Determination of chitosan content by Fourier transform infrared spectrometry
2013-11-21TANGBinghua
TANG Bing-hua
(Zhanjiang Life Source Health Products Company Ltd., Zhanjiang 524000, Guangdong, China)
Chitosan, 2-amino-2-deoxy-b-1,4-glucan prepared by N-deacetylation of chitin, is a biodegradable and non-toxic polysaccharide with low immunogenicity[1]. As the most important derivate of chitin, chitosan possesses many important characteristics such as defined chemical structure, special physical and biological function, biodegradability, and biocompatibility, and it can be easily processed into various products including flakes, fine powders, beads, membranes, sponges, cottons, fibers, and gels[2-5]. Therefore, in the 21st century, chitosan faces new opportunities to contribute to functional materials and environmentally friendly materials thereby meeting the diverse needs of today’s society. In this sense, it is imperative to develop novel efficient methods for the determination of chitosan.
Commonly used methods for the determination of chitosan content include chromatography, spectrophotometry, nuclear magnetic resonance spectroscopy, colorimetric assay and fluorimetric technique, or a combination of these methods[6-10]. Chromatography has a high sensitivity and can be adopted to provide accurate and reliable results, but it is tedious and time-consuming. Spectrophotometry, as a traditional method, has been repeatedly modified since its naissance. In this respect, further modification to spectrophotometry may be of significance for facile determination of chitosan. Therefore, in the present research we make use of nonlinear modeling of Fourier transfer infrared spectrometry (FT-IR) to establish a simple and sensitive method for the quantitative determination of chitosan.
1 Experimental
1.1 Materials and reagents
Chitosan with a deacetylation degree of 90% was purchased from Guoyao Biochemical Company Ltd (Shanghai, China). Commercial potassium bromide is of analytical grade.
1.2 FT-IR analysis
A PerkinElmer Spectrum 100 FT-IR spectrometer was performed to determine the content of chitosan. Chitosan samples were prepared in potassium bromide disks. The disks were obtained by compacting with a hydrostatic press at a force of 10 tons for 1 min. The FT-IR spectrum was recorded with a resolution of 4 cm-1at room temperature over 16 cumulative scans in a wave number range of 4000-450 cm-1.
1.3 Preparation of chitosan samples with standard content
As-received chitosan was ground in a mortar and mixed evenly with potassium bromide after accurate weighing. The standard content of chitosan was ranged from 0.5% to 4.0% with a 0.5% gradient. As-pressed chitosan tablets with controlled weight of 0.10 g per piece were prepared.
2 Results and discussion
2.1 FT-IR analysis of chitosan
Since each specific chemical bond often has a unique energy absorption, FT-IR spectrometry has been extensively applied to identify the presence of certain functional groups or chemical bonds in a molecule. Fig.1 shows the FT-IR spectrum of chitosan. There is a strong and broad absorbent band near 3 435 cm-1, and it can be assigned toν(N―H),ν(O―H) andν(―NH2) in chitosan. The stretching vibrations in the range of 2 920-2 865 cm-1confirm the presence of methyl group in NHCOCH3, methylene group in CH2OH and methenyl group (―CH) in pyranose ring. The absorption bands at 1 635 cm-1and 1 595 cm-1are assigned as amide I and ―NH vibrations, respectively[11]. The sharp band at 1 389 cm-1is attributed to the bending vibration of methenyl group (―CH) in pyranose ring. The absorption bands within 1 157-550 cm-1region confirm the presence of ―CH3, ―CH2and ―CH groups as well as primary and secondary ―OH groups attached to the pyranose ring and oxygen atoms in ether groups[12].
The absorption bands of methenyl group (―CH) in pyranose ring are the characteristic absorption peak of chitosan. Namely, chitosan with different contents show different intensities of absorption bands at 1 389 cm-1and 2 865 cm-1(Fig.2), so the FT-IR spectra of various chitosan samples can be used for their quantitative analysis.

Fig.1 FT-IR spectrum of chitosan

Fig.2 FT-IR spectrum of chitosans with different content
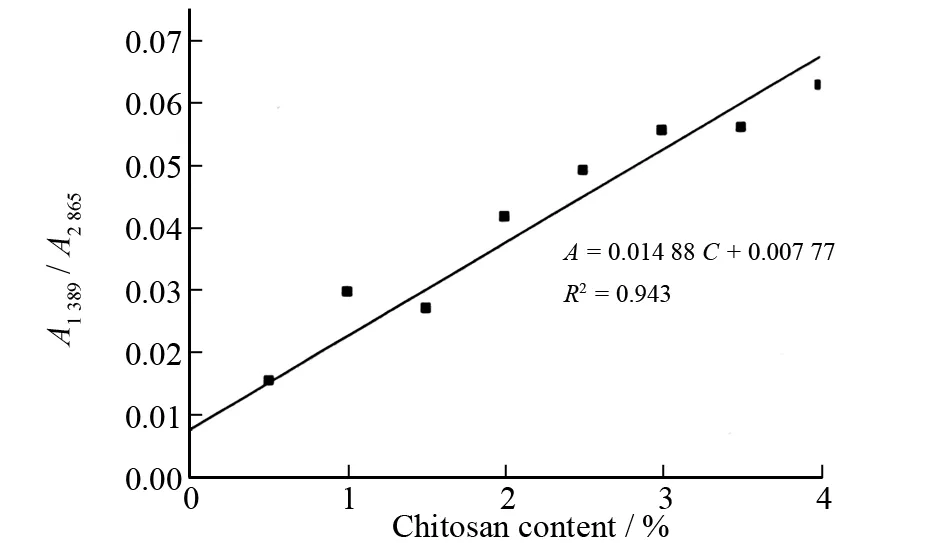
Fig.3 Calibration plot of FT-IR method for determination of chitosan content
2.2 Preparation of standard curve for determination of chitosan content
The areas of the absorbance peaks of methenyl group (―CH) in pyranose ring at 1 389 cm-1and 2 865 cm-1were determined to draw standard curves for the determination of chitosan content. The chitosan content standard curve was developed and constructed by calibrating the area ratios (A=A1 389/A2 865) at varying chitosan content. Linear model is a kind of traditional quantitative model widely used in the quantitative analysis, because of its convenience. The standard quality content of chitosan is taken as theXaxis, while the absorbance area ratio (A) is taken as theYaxis to establish the linear model. A linear plot of the determined values is presented in Fig.3. The resulting plot indicates that there is an excellent correlation between varying chitosan content (C) and their associated absorbance areas ratios (A). The regression equation isA= 0.014 88C+ 0.007 77 with anR2of 0.943.
2.3 Validation of the FT-IR method in determining chitosan content of pharmaceutical formulation
The content of chitosan in pharmaceutical formulation of chitosan capsules was determined with the established FT-IR method so as to validate its adaptability and usability. As shown in Fig.3, the relative error of chitosan content between the one declared in label and that determined in formulation is in the range of 0.6% to 0.8% (average 0.7%), which indicates that the proposed method is suitable for determination of chitosan in real samples.
Conclusions: A novel FT-IR spectrometric method has been established based on linear model of quantitative analysis to quantitatively determine chitosan content while the absorbance area ratios (A) of the methenyl group in pyranose ring at 1 389 cm-1and 2 865 cm-1are adopted as the quantitative references. Validation tests show that the established method can be well adopted to determine chitosan content in real pharmaceutical samples.
:
[1] RAVO KUMAR M N V, MUZZARELLI R A A, MUZZARELLI C, et al. Chitosan chemistry and pharmaceutical perspectives [J]. Chem Rev, 2004, 104: 6017-6084.
[2] GE H C, LUO D K. Preparation of carboxymethyl chitosan in aqueous solution under microwave irradiation [J]. Carbohydr Res, 2005, 340: 1351-1356.
[3] KASAAI M R. A review of several reported procedures to determine the degree of N-acetylation for chitin and chitosan using infrared spectroscopy [J]. Carbohydr Polym, 2008, 71: 497-508.
[4] BERGER J, REIST M, CHENITE A, et al. Pseudo-thermosetting chitosan hydrogels for biomedical application [J]. Int J Pharm, 2005, 288: 197-206.
[5] KURITA K. Controlled functionalization of the polysaccharide chitin [J]. Prog Polym Sci, 2001, 26: 1921-1971.
[6] KASAAI M R. Determination of the degree of N-acetylation for chitin and chitosan by various NMR spectroscopy techniques: A review [J]. Carbohydr Polym, 2010, 79: 801-810.
[7] BADAWY M E I. A new rapid and sensitive spectrophotometric method for determination of a biopolymer chitosan [J]. Int J Carbohydr Chem, 2012: 1-7.
[8] TSUJI A, KINOSHITA T, HOSHINO M. Microdetermination of hexosamines [J]. Chem Pharm Bull, 1969, 17: 217-218.
[9] TSUJI A, KINOSHITA T, HOSHINO M. Analytical chemical studies on animo sugars. II. Determination of hexosamines using 3-methyl-2-benzothiazolone hydrazone hydrochloride [J]. Chem Pharm Bull, 1969, 17: 1505-1510.
[10] EIKENES M, FONGEN M, ROED L, et al. Determination of chitosan in wood and water samples by acidic hydrolysis and liquid chromatography with online fluorescence derivatization [J]. Carbohydr Polym, 2005, 61: 29-38.
[11] SAJOMSANG W, GONIL P, TANTAYANON S. Antibacterial activity of quaternary ammonium chitosan containing mono or disaccharide moieties: Preparation and characterization [J]. Int J Biol Macromol, 2009, 44: 419-427.
[12] LIM S H, HUDSON S M. Synthesis and antimicrobial activity of a water-soluble chitosan derivative with a fiber-reactive group [J]. Carbohydr Res, 2004, 339: 313-321.
