reduction and Refinement).Replacement requires sentient animals to be substituted with non-sentient alternatives wherever possible and reduction requires that animal numbers be reduced to the minimum consistent with the scientific objective of the study.However,there are a number of aspects to refinement and the conceptneedsto be carefully defined.Russelland Burch defined itas“any decrease in the incidence or severity of inhumane procedures applied to animals…… to reduce to an absolute minimum the amount of distress imposed”(paraphrased from Russell and Burch,1959).This definition focuses on minimising harms to animals and whilst this is an important aspect of refinement,the concept of positively improving animal welfare with enrichment and through improvements to housing and care is also an important consideration.Refinement in itself can be easy to implement and is done so in many laboratories worldwide①The RSPCA is a UK organisation,consequently the perspective given in this paper will reflect a UK view,based on our interactions with UK establishments..In many cases,refinement is reactive and implemented via small changes to protocols and models following observations of poor welfare.It is essential to be proactive and initiate refinement at the planning stage of a research programme.In this paper we shalloutline some fundamentalsteps towards implementation of refinement.
2 Basic principles
Refinement is integral to high quality science and
* elliot.lilley@rspca.org.uk.should be considered from the very startofany scientific program that may use animal models or procedures.There are five key principles that need to be applied in order to successfully implement refinement:
● The link between improved animal welfare and better science needs to be recognised.
● It is essential to be able to identify‘factors’thatcould cause pain,suffering ordistress and consider how you could refine them.
● It is important to be able to recognise when an animal is suffering,particularly the subtle signs.
● Levels of suffering need to be assessed,categorised and prioritised.
● There needs to be a strategy to avoid or minimise suffering.
2.1 Recognising the link between animal welfare and scientific quality
Refinement,with resulting improved animal welfare is broadly acknowledged to be essential for high quality science.This was well articulated by Russell and Burch:
“itis widely recognised thatthe humanest possible treatment of experimental animals,far from beingan obstacle,isactually a prerequisite for successful animal experiments”
(Russell&Burch 1959)and has been repeated by many others since(Baumans,2005;Lloyd,et al.,2008).
When trying to define what constitutes positive animal welfare it may be useful to use the principles of the“Five Freedoms”.First set out by the British Farm Animal Welfare Council in 1979(FAWC,1979)the five freedoms are:
● Freedom from hunger and thirst
● Freedom from discomfort
● Freedom from pain,injury and disease
● Freedom to express normal behaviour
● Freedom from fear and distress.
Scientific research can impact on all of these freedoms and as such can cause significant impairment of welfare(Lloyd,et al.,2008).Any deviation from the five freedoms is a potential source of suffering.When setting out to refine a protocol,minimising the impact on each of these“Five Freedoms”is a key principle.
The quality of experimental science is dependent on three critical factors:
● There should be a clear hypothesis to be tested.
● The experiment should be designed and powered in order to provide clear results.
● Variables that are not under investigation should be strictly controlled.
Poor animal welfare is a confounding factor in an experiment and will therefore increase variability and error(Poole,1997).There are many factors that can affect welfare.Restricted cage size and inappropriate flooring can adversely affect physiological parameters such as blood pressure,heart rate and body temperature(Sherwin,2007).Cage cleaning methods and routines can be a source of stress(Meller,et al.,2011)and stress can affect immune function(Khansari,et al.,1990).Appropriate standards of housing and care should be enforced with additional provisions,in addition to usual good practice①http://www.rspca.org.uk/sciencegroup/researchanimals/reportsandresources/housingandcare.,if necessary.
It is critical therefore that all sources of suffering that can occur in an experiment are identified and their impact minimised.
2.2 Identifying factors
We need to be aware that there are multiple potential sources of suffering for animals used in a scientific procedure.These include the procedure itself(including handling, restraint, identification,sampling and administration of substances;Morton,et al.,2001),housing,husbandry and care,transport,identification procedures and euthanasia.Refinement should be applied to the lifetime experience of the animal(Wolfensohn & Anderson 2012).For example:Non-human primates are highly intelligent and social animals who should live in a complex environment with a well-defined social structure.If animals like this are then transported,singly housed in small transport cages,over a long distance the stress caused by the transport conditions is likely to result in a high level of suffering before any scientific procedure has been started.
Each step of the scientific procedure(including housing and care,transport and euthanasia)should be examined,potential sources of suffering identified and cleardetailed stepsprovided in orderto reduce suffering(a detailed worked example of a welfare assessment protocol is provided in section 3.2).
2.3 Recognising suffering
The single most critical factor in the ability to recognise suffering in laboratory animals is the understanding ofwhat“normal” appearance and behaviour is for the species and strain of animal being used.It is critical to be familiar with the full range of“normal” behaviourslikely to be seen within a laboratory setting in orderto begin to recognise potential welfare problems.In rats for example,one should be aware of the normal activity patterns(play,feeding,nest building,grooming,interaction with cage mates,interaction with enrichment, sleep)for the strain and sex being studied,before initiating a project.Excessive grooming can be an indication of a welfare issue but unless the investigator is aware what normal grooming looks like,how will they be able to recognise it?Here,as in all areas of successful animal welfare assessment,a good relationship between the principle investigator and the day-to-day animal care staff is essential.Many senior animal care staff have amassed an extensive understanding of normal behaviour exhibited by laboratory animal species and can offer valuable advice for successfulwelfare assessment.There have been some excellent recent publications detailing pain recognition in commonly used laboratory animal species(Langford et al.,2010;Sotocinal et al.,2010;Keating et al.,2012).Facial“grimace”scales have been developed for rats,mice and rabbits and can be used to identify nociceptive pain in these species easily and earlier than many common overt behavioural changes(e.g.lameness,vocalisation,licking, scratching).Also,poor nest building and general maintenance of their surroundings have been highlighted as indicators of reduced welfare.“Normal”mice will build a distinct nest in their home cage and defecate outside of the nest.Sick mice have been shown to neglect nest building and to defecate indiscriminately throughout the cage(Arras,et al.,2007).
Although recognising and alleviating pain is undoubtedly important(Hawkins,2002),animals can also suffer from fear,anxiety,boredom and stress.There are a vast range of behavioural indicators of suffering that need to be considered and looked for.These include:movement patterns (circling or excessive/inappropriate use of enrichment items may indicate stereotypy),feeding/drinking patterns(even modest weight loss or dehydration can have powerful welfare implications in juvenile,pregnant or elderly animals)andinteractions withcagemates(both excessive interaction e.g. barbering and reduced interaction e.g.isolation can indicate welfare issues).
2.4 Assessing and categorising suffering
Once the potential sources of suffering have been identified they need to be categorised so that the level of suffering can be determined and potential refinement can bedefined (Hawkinsetal 2011).In the European Union,laboratory animal welfare legislation(Directive 2010/63/EU;European Commission,2010)subdivides levels of potential suffering into three categories:mild,moderate and severe.All suffering is important but severe suffering is clearly of the highest concern.These categories can be useful to help define the total upper limit of suffering experienced by an animal during a procedure.They can also help to understand how,over time,levels of suffering can change.For example:acute weight loss of 5~10%may be mild or moderate depending on the age and health status of the animal,but if weight loss continues to >25%the suffering will be severe.By categorising suffering,humane endpoints(see below)can be established.Categorising suffering also helps to determine where refinementresourcesneed to be allocated and procedures with the highest potential for suffering can beprioritised forurgentevaluation.However,all suffering is important and procedures with lower overall severity still need to be refined.
2.5 Avoiding and minimising suffering
The easiest way to eliminate suffering in the laboratory is not to perform the procedure.This may sound over simplistic but over recent years development of more humane animal models has accelerated and in vitro human tissue/organ systems with much greater complexity and translational validity are now being produced.If a particular scientific question cannot be appropriately answered withoutthe use ofliving animals,suffering must be reduced through refinement of the procedure.Minimising or avoiding suffering can be achieved by changing the protocol(e.g.changing the dose volume,type or site of adjuvant injection),providing analgesia(see below),providing palliative care(e.g.by providing additional/modified bedding,providing more accessible nutrition,returning animals to their normal social group following the procedure)or by identifying and implementing a humane endpoint.
2.6 Humane endpoints
Humane endpoints are specific and identifiable early indicators of suffering that,taking into account the scientific objectives of the experiment,direct the researcher or animal care technician to take action to reduce or end suffering(National Research Council,2008;Franco et al.,2012).In practice,this means that animals may be removed from a study,given pain relief or be killed using a humane method.When establishing a humane endpoint two factors need to be considered and “balanced” from an ethical perspective.On the one hand,the procedure needs to generate meaningful scientific data and on the other hand the suffering of the animals being used needs to be minimised.For example:in the DSS(Dextran sodium sulphate)model of inflammatory bowel disease weight loss can be extensive and rapid.A typical humane endpoint for weight loss in many procedures would be a maximum of 20% (of initial weight).However,in the DSS model there can be an acute,weight loss of between 25~30% followed bya subsequent recovery in some strains of mouse.If the 20% humane endpoint was used for this model all control DSS animals would have to be euthanized.However ifa modified endpointwasused where animals were euthanized if they showed weight loss of greater than 25% for more than 24h,the suffering could be moderated and data still obtained.
Suffering can also be reduced through the use of appropriate analgesia.Analgesia should be provided in all cases where pain may be experienced and only withheld if there is clear evidence that the scientific objectives will be impossible to achieve otherwise.Pilot studies should be conducted to assess suitable analgesic regimes and a range of analgesic agents should be tested (e.g. local anaesthetic creams, opioid analgesics and NSAIDS).
3 Practical steps to implementing refinement
In order to implement refinement,where should one start?Welfare assessment is crucial and this should be carried out with a team-based approach.In a well run research establishment there are a number of key people.People with important role to play in maintaining welfare standards.The study director or principle investigator is responsible for the study design and allocation and coordination of resources to enable the research(including staff,equipment,laboratory space and animals).The hands-on scientist(or a team of scientists)performs the study.The animal care staff provide the day to day husbandry and care for the animals(there may also be a specific senior animal technologist with overall responsibility for welfare of animals within the facility).There should also be input from a veterinarian with expertise in laboratory animal care.All of these individuals need to know what their responsibilities are priorto,during and afteran experiment (including planning, transport,husbandry,scientific procedure,fate of the animals,scientific and regulatory paperwork).One way of ensuring that each team member knows what their responsibilities are is to produce a procedure-specific welfare assessment protocol(Hawkins et al 2011).
3.1 Writing a welfare assessment protocol
No scientist would start an experiment without a plan,in the form of a dedicated protocol defining the critical steps required to perform a successful study.Equally,from both a scientific and animal welfare point of view,the experiment should not be started without a clear understanding of the potential sources of suffering from every step of the procedure and the steps that should be taken to ameliorate them.This requires a critical assessment of every aspect of the experimental procedure to predict likely sources of suffering.The published literature can be helpful to predict welfare issues but often specific welfare information is not adequately reported.Discussions with colleagues working in similar research fields can be helpful as well as seeking input from veterinarians and experienced animal care staff.If the particular procedure has not been performed previously,a pilot study designed specifically to identify welfareissues can bevery useful.
What does a welfare assessment protocol look like?A worked example is shown below.
3.2 Experimental autoimmune encephalomyelitis:a hypothetical example of a welfare assessment protocol
3.2.1 General information
Experimental Autoimmune Encephalomyelitis(EAE)is used to model various aspects of Multiple Sclerosis(MS)in a number of species including rodents and primates.MS is a complex neurological disorder that occurs in young adults.Its key features include inflammation,demyelination and axonal loss(Baxter,2007).
EAE involves generating immune system activity targeted at myelin,which induces inflammation in the central nervous system and opening of the blood brain barrier.This can causea severe neurological syndrome in the animal model,which should be followed by a partialrecovery during the firstchronic remitting relapsing phase.This phase is associated with inflammation and reversible demyelination.After 9~10 weeks,the animal will enter the progressive form,which is associated with chronic demyelination and axonal loss.
3.2.2 Producing the welfareassessment protocol
In this hypothetical example,EAE was induced in four male and four female Biozzi ABH mice(Baker,et al.,1990),in order to evaluate a potential therapy for MS.At the initial project planning stage,the user considered each possible adverse event for the animals and identified potential causes of suffering,in discussion with the animal technologist and care staff and attending veterinarian. They researched refinements and these were implemented in the project as in Table 1.The mice were socially housed in singlesex groups of four,each comprising two EAE and two healthy individuals.They were provided with solid flooring,sawdust litter,sufficient refuges and nesting material,and chew blocks.The prospective severity of the procedure was judged to be severe,as the procedures were expected to cause severe impairment of the animals'general wellbeing and condition.
In this example Table 1 forms the basis of the welfare assessmentprotocol.Itlists the protocol specific potential sources of suffering and suggests both potential refinements and humane endpoints for each of these.However,this on its own,is not enough to effectively manage and reduce suffering.There needs to be a clear and simple method of assessment for both the scientists and animal care staff so that welfare issues can be identified and acted upon.In many cases a score sheet can be used.An example,is given in Table 2.In this example score sheet observations are made in a series of broad categories (e.g.appearance,body function environment,etc)and then subdivided into specific clinical signs or behaviours(e.g.weight,bladder control,nest building,etc).These signs or behaviours need to be relevant to the potential sources of suffering identified in Table 1.In order to make a quantitative assessment of the degree of suffering associated with each of the clinical signs or behaviours in Table 2,there needs to be a consideration of the potential range of manifestations that may present during the procedure and to establish a scoring system to reflect relative severity(Ullman-Cullere& Foltz,1999).For example:In this EAE model weight loss may occur and may range from mild to severe.Here,we may suggest that mild weight loss of less than 10%would score 1,moderate weight loss of 10~20%would score 2 and severe weight loss of 20~35%would score 3.A suggested scoring system for the EAE example is given in Table 3.
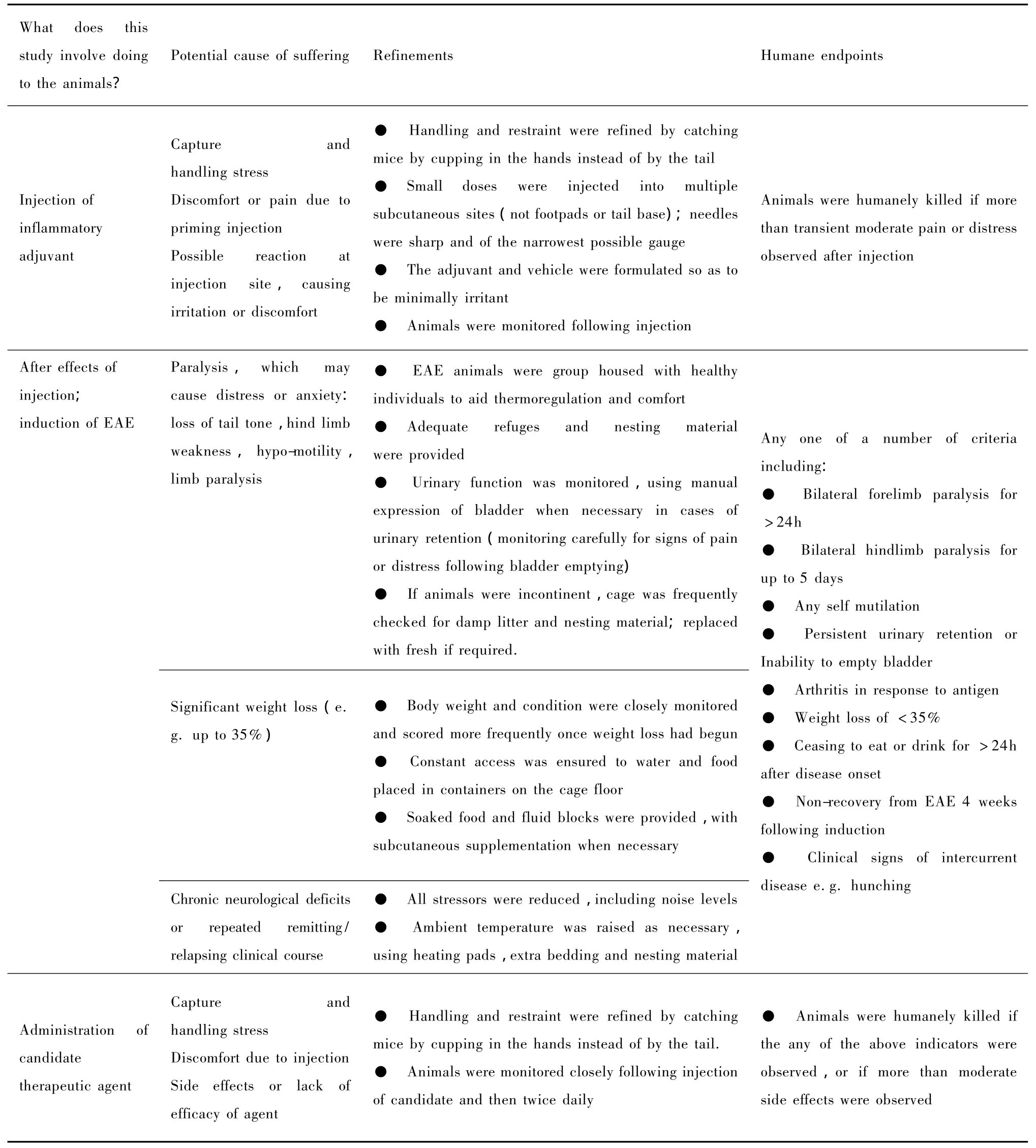
Tab.1 Potential causes of suffering and refinements in EAE mice

Tab.2 Score sheet used for EAE mice
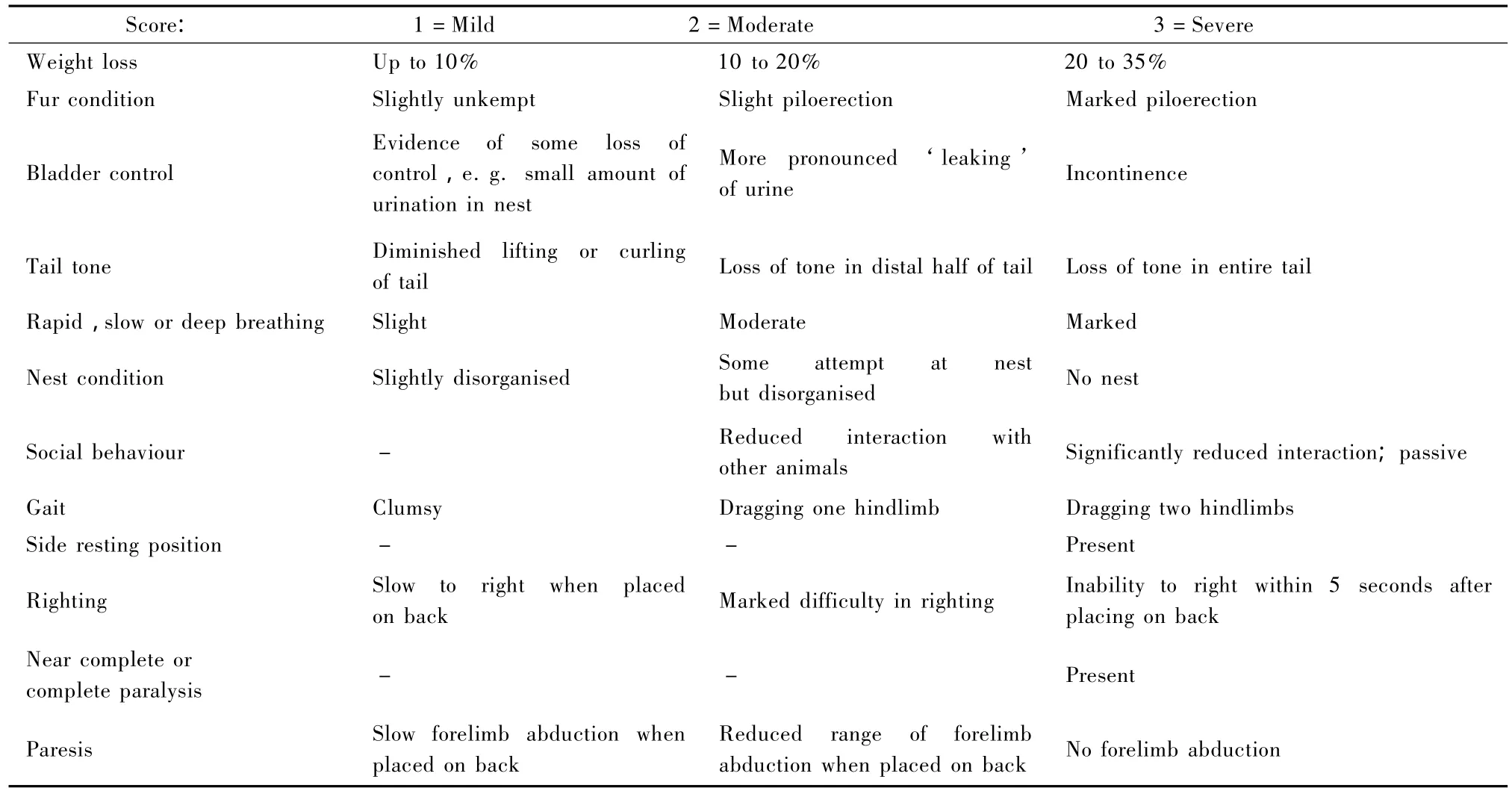
Tab.3 Assessment system for indicators in EAE clinical score sheet
Once the complete welfare protocol,including a scoring system,is in place the experimentcan proceed.However,the refinement process should not stop.Refinement is a continuous,evolving process and new welfare issues and opportunities to refine will often present themselves during an experiment.Within the context of the EAE example,what would this look like?
3.2.3 Experimental observations and analysis
During the study mice were monitored by the animal technologists and care staff using a clinical score sheetsystem thathad been tailored to the protocol following discussion with the users,animal technologists and care staff and veterinarian(Table 2).This included parameters relating to weight,fur condition,tail tone,bladder control,righting,gait,paresis and advanced signs(side resting position;near complete paralysis;rapid,slow or deep breathing).As the project involved severe procedures,animals were very closely monitored and ongoing reviews of severity were regularly conducted by the user,in discussion with the ethics committee, animal technologist and designated veterinarian.
At the end of the procedure,the score sheet was reviewed for each individual to see how highly the indicators had scored and how this had changed over time.
● Two mice lost 8%of their body weight,had slightly matted fur and slow forelimb abduction,but scored‘2’for all other indicators for the first 5 days of the project.Their scores then reverted to‘1’or‘0’for each indicator for the remainder of the study.Severity=MODERATE.
● Three mice lost between 22 and 32%of their body weight and scored a combination of‘3’,‘2’and‘1’ throughoutthe procedure. Severity =SEVERE.
● One mouse lost 37%of his body weight and was humanely killed.Severity=SEVERE.
● Two mice lost 15 and 18% of their body weight respectively,and scored a combination of‘2’and‘3’for all other indicators for the first 4 days of the study.They then scored a combination of‘1’and‘2’for the rest of the project.Severity=SEVERE.
Paralysis was not observed and it proved to be too difficult to remotely assess respiratory rate without overly stressing the animals,so both of these were deleted from the record sheets.Increased time in the refuge was frequently noted in the free text boxes as an early indicator of suffering,so this was added to the sheets for future projects.
Following their retrospective assessment of severity,the usersconsulted with colleaguesand searched the literature for further refinements.The following additional refinements were identified:
● Pre-feeding animals with high-energy supplement foods,such as jelly and condensed milk,before administering the adjuvant.This was introduced to help the animals to cope with weight loss during the procedure.
● Using a lower dose of adjuvant,this was instigated to reduce local irritation following adjuvant injection.
● Using an alternative study protocol so that the duration of the project could be reduced
These were added to the protocolforfuture studies,with the intention of comparing actual severity levelsto see whether the refinements had been effective.
In this example,a“refinement loop”has been demonstrated(Lloyd et al.,2008).This implies a continuous cycle of assessment of suffering,identification of refinement, implementation of refinement and back to assessment of suffering and so on.This should always be the case;welfare science is aconstantly developing field and new refinement principles are being regularly reported.A constant appraisal of the literature is essential to stay abreast with the current state of play with regard to laboratory animal welfare.It is equally important that knowledge gained from refinement is published so that as many animals as possible benefit from any welfare benefits and reduced suffering.
3.2.4 Closing remarks
Refinement of procedures and models that use living animals makes a real difference to both scientific validity,data quality and animal welfare.This report outlines practical steps that can be taken to implement refinement in scientific procedures that used animals and the authors hope that these principles will be taken,implemented and further developed bythe scientific community.
Baker,D.,O'Neill,J.K.,Gschmeissner,S.E.,Wilcox,C.E.,Butter,C.& Turk,J.L.(1990)Induction of chronic relapsing experimental allergic encephalomyelitis in Biozzi mice.Journal of Neuroimmunology.28,261-270.
Baxter,A.G.(2007)The origin and application of experimental autoimmune encephalomyelitis.Nature Reviews Immunology.7,904-912.
European Commission(2010).DIRECTIVE 2010/63/EU OF THE EUROPEANPARLIAMENTANDOFTHECOUNCILof22 September 2010 on the protection of animals used for scientific purposes.Official Journal of the European Union.L276/33.
Franco,N.H.,Correia-Neves,M.& Olsson,A.S.(2012)How humane is your endpoint?PLoS Pathog 8(1):e1002399.doi:10.1371/journal.ppat.1002399.
Hawkins,P.(2002)Recognizing and assessing pain,suffering and distress in laboratory animals:a survey of current practice in theUK with recommendations.Laboratory Animals,36,378 -395.
Hawkins,P.,Morton,D.B.,Burman,O.,Dennison,N.,Honess,P.,Jennings,M.,Lane,S.,Middleton,V.,Roughan,J.V.,Wells,S.& Westwood,K.,(2011).A guide to defining and implementing protocols for the welfare assessment of laboratory animals:eleventh report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement.Laboratory Animals,45,1-13.
Keating,S.C.J.,Thomas,A.A.,Flecknell,P.A.& Leach,M.C.(2012).Evaluation of EMLA Cream for Preventing Pain during Tattooing of Rabbits:Changes in Physiological.
Behavioural and Facial Expression Responses. PLoS ONE, 7(9),e44437.
Langford,D.J.,Bailey,A.L.,Chanda,M.L.,Clarke,S.E.,Drummond,T.E.,Echols,S.,Glick,S.,Ingrao,J.,Klassen-Ross,T.,LaCroix-Fralish,M.L.,Matsumiya,L.,Sorge,R.E.,Sotocinal,S.G.,Tabaka,J.M.,Wong,D.,van den Maagdenberg,A.M.J.M.,Ferrari,M.D.,Craig,K.D.&Mogil,J.S.(2010).Coding of facial expressions of pain in the laboratory mouse.Nature Methods,7(6),447 -453.
Lloyd,M.H.,Foden,B.W.& Wolfensohn,S.E.(2008).Refinement:promoting the three Rsin practice.Laboratory Animals,42,284 -293.
Meller, A., Kasanen, I., Ruksenas, O., Apanaviciene, N.,Baturaite,Z.,Voipio,H.M.& Nevalainen,T.(2011).Refining cage change routines:comparison of cardiovascular responses to three different ways of cage change in rats.Laboratory Animals,45,167-173.
Morton,D.B.,Jennings,M.,Buckwell,A.,Ewbank,R.,Godfrey,C.,Holgate,B.,Inglis,I.,James,R.,Page,C.,Sharman,I.,Verschoyle,R.,Westall,L.& Wilson,A.B;(2001)Refining procedures for the administration of substances;Report of the BVAAWF/FRAME/RSPCA/UFAW jointworkinggroupon refinement.Laboratory Animals.35,1 -41.
National Research Council(2008).Recognition and Alleviation of Distress in Laboratory Animals. Washington, DC:National Academies Press.
Russell,W.M.S.& Burch,R.L.(1959).The Principles of Humane Experimental Technique.Methuen:London.
Sotocinal,S.G.,Sorge,R.E.,Zaloum,A.,Tuttle,A.H.,Martin,L.J.,Wieskopf,J.S.,Mapplebeck,J.C.S.,Wei,P.,Zhan,S.,Zhang,S.,McDougall,J.J.,King,O.D.& Mogil,J.S.(2011).The Rat Grimace Scale:A partially automated method for quantifying pain in the laboratory ratvia facialexpressions.Molecular Pain,7,55-64.
Ullman-Cullere,M.H.& Foltz,C.J.(1999).Body Condition Scoring:A Rapid and Accurate Method for Assessing Health Status in Mice.Laboratory Animal Science.49,319 -323.
Wolfensohn,S.& Anderson,D.(2012).Lifetime experience and cumulative suffering:A report on assessment of lifetime experience and cumulative severity under EU Directive 2010/63.LASA Forum Summer issue.
