PH6/TPEE热塑性弹性体的制备
2013-10-11易春旺彭治汉王华平王朝生
易春旺,彭治汉,王华平,王朝生
(1.湖南师范大学化学化工学院,中国长沙 410081;2.上海东华大学教育部高性能纤维产品重点实验室,中国上海 201620)
1 Introduction
Thermoplastic elastomers(TPEs)[1-6],whether based on block copolymers prepared by chemical reactions or blends,generally consist of two phases,where one part is rubber(soft phase)provides good plasticity to the material,another part is plastic(hard phase)endows the material with outstanding elasticity.
The most important approach to obtain TPEs is using chemical reaction[7-9].Another approach involves melt blending elastomeric materials with cheaper rigid plastics,which could improve the thermal performances and reduce the price.However,most polymer blends consist of thermodynamically immiscible components and the resul-ting multi-phase morphology has a considerable influence on the mechanical properties of blends.To increase the compatibility of components and improve their performance,cross linking agents and compatibilizers are widely used in preparing TPEs blends.
Recently a more attractive approach by using chemical reactivity of component polymers to achieve TPEs with controlled morphology and performances was developed[10-12].Polyaimdes with amino/carboxyl end-groups,for example Polyamide-6(PA6)was often blended with other TPEs,the new formed chemical bonds between matrix and dispersed particles are feasible to improve the miscibility and mechanical properties of blends without additional compatibilizer[13-17].
As an important member of the TPEs family,thermoplastic ester are often blended with rubbers,resins,other thermoplastic elastomers and etc to modify their mechanical properties[18-19].Thus in this study,TPEEs consisting of poly(tetrahydrofuran terephthalate)hard segment and poly(tetramethylene glycol)soft segment were blended with PA6,the morphology and mechanical properties of blends have been studied in detail.
2 Experimental
2.1 Materials
TPEEs with brand name of HP35(Iv=0.79 dl g-1)were received from Sinotex Investment& Development Co.,Ltd.Two kinds of PA6 were investigated:round PA6 chips with extractables of 11.2 wt%and ηrof 2.22(socalled unextracted PA6,henceforth denoted as UPA,were presented by Ningbo Shunlong Polyamide Co.,Ltd.);PA6(round chips,ηr=2.45,extractables mass ratio 0.35 wt%)were obtained by removing extractables from UPA6 in refluxing hot water at 100℃.
2.2 Blending
TPEEs were mixed with UPA6 and PA6 in mass ratio(UPA6/PA6:TPEE)of 1∶9,1∶4 and 3∶7 in advance and dried in vacuum oven at 100℃ for at least 12 h.These mixtures were then extruded by co-rotating twin screw extruder(TSE-18A,D=18 mm,L/D=36)equipped with vacuum suction and cut into regular chips.Chips were then dried in vacuum oven at 100℃ for 12 h for following molding processing.
2.3 Analysis
The fracture surface morphology of the polymer blends was observed under a scanning electron microscope(JSM-5600 LV,Japan).Strain-stress properties were tested by WDW3020 electric universal testing machine at room temperature,the crosshead speed was varied from 20 mm·min-1to 40 mm·min-1.DMA was tested at strain of 0.1%and a heating rate of 3 ℃·min-1using a Myrenne ATM3 torsion pendulum.The glass transition temperature(Tg)was given by the peak value of tan delta curve.
3.1 Morphology
Fig.1 shows the morphology of UPA6/HP35 and PA6/HP35 blends,both blends indicate good compatibility as the content of hard phase reaches 20 wt%,small fine particles disperse in the HP35 matrix evenly.As far as we know,there are about 10 wt%extractables including monomer,linear oligomers and cyclic oligomers in UPA6.The cyclic dimmer is easily accumulated and aggregates with other components and results in the UPA6 particles(Fig.1(d))appearing larger than PA6 particles(Fig.1(a)).
The size of dispersed particles changes very little when the content of UPA6 varies from 10 wt%to 30 wt%,the blend presents a good morphology as shown in Fig.1(d ~ f).However the PA6 particle size increases significantly with the increase of PA6 content,while at the same time the fracture surface morphology indicated in Fig.1(b)and Fig.1(c)is very poor,and many deep holes attributed to the dripping of PA6 particles are observed in fracture surface.

Fig.1 SEM images of fracture surface for blends of(100 - χ)wt%HP35 and χ wt%of UPA6 or PA6: χ of(a),(b)and(c)=10,20,30(PA6);χ of(d),(e)and(f)=10,20,30(UPA6)
3.2 Mechanical properties
3.2.1 Tensile deformation behavior The elasticity and mechanical properties for all PA6/HP35 blends decrease significantly as shown in Tab.1.PA6 and HP35 are immiscible in blends without a compatibilizer.UPA6/HP35 shows better elasticity over the whole ratio range:the maximum elongation at break reaches 486%when the content of UPA6 is only 10 wt%.As UPA6 content increases to 20 wt%,the blend presents the highest breaking strength of 23.5 MPa.

Tab.1 Thermal properties of blends sample
3.2.2 Dynamic mechanical analysis Fig.2 indicates the Tan delta curves of blends,there are two peaks for all blends.The lower one is related to the rubber phase(here denoted as Tg1),another one is attributed to plastic phase(here denoted as Tg2).Tg1values of UPA6/HP35 shift to a far lower temperature than that of HP35 as shown in Fig.2.The value of Tg1decreases from-44.8℃ to-50℃ and thus forms a reversal shift of Tg1as UPA6 content increases,meanwhile Tg2values also shift to lower temperature and show peaks at temperature of 30.2 ~32.4 ℃ when UPA6 content varies from 10 wt%to 30 wt%.However,Tg1values of PA6/HP35 are over zero and peaks appear at temperature of 10.5~13.8℃,more surprisingly the Tg2values are over 97℃ and 40℃ higher than that of PA6.
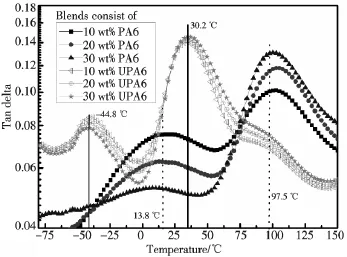
Fig.2 The Tan delta curves of blends
4 Conclusion
In this paper we studied the morphology,mechanical properties of blends prepared by UPA6,PA6 and HP35 in mass ratio of 10~30 wt%.Extractables in UPA6 play an important role as compatibilizer in the UPA6/HP35 blends.The elongation rate at break for UPA6/HP35 is higher than 300 wt%over the whole ratio range and the highest value reaches 485%when UPA6 is 10 wt%.However,blends prepared by PA6 and HP35 only show plastic characteristics and the immiscible phases damage their morphology.A more interesting phenomenon in this study is the reversal shift of glass transition temperature for rubber phase in UPA6/HP35,hence there is need for a more detailed study in the future.
[1]RZYMSKI W M,RADUSCH H J.New thermoplastic elastomers[J].Polymers,2005,50(4):221-256.
[2]REISCH M S.Stretchable plastics continue to expand[J].Chem Eng News,2005,83(42):25-26.
[3]KRIJGSMAN J,HUSKEN D,GAYMANS R J.Synthesis and properties of thermoplastic elastomers based on PTMO and tetraamide[J].Polymer,2003,44(25):7573-7588.
[4]PUSKAS J E,CHEN Y.Biomedical application of commercial polymyers and novel polyisobutylene-based thermoplastic elastomers for soft tissue replacement[J].Biomacromolecules,2004,5(4):1141-1154.
[5]DONG J Y,HONG H,CHUNG T C,et al.Synthesis of linear polyolefin elastomers containing divinylbenzene units and applications in cross-linking,functionalization,and graft reactions[J].Macromolecules,2003,36(16):6000-6009.
[6]AUSTIN T C C,WILLIAM J F N R G N.Polyester amides suitable for injection molding:US,4129715[P],1978-06-09.
[7]YI C,PENG Z,WANG H,et al.Synthesis and characteristics of thermoplastic elastomer based on polyamide-6[J].Polym Int,2011,60(12):1728-1736.
[8]VAN DER SCHUUR M,NOORDOVER B,GAYMANS R J.Polyurethane elastomers with amide chain extenders of uniform length[J].Polymer,2006,47(4):1091-1100.
[9]BEGENIR A,MICHIELSEN S,POURDEYHIMI B.Melt-blowing thermoplastic polyurethane and polyether-block-amide elastomers:Effect of processing conditions and crystallization on web properties[J].Polym Eng Sci,2009,49(7):1340-1349.
[10]TAN J,DING Y M,HE X T,et al.Abrasion resistance of thermoplastic polyurethane materials blended with ethylene-propylene-diene monomer rubber[J].J Appl Polym Sci,2008,110(3):1851-1857.
[11]OKADA O,KESKKULA H,PAUL D R.Mechanical properties of blends of maleated ethylene-propylene rubber and nylon 6[J].Polymer,2001,42(21):8715-8725.
[12]WANG J S,CHEN X D,ZHANG M Q,et al.Polyurethane/polyolefin blends:Morphology,compatibilization and mechanical properties[J].Polym Polym Comp,2006,14(1):1-11.
[13]KIM G H,HWANG S S,CHO B G,et al.Reactive extrusion of polypropylene and nylon blends from commingled plastic wastes[J].Macromolecular Symp,2007,249-250(1):485-492.
[14]KITAYAMA N,KESKKULA H,PAUL D R.Reactive compatibilization of nylon 6/styrene-acrylonitrile copolymer blends:Part 2.Dispersed phase particle size[J].Polymer,2000,41(22):8053-8060.
[15]KITAYAMA N,KESKKULA H,PAUL D R.Reactive compatibilization of nylon 6/styrene-acrylonitrile copolymer blends:Part 1.Phase inversion behavior[J].Polymer,2000,41(22):8041-8052.
[16]KITAYAMA N,KESKKULA H,PAUL D R.Reactive compatibilization of nylon 6/styrene-acrylonitrile copolymer blends:Part 3.Tensile stress-strain behavior[J].Polymer,2001,42(8):3751-3759.
[17]OKADA O,KESKKULA H,PAUL D R.Nylon 6 as a modifier for maleated ethylene-propylene elastomers[J].Polymer,1999,40(10):2699-2709.
[18]LEE T Y,LEE C H,CHO S,et al.Enhancement of physical properties of thermoplastic polyether-ester elastomer by reactive extrusion with chain extender[J].Polym Bull,2011,66(7):979-990.
[19]CHO S,JANG Y,KIM D,et al.High molecular weight thermoplastic polyether ester elastomer by reactive extrusion[J].Polym Eng Sci,2009,49(7):1456-1460.
