Mechanical Stimulus Inhibits the Growth of a Bone Tissue Model Cultured In Vitro△
2013-11-18ZongmingWanLuLiuJianyuLiRuixinLiYongGuoHaoLiJianmingZhangandXizhengZhang
Zong-ming Wan,Lu LiuJian-yu LiRui-xin LiYong GuoHao LiJian-ming Zhangand Xi-zheng Zhang*
1Institute of Medical Equipment,Academy of Military Medical Sciences of Chinese People's Liberation Army,Tianjin 300161,China
2Department of Pharmacology,Logistics College of Chinese People’s Armed Police Forces,Tianjin 300162,China
AS a composite material,formation and growth of the bone tissue is a complex process involving molecular,cellular and biochemical metabolic changes.But compared with other tissuesin vivo,the structure of bone tissue is much denser,and stress environment and biological environment play important roles in the formation of its structure and function.1In the 19th century,Julius wolff has suggested that external mechanical loading can affect the shape and structure of the bone.2The mechanical steady-state theory raised by Frost in 1987 had provided a better explanation to Wolff’s law at the tissue level.3Our previous researches have revealed that the mechanical strain can apparently regulate the MC3T3-E1 cells,a mouse monoclonal pre-osteoblastic cell line,to differentiate to the osteoblastsin vitroand regulate the bone reconstructionin vivo.4-11Bone growthin vivois influenced by a combination of biochemical,mechanical,and other non-biological stimulations.When we want to illustrate how the stress affects bone growthin vivo,other factors or conditions may interfere with the results.Therefore,to investigate the roles of individual mechanical factor in bone growth,cancellous bone explant modelsin vitrocan sweep away the interfering factors.Two studies have reported that a three-dimensional bioreactor system (Zetos™) could allow the controlled application of force and/or compression to three-dimensional explant models of cancellous bone maintained within specific chambers exvivo.12,13
Unlike two-dimensionalin vitrocell models,the explant models maintain thein vivothree-dimensional morphology and intercellular connections in the bone tissue.In this study,a rabbit cancellous bone explant culture model was constructed and used to investigate its response to physiological mechanical load.Our general assumption is that the physiological mechanical load can increase the reconstruction of rabbit cancellous bone explant culture modelin vitro,and based on it,we will culture the tissue engineering model with mechanical loadin vitro.The apoptosis in this bone model which was subjected to the pathological mechanical stress was specified for such hypothesis.
MATERIALS AND METHODS
Rabbits
Naturally mated 3-month old male New Zealand white rabbits were obtained from the Laboratory Animal Center of Academy of Military Medical Sciences.The care and use of animals were in accordance with the governmental guidelines for the care and use of laboratory animals.
Preparation of rabbit cancellous bone explant culture model
Under sterile conditions,rabbit femoral head were cut into 1-mm-thick cancellous bone tissue slices.After the cortical bone and adipose tissue were removed,these slices were made into about 8 mm in diameter and rinsed in sterile phosphate buffered saline (PBS) for 3 times.Thereafter,these bone slices were cultured with Dulbecco's Modified Eagle's medium (DMEM),containing 15% fetal bovine serum suitable for tissue living,in a new dynamic load and circulating perfusion bioreactor system which was independently developed by the Chinese Academy of Military Medical Sciences.14Having been cultured for 0,3,5,and 7 days,the cancellous bone explant model was identified in morphology by hematoxylin-eosin (HE) staining and scanning electron microscopy (provided by Nankai University,Tianjin,China).DMEM containing 100 U/mL penicillin,100 µg/mL streptomycin,and fetal bovine serum was obtained from HyClone,USA.
MicroCT scanning and finite element analysis
At the same time,the cancellous bone explant culture model was multi-level scanned on a high-resolution microCT(Skyscan 1076 X-ray Microtomography,Belgium) in Beijing University of Aeronautics and Astronautics,China.Then the finite element models were input into AYSYS 12.0 software.During finite element analysis,a mechanical load of 1000,2000,or 3000 με was imposed onto the three-dimensional model by imitating thein vivophysiological stress range of bone.
Application of mechanical load on bone explant model
After cultured for 1 day,the bone explant model was stressed by dynamic load in the circulating perfusion bioreactor system which was made with piezoelectric ceramics,and controlled by installed computer software.Through the piezoelectric ceramic-loading rod transferring,these model samples were subjected to a sinusoidal stress loading of 1000,2000,3000,and 4000 με respectively for 30 minutes per day for 5 days.The mechanical loading could lead to producing a compressive displacement in the bone tissue.Based on the compressive displacement (μm)which was displayed by the computer and the thickness of the bone tissue (m),the intensity of mechanical stress (με)in the bone tissue could be calculated by the following formula:strengthen of mechanical stress (με)=compressive displacement (μm)/ thickness of the bone tissue (m).
Alkaline phosphatase (AKP) activity analysis
After mechanical load,the cancellous bone explant model was rinsed for 3 times in PBS (unstressed bone tissues as the control group were incubated under the same conditions for the maximum period of mechanical loading application),then cut into about 1 mm3sized cubes and tardily homogenized in RIPA lysis buffer (400 μl) containing protease and phosphatase inhibitors at 4˚C.Thereafter,the extract of protein lysates was collected,and the total protein assay was performed using BCATMProtein Quantification kit (Nanjing Jiancheng Bioengineering Institute,China).According to manufacture’s instructions of AKP Activity Assay kit (Nanjing Jiancheng Bioengineering Institute),the absorbance (OD) value of AKP in the total protein with equal dose was detected.We calculated the AKP activity with the following formula:AKP (U/gprot)=(SOD/StOD)×St phonel quantity (0.003 mg)/S protein quantity (g);where SODis sample OD value,StODis standard substance OD value,St phonel quantity is standard substance phonel quantity,S protein quantity is sample protein quantity.
Tartrate-resistant acid phosphatase (TRAP) activity analysis
The extraction and quantification of total protein in the bone explant model was performed as described above.OD values were measured at 530 nm ultraviolet spectra.Thereafter,we calculated the TRAP activity (U/gprot)basing on the manufacture’s instructions of TRAP Assay kit(Nanjing Jiancheng Bioengineering Institute).
DNA Ladder detection
After mechanical load,the bone explant model was rinsed with ice cold PBS and cut into 1 cm3in size,then homogenized on ice for 10 minutes.Thereafter,the DNA in the model samples was extracted using the DNA Ladder Assay kit (Nanjing Biobox Biotech Co.,Ltd.,China).The DNA fragments were visualized under ultraviolet light after being separated by electrophoresis on a 1.5% agarose gel by comparing with a 200-bp DNA ladder marker.
Caspase-3/8/9 activity detection
According to manufacturer’s instructions of Caspase-3/8/9 Activity Detection kit (Nanjing Biobox Biotech.Co.,Ltd.),the protein in the bone explant model was extracted after these samples being subjected to mechanical loading for 5 days.Aliquots (50 µl) of the protein were removed and placed in a 96-well microplate containing caspase-3/8/9 reaction substrate,and the microplate was incubated at 37˚C for 4 hours.The OD value was measured at 405 nm(TECAN,Switzerland),and the caspase-3/8/9 activity was determined through ODsample/ODcontrol.
Statistical analysis
All statistical analyses were performed using Statistics Package for the Social Sciences (SPSS) 11.0 (IBM SPSS,Inc.,Chicago,IL,USA).The means and standard deviation were calculated for data from three separate experiments with triplicate samples.Statistical significance was determined with a two-tailed Student'sttest.The difference was considered as statistically significant atP<0.05.
RESULTS
Identification of rabbit cancellous bone explant model in vitro
The cancellous bone tissue slices can live for 7 days in the DMEM containing 15% fetal bovine serum under dynamic load in a circulating perfusion bioreactor system.HE staining showed that the histomorphology of the cancellous bone tissue slices cultured for 3,5,and 7 days had no apparent difference compared with fresh slices.Osteocytes were trapped within the bone trabeculae with distinctly visible nucleus in cells (Fig.1).Electron microscope scan also obviously detected living cells in the bone tissue cultured for 3,5,and 7 days.However,there were not cells to be found on uncultured bone tissue because cells were covered by lots of adipose tissues (Fig.2).
Results of MicroCT scanning and finite element analysis
Figure 3 shows the strain distribution in the cancellous bone explant model under 1000,2000,and 3000 με load,respectively.The strain at the nodes of the bone explant model with 1000,2000,and 3000 με load was mainly lower than 2000 με.When the bone explant models received 3000 με load,the strain of about 2000 nodes was higher than 4000 με,however the range of physiological strain on bonein vivois lower than 3000 με.Therefore,we selected the mechanical load of 1000,2000,3000,and 4000 με respectively to treat the explant culture model of the bone tissuein vitro.
AKP and TRAP activity
As displayed in the Table 1,mechanical load of 3000 and 4000 με for 30 minutes per day for 5 days significantly reduced the activities of AKP and TRAP in the bone explant model compared with the control group (allP<0.05).However,there was no significant difference in the activity of AKP or TRAP between 1000 με group and 2000 με group (allP>0.05).
DNA ladder

Figure 1.Photomicrographs of hematoxylin-eosin stained slices of rabbit cancellous bone cultured for 0 day (A),3 (B),5 (C),and 7 days (D) respectively in vitro.HE staining ×20

Figure 2.Results of electron microscopy scan of rabbit cancellous bone cultured for 0 day (A),3 (B),5 (C),and 7 days (D)respectively in vitro.×1000
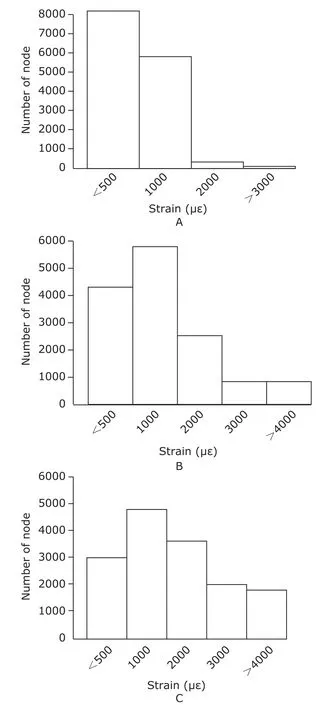
Figure 3.Distribution of strain in the cancellous bone explant model under 1000 (A),2000 (B),and 3000 με load (C),respectively.
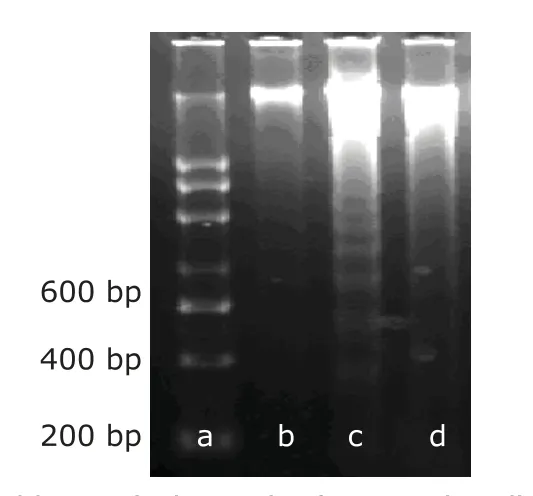
Figure 4.DNA ladder analysis result of apoptotic cells by agarose gel electrophoresis.
As showed in Figure 4,DNA extracted from the cancallous bone explant model samples was fragmented into multiple fragments with lengths as integer multiples of approximately 180-200 base pairs.The DNA ladders were seen after mechanical load of 3000 and 4000 με (Fig.4,Lanes c and d).But no obvious DNA ladder patterns were observed in the control group during the entire experiment.
Caspase activity
The protein activity levels of caspase-3/8/9 in the mechanical load groups were significantly increased (allP<0.05) compared with the control group (Table 2).

Table 2.Comparisons of caspase-3/8/9 activities in the bone explant culture model receiving 3000 and 4000 με mechanical loads for 5 days§ (n=3)
DISCUSSION
The bone is an extremely complex organ composed of compact bone tissue containing cellular component and matrix component.It is difficult to culture bone tissue in simplifiedin vitroenvironment.We cultured the cancellous bone explant model in an environment suitable for tissue growth with dynamic load and circulating perfusion.This protocol provided the cancellous bone cells with O2,nutrient and mechanical load.The morphology of the model visualized by HE staining and scanning electron microscopy after 7 days of culture was normal.
Mechanical load is a key physiological regulatory mechanism for bone formation and is essential for the maintenance of skeletal architectural integrity and bone strength.According to the“mechanical steady-state theory”raised by Frost,3thein vivophysiological mechanical stress of bone ranges from 1000 to 4000 με.In order to test the effect of mechanical load on growth of the rabbit cancellous bone explant modelin vitro,these bone tissue samples were subjected to mechanical stress of 1000,2000,3000,and 4000 με,respectively.We found that the mechanical stress of 3000 and 4000 με could simultaneously inhibit the activities of AKP and TRAP,reflecting proliferation and differentiation of osteoblasts and osteoclasts respectively.
As it is known that on the one hand osteoblasts and osteoclasts are the effector cells which are responsible for bone formation and bone resorption respectively,15-17and mechanical stress can directly affect their developmentsin vivoorin vitro.18,19On the other hand,osteocytes,known as the mechanosensors,have two key features of ability to detect mechanical stimuli and to send signals to other effector cells that regulate bone formation and resorption.20-24Other experimental studiesin vitroandin vivoalso revealed the osteocytes network is the main sensor detecting strain in the bone tissue.25These cells,embedded in their lacunocanalicular system and largely distributed in bone,interconnecting by gap junctions between their cell processes,connecting with cells of the bone surface and of the bone marrow,are ideal place to sense bone loading and to direct remodelling according to strains.26Osteocytes are sensitive to biomechanical stress,particularly to fluid flow and shear stress induced by loading in the lacunocanalicular system.27,28Youet al29indicated that osteocytes may function as mechanotransducers by regulating local osteoclastogenesisviasoluble signals mechanically stimulated when co-cultured with osteoclasts.Likewise,some studies showed that sclerostin,an osteocytic protein belonging to the transforming growth factor/bone morphogenetic protein family which can inhibit Wnt signaling,is decreased by mechanical stimulation.Wnt has an essential role in osteoblast proliferation and differentiation,and the decrease of sclerostin could be a major signal to increase bone formation in response to loading.30-32
In this study,we detected apoptosis of bone cells in the cancellous bone explant model.Apoptotic cell death and subsequent removal of apoptotic cell debris by specialized phagocytic cells occur in numerous tissues during development33-36and in response to injury.37-41Our experiment showed by DNA ladder assay that there were apparent apoptosis of cells in the bone tissue subjected to mechanical stress compared with the control group.The appearance of the DNA ladder bands further showed that mechanical stimulation led to the degradation of nuclear chromatin DNA to nucleosome fragments,which was characteristic of early apoptosis.42Caspase-3,-8 and -9 are members of the caspase proteases family which has been known to play a major role in the execution phase of apoptosis.A series of members of caspase proteases family are all activated by some stimulating factors to participate in the apoptosis occurrence.It has been shown that the caspase-3 is the key protease among caspase family,because it is activated by a variety of apoptotic stimulating factors at the beginning of apoptosis,then the activated caspase-3 protease may act on some of the other caspase members to be activated in cascade,causing cell morphological changes,and to the end apoptosis to be completed.43Our experiment indicated that the mechanical stimulation of 3000 με could activate the apoptosis programviacaspase-dependent route.
In summary,the cancellous bone explant models from the rabbit femoral head could be alive at least for 7 days in the dynamic load and circulating perfusion bioreactor system,and pathological mechanical loading (3000 and 4000 με) could inhibit the proliferation or differentiation of bone cells,which might be a caspase-mediated process that lacks cell type specificity.Pathological mechanical load might affect bone tissue growth by cellular apoptosisin vitro.
1.Rubin C,Turner AS,Bain S,et al.Anabolism low mechanical signals strengthen long bones.Nature 2001;412:603-4.
2.Dibbets JMH.One century of Wolff’s law.In:Carlson DS,Goldstein SA,editors.Bone dynamics in orthodontic and orthopaedic treatment.Center for human growth and development.Ann Arbor:University of Michigan Press;1992.p.1-13.
3.Frost HM.Bone“mass”and the“mechanostat”:A proposal.Anat Rec 1987;219:1-9.
4.Wang L,Zhang X,Guo Y,et al.Involvement of BMPs/Smad signaling pathway in mechanical response in osteoblasts.Cell Physiol Biochem 2010;26:1093-102.
5.Chen XY,Zhang XZ,Guo Y,et al.The establishment of a mechanobiology model of bone and functional adaptation in response to mechanical loading.Clin Biomech (Bristol,Avon) 2008;23:S88-95.
6.Yan YX,Gong YW,Guo Y,et al.Mechanical strain regulates osteoblast proliferation through integrin-mediated ERK activation.PLoS ONE 2012;7:e35709.
7.Ma R,Zhu D,Gong H,et al.High-frequency and low-magnitude whole body vibration with rest days is more effective in improving skeletal micro-morphology and biomechanical properties in ovariectomised rodents.Hip Int 2012;22:218-26.
8.Gong H,Zhu D,Gao J,et al.An adaptation model for trabecular bone at different mechanical levels.Biomed Eng Online 2010;9:32.
9.Zhang C,Zhang X,Dong X.A loading device suitable for studying mechanical response of bone cells in hard scaffolds.J Biomed Mater Res B Appl Biomater 2009;91:481-8.
10.Zhang C,Zhang X,Dong X,et al.Bone modeling adaptation as a method for promoting development of bone tissue engineered constructin vitro.Med Hypotheses 2007;69:178-81.
11.Chunqiu Z,Xizheng Z,Han W,et al.Direct compression as an appropriately mechanical environment in bone tissue reconstructionin vitro.Med Hypotheses 2006;67:1414-8.
12.Jones DB,Broeckmann E,Pohl T,et al.Development of a mechanical testing and loading system for trabecular bone studies for long-term culture.Eur Cell Mater 2003;5:48-59.
13.Endres S,Kratz M,Wunsch S,et al.Zetos:A culture loading system for trabecular bone.Investigation of different loading signal intensities on bovine bone cylinders.J Musculoskelet Neuronal Interact 2009;9:173-83.
14.Chen XZ,Shi CH,Li RX,et al.Design of a new dynamic load and circulating-perfusion bioreactor system.J Med Biomech 2011;26:441-7.
15.Burger EH,Klein-Nulend J.Mechanotransduction in bone—role of the lacunocanalicular network.FASEB J 1999;13:S101-12.
16.Cowin SC,Moss-Salentijn L,Moss ML.Candidates for the mechanosensory system in bone.J Biomech Eng 1991;113:191-7.
17.Burr DB,Martin RB,Schaffler MB,et al.Bone remodeling in response toin vivofatigue microdamage.J Biomech 1985;18:189-200.
18.Iqbal J,Zaidi M.Molecular regulation of mechanotransduction.Biochem Biophys Res Commun2005;328:751-5.
19.Enlow DH.Functions of the Haversian system.Am J Anat 1962;110:269-305.
20.Schaffler MB.Role of bone turnover in microdamage.Osteoporos Int 2003;14:73-80.
21.Yellowley CE,Li Z,Zhou Z,et al.Functional gap junctions between osteocytic and osteoblastic cells.J Bone Miner Res 2000;15:209-17.
22.You L,Cowin SC,Schaffler MB,et al.A model for strain amplification in the actin cytoskeleton of osteocytes due to fluid drag on pericellular matrix.J Biomech 2001;34:1375-86.
23.You LD,Weinbaum S,Cowin SC,et al.Ultrastructure of the osteocyte process and its pericellular matrix.Anat Rec 2004;278:505-13.
24.Bonewald LF.Mechanosensation and transduction in osteocytes.Bonekey Osteovision 2006;3:7-15.
25.Kamioka H,Honjo T,Takano-Yamamoto T.A three dimensional distribution of osteocyte processes revealed by the combination of confocal laser scanning microscopy and differential interference contrast microscopy.Bone 2001;28:145-9.
26.Klein-Nulend J,van der Plas A,Semeins CM,et al.Sensitivity of osteocytes to biomechanical stressin vitro.FASEB J 1995;9:441-5.
27.Weinbaum S,Cowin SC,Zeng Y.A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses.J Biomech 1994;27:339-60.
28.Knothe Tate ML,Steck R,Forwood MR,et al.In vivodemonstration of load-induced fluid flow in the rat tibia and its potential implications for processes associated with functional adaptation.J Exp Biol 2000;203:2737-45.
29.You L,Temiyasathit S,Lee P,et al.Osteocytes as mechanosensors in the inhibition of bone resorption due to mechanical loading.Bone 2008;42:172-9.
30.Robling AG,Niziolek PJ,Baldridge LA,et al.Mechanical stimulation of bonein vivoreduces osteocyte expression of Sost/sclerostin.J Biol Chem 2008;283:5866-75.
31.Sawakami K,Robling AG,Ai M,et al.The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment.J Biol Chem 2006;281:23698-711.
32.Bonewald LF,Johnson ML.Osteocytes,mechanosensing and Wnt signaling.Bone 2008;42:606-15.
33.Boudreau N,Sympson CJ,Werb Z,et al.Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix.Science 1995;267:891-3.
34.Earnshaw WC.Nuclear changes in apoptosis.Curr Opin Cell Biol 1995;7:337-43.
35.Kerr JF,Wyllie AH,Currie AR.Apoptosis:A basic biological phenomenon with wide-ranging implications in tissue kinetics.Br J Cancer 1972;26:239-57.
36.Wyllie AH,Kerr JF,Currie AR.Cell death:The significance of apoptosis.Int Rev Cytol 1980;68:251-306.
37.Wilson SE,Mohan RR,Hong J,et al.Apoptosis in the cornea in response to epithelial injury:Significance to wound healing and dry eye.Adv Exp Med Biol 2002;506:821-6.
38.Desmoulière A,Badid C,Bochaton-Piallat ML,et al.Apoptosis during wound healing,fibrocontractive diseases and vascular wall injury.Int J Biochem Cell Biol 1997;29:19-30.
39.Bederson JB,Levy AL,Ding WH,et al.Acute vasoconstriction after subarachnoid hemorrhage.Neurosurgery 1998,42:352-60.
40.Rodriguez M,Lucchesi BR,Schaper J.Apoptosis in myocardial infarction.Ann Med 2002;34:470-9.
41.Ruoslahti E,Reed JC.Anchorage dependence,integrins,and apoptosis.Cell 1994;77:477-8.
42.Loo DT,Rillema JR.Measurement of cell death.MethodsCell Biol 1998;57:251-64.
43.Philchenkov A.Caspases:Potential targets for regulating cell death.J Cell Mol Med 2004;8:432-44.
杂志排行
Chinese Medical Sciences Journal的其它文章
- Primary Endodermal Sinus Tumor in the Posterior Cranial Fossa:Clinical Analysis of 7 Cases
- Coronary Artery Perforation Complicated With Acute Aortic Valve Regurgitation During Percutaneous Coronary Intervention:Report of Two Cases
- CORRECTION
- Recurrent Seizures Manifestations in a Case of Congenital Hypoparathyroidism:a Case Report
- What Moved into the Lung? An Unusual Case of Foreign Body Migration
- Reconstruction of the Medial Patellofemoral Ligament Using Hamstring Tendon Graft With Different Methods: a Biomechanical Study
