石墨烯/硫酸铅复合材料的制备及其在铅酸电池中的电化学性能
2013-09-15马荆亮王殿龙方明学
马荆亮 王殿龙*, 陈 飞 方明学
(1哈尔滨工业大学化工学院,哈尔滨 150001)
(2天能集团,沭阳 223600)
0 Introduction
Recently lead acid battery has come back to the sight of scientists and research institutions when carbon-lead battery is invented to meet the requirements of hybrid electric vehicles (HEV).In a long period of time,lead-acid battery has been widely used in the fields like transportation,military defense,communication,and energy storage because of its great advantages like mature technology,low cost and good reliability.Nevertheless,low energy density and short service life restrict its further development in electric vehicles,especially under the high rate partial state-of-charge when valve-regulated lead acid battery fails prematurely due to sulfation and gas evolution on negative plates[1].Conventional improvement of negative active materials is to dope active materials with suitable additives like expanders,nucleators and conductors[2-5],but the materials and structure design of lead acid batteries do not change much.Ultrabattery with a novel composite negative plate by combining a carbon-based supercapacitor and a lead-acid battery promotes the performance of lead-acid battery remarkably[6-9].Its breakthrough of performance depends on choices of appropriate carbon materials adapting acid environments and matching chargingdischarging potential of conventional negative plate,meanwhile,valid inhibitant of hydrogen evolution is also important.
Graphene-based materials are quite intriguing due to their extraordinary performance of high surface area,electronic conductivity and electrochemical properties[10].Recently,it has been reported much about graphene composites used as anode materials of lithium battery which highly improve its performance through providing good electronic contact and accommodating large specific surface area[11-13].If we can apply graphene into lead-acid battery,it may play an important role on improving capacity and rate performance of lead-acid battery by means of enhancing conductivity, capacitive effect, and dispersion of the lead sulfate,etc.
Lead sulfate can lead to the failure of lead acid battery under high rate partial state of charge,but it can also reduce the battery manufacture processes like formation process when lead sulfate is directly used as electrode materials.Lead sulfate is also very simple to synthesize,cheap and has good batch stability for production.Yan et al.[14]investigated the feasibility of lead sulfate used as positive active materials of lead acid battery and concluded this materials could satisfy the demands of electrical bicycle.Zhang et al.[15]found lead sulfate prepared from lead can be transformed to active stateby inversecharging.Chen et al.[16]published a patent about lead sulfate-graphene composite electrode material.However,few people has used lead sulfate as negative active materials of lead acid battery let alone applied in HEV according to the literature[1].If graphene with large surface area and good conductivity could be used as dispersant of lead sulfate,agglomerate of lead sulfate may be avoided.Based on this consideration,we choose graphene nano sheets(GNS)as carbon source to prepare PbSO4/GNS composites followed with investigation of their morphologies,structures,electrochemical characteristics and battery performance.
1 Experimental
1.1 Preparation of samples
Graphite oxide (GO)sheets were obtained by Hummers method[17],afterwards graphene nano sheets were prepared through thermal exfoliation of graphite oxide[18].Graphene nano sheets were mixed with certain amount of Pb(CH3COO)2·3H2O,followed by ultrasonication in distilled water and impregnation under normal temperature and pressure(100 kPa and 25℃)for 96 h.After the impregnation,the mixture was filtered and put into dilute sulphuric acid for 12 h to precipitate.Finally,PbSO4/GNS composites were obtained by extraction filtration,then washed with distilled water,and dried in air at 50℃.
1.2 Characterization
The powder XRD measurements of synthetic composites and negative pastes were conducted on a D/max-r B X-ray powder diffractometer by using a graphite monochromator with Cu Kα radiation (λ=0.154 18 nm)at 45 kV and 50 mA.The data were collected between angles(2θ)of 10~90°at a scanning rate of 5°·min-1.A Quanta 200 (FEI,American)scanning electron microscope was used for low resolution SEM analysis for synthetic composites and negative plates while a S4800 HSD (Hitachi,Japan)scanning electron microscope was used for high resolution SEM analysis for synthetic composites.
1.3 Electrochemical test
Pastes on the negative plate were made by mixing the synthetic composites,acetylene black,and polytetrafluoroethylene (PTFE,10wt%),in a weight ratio of 80∶15∶5.The negative plate grid was prepared from a normal plate grid which was cut into single grids and processed under 10 MPa pressure for 1 min to form an average dimension of 8 mm ×8 mm ×0.5 mm.The composite electrodes were obtained by pasting the negative paste on the homemade plates and drying in air at 60 ℃ for 4 h.Preparation of electrodes with graphene nano sheets and lead sulfate was the same as above.
The cyclic voltammetry (CV)method operated with a three-electrode system was used to analyze the electrochemical behavior of graphene nano sheets and synthetic composites.The working electrode(8 mm×8 mm)was an electrode with graphene nano sheets(active materials)and PbSO4/GNS composites.The counter electrode and reference electrode was commercial positive plate electrode and saturated Hg/Hg2SO4electrode,respectively.The electrolyte was sulfuric acid solution with a density of 1.347 g·cm-3.All tests were conducted at room temperature using a CHI 630b electrochemical system(Shanghai Chenhua Instrument Co.,Ltd.,China).The voltage range was 0~-1.5 V for the synthetic composites and 0~-0.95 V for the graphene nano sheets while the scanning rate changed from 1 mV·s-1to 20 mV·s-1.
Single-cell flooded lead-acid batteries,whose capacity was limited by negative plate capacity,were assembled with one prepared negative plate and one commercial positive plate from Zhejiang Tianneng Battery Company Ltd.
Charge-discharge tests of the batteries at current density of 100,200 and 300 mA·g-1in voltage range of 1.6~2.4 V at room temperature were performed in H2SO4electrolyte with density of 1.347 g·cm-3using CT-3008W battery testing system (Shenzhen Neware electronic Co.,Ltd.,China).
2 Results and discussion
2.1 Characterization of the PbSO4/GNS composites
Fig.1 presents the XRD patterns of the PbSO4/GNScomposites and lead sulfate.Lead sulfate prepared from precipitation of lead acetate and sulfuric acid display many peaks in accord with the sharp peaks of PbSO4/GNS composites.This demonstrates the existence of lead sulfate in the composites.According to the literature[18],the diffraction peaks located at 2θ of about 22°can be attributed to graphene nano sheets.The results indicate that the obtained composites consist of lead sulfate and graphene nano sheets.
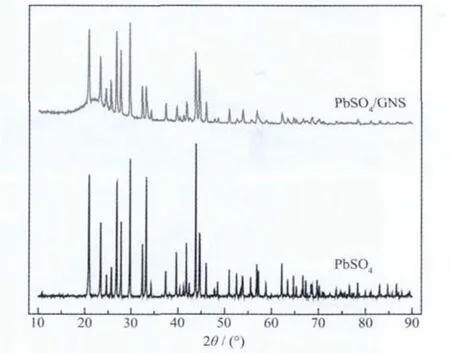
Fig.1 XRD patterns of the PbSO4/GNScomposites and PbSO4
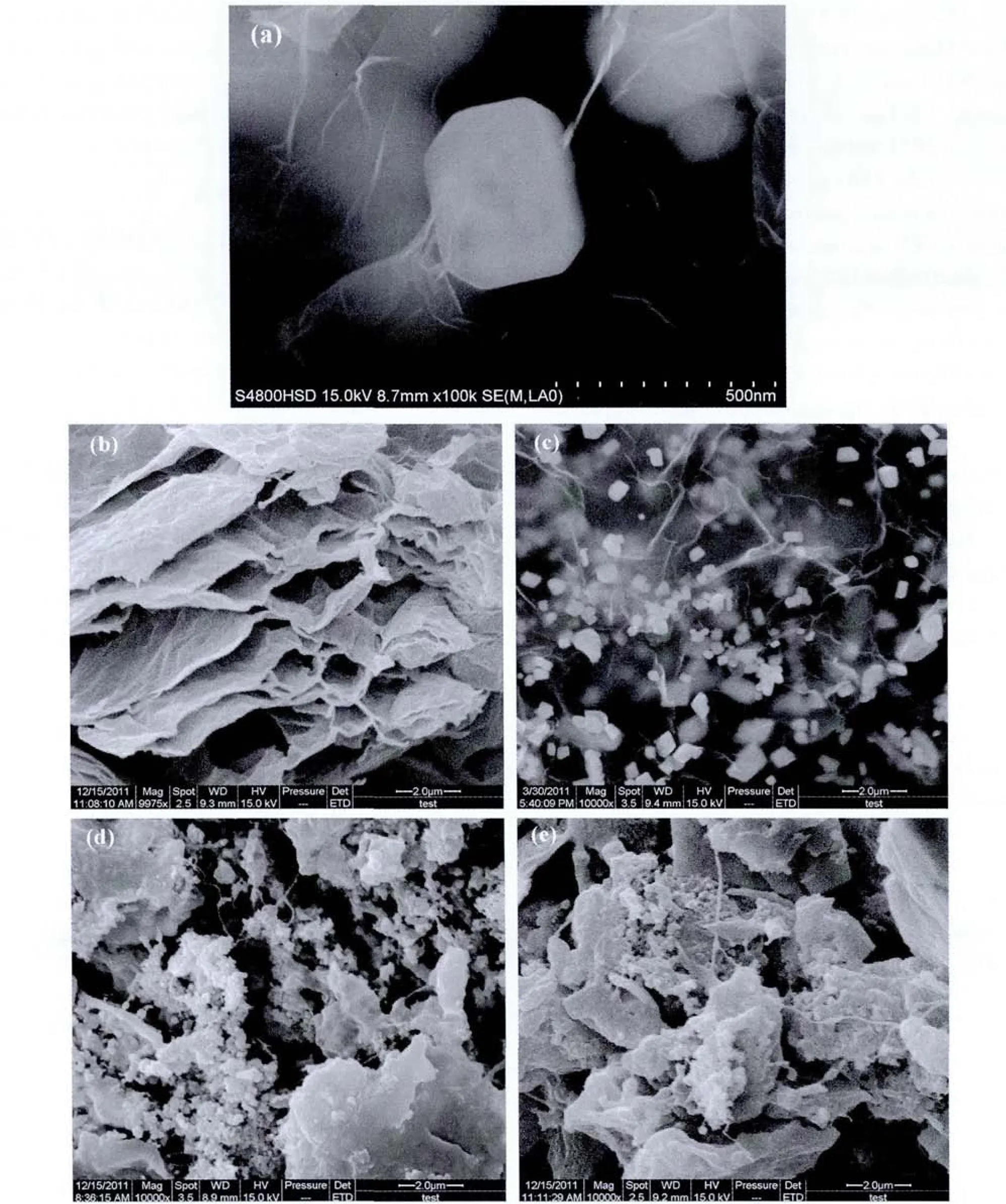
Fig.2 SEM images of GNS/PbSO4 composites(a)and(c),GNS(b),composites after charge(d)and discharge(e)
Fig.2 shows SEM pictures of PbSO4/GNS composites.A structure with a PbSO4particle inserted into graphene sheets can be clearly observed in Fig.2(a).Compared with pure graphene nano sheets in Fig.2(b),it can be seen that white particles of PbSO4are well distributed among the nano sheets in Fig.2(c).Even after high rate charging and discharging cycles,the electrodes mainly consisted of PbSO4/GNS composites still represent great dispersion of Pb(shown in Fig.2(d))or PbSO4(shown in Fig.2(e))particles among the graphene nano sheets instead of agglomerate, especially the decentralized PbSO4particles would influence the performance of lead acid battery markedly.These results indicate why graphene nano sheets could improve performance of negative plates.Since graphene nano sheets have good electronic conductivity and can distribute lead sulfate particles,lead sulfate on negative plates is easy to convert and keep small size instead of agglomerate.In the optimal conditions,mass fraction of PbSO4in the composites is about 70%.
2.2 Electrochemical testing
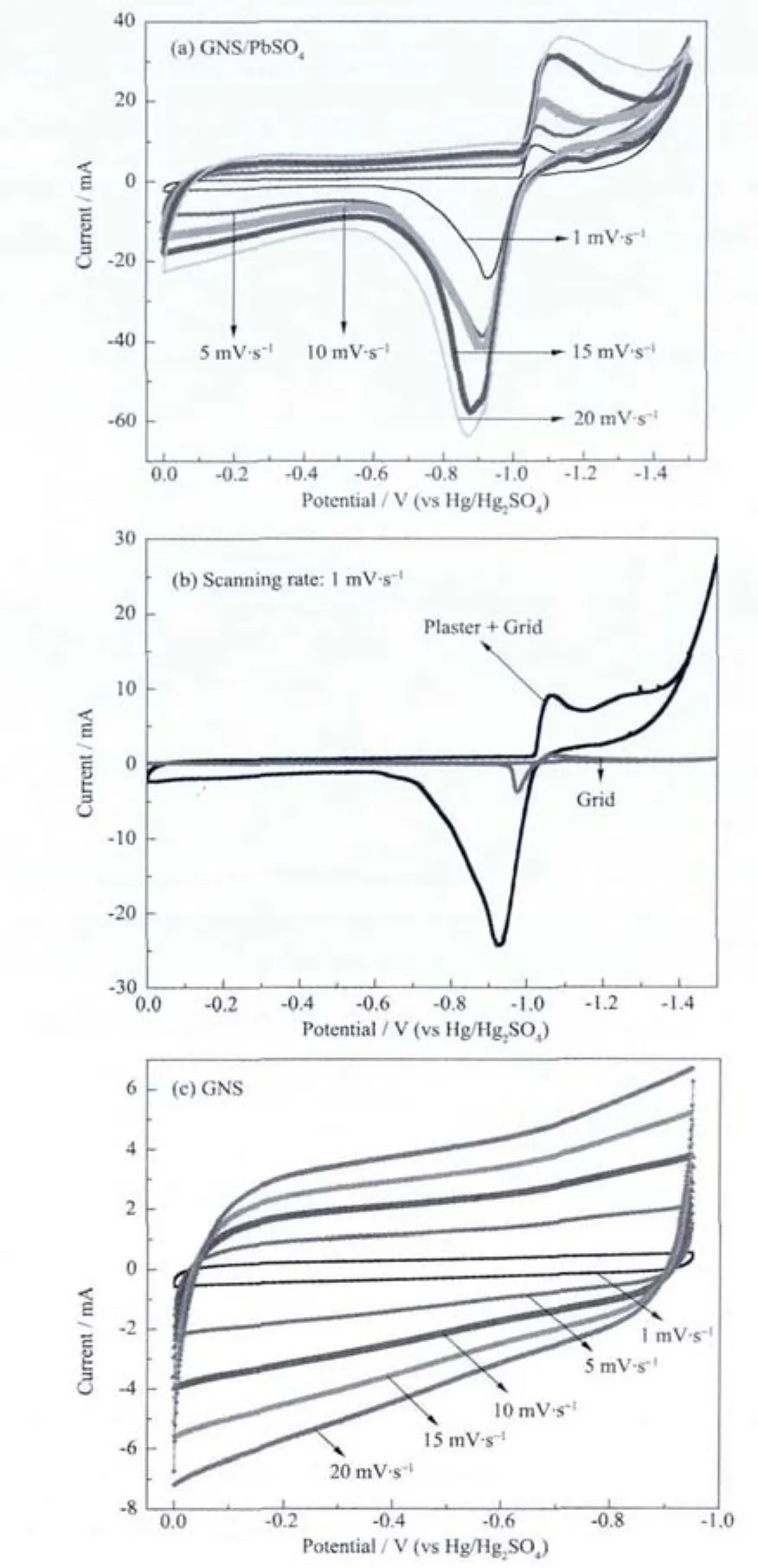
Fig.3 CV curves of composites plate(a),compare of plates with and without plaster(b),GNSplate(c)at different scanning rates
Fig.3a shows the oxidation and reduction peaks of composites plate.The peaks are shifted when the scanning rate changes from 1 mV·s-1to 20 mV·s-1.Along with the changes of oxidation and reduction current peaks,oxidation and reduction potential differential increases a little indicating the systems is almost reversible.Further,anode current at high potential zone deviates from zero line of current and the extent of excursion amplifies as the scanning rate increases.The cyclic voltammetry (CV)curves of composites plate and pure lead grid are compared in Fig.3b,which shows that current peaks of composites plate are 8 times the values of pure lead grid.This indicates the composites will release the main current.CV curves in Fig.3c show proximate rectangular shape of the cyclic voltammetry curves reflecting the capacity effect of graphene nano sheets.The current of graphene nano sheets is less than that of composites in the same potential region which could be caused by the dissociation of hydrogen atoms formed at reduction process when the scanning rate is more negative.The results imply that lead sulfate combined with graphene nano sheets can form a leadcarbon plate like an ultrabattery.When negative plates are under high rate current,the graphene nano sheets with capacitive character may act as a buffer to help negative plates absorb high density current so that sulfation can be prevented on the negative plates.Additionally,cathode current of hydrogen evolution also increases considerably caused by doped carbon materials like graphene and acetylene black whose over potential of hydrogen evolution is low.
Fig.4 shows the charge-discharge curves of batteries with negative active materials of synthetic composites,lead sulfate and graphene nano sheets at the fifth cycle.As shown in Fig.4,the chargedischarge voltage terrace of battery with PbSO4/GNS plate is the same as that of battery with graphene nano sheets plate,and the terrace for the above batteries is 0.1 V higher than that of the battery with PbSO4plate.This can be explained by the mixed potential formed on a hybrid negative electrode with carbon materials[19].The charging efficiency of the battery with graphene nano sheets and PbSO4/GNS plates is about 80%while that of the battery with PbSO4plate approaches 100%confirming the effect of hydrogen evolution shown in Fig.3a.It can be seen that charging voltage of the battery with PbSO4and graphene nano sheets plate is easy to reach 2.4 V while it is hard for synthetic composites.This may be that impurities and surface of graphene nano sheets could store many protons or solvated protons resulting in hydrogen evolution (the final charging voltage of the battery with this plate is restricted to 2.35 V).The capacity sum of lead sulfate and graphene nano sheets is nearly equal to the capacity of PbSO4/GNS revealing the capacity contribution from graphene nano sheets under high rate charging and discharging.Otherwise,the initial and final discharging voltages of the battery with PbSO4plates are distinctly different from that of the battery with composites plate whose initial voltage drops abruptly after releasing a certain amount of capacity and final voltage drops much slower.These results could both be ascribed to the addition of graphene nano sheets which can alleviate the accumulation of lead sulfate rather than the growth of PbSO4crystals and rapid failure of battery by giving scope to their dispersive,capacitive and conductive effects.
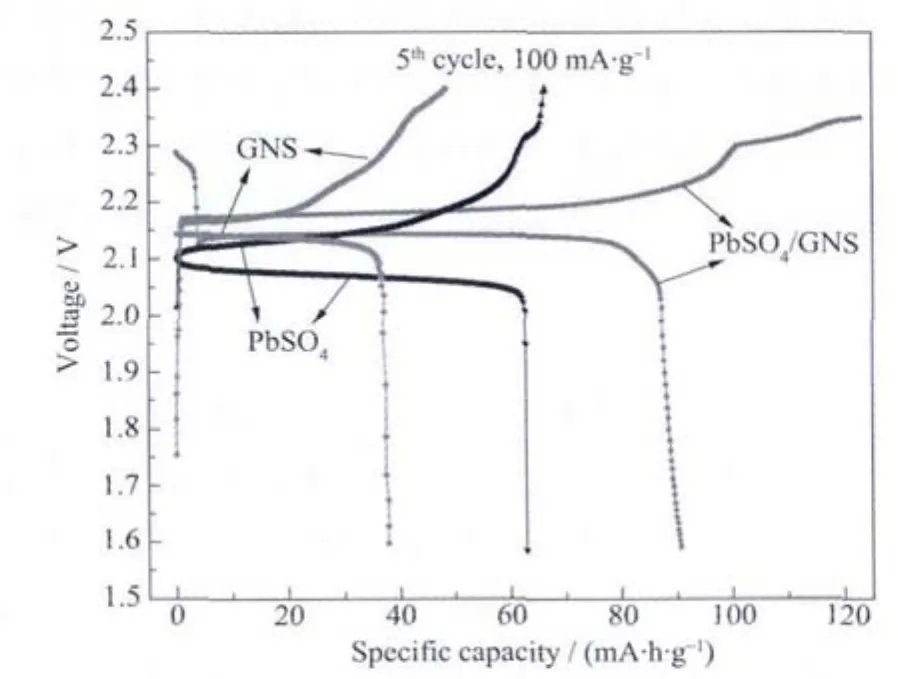
Fig.4 Charge-discharge curves of PbSO4/GNS composites,PbSO4 and GNSat the current density of 100 mA·g-1
Fig.5 presents cycle performance comparison of batteries with active materials of PbSO4/GNS composites and pure PbSO4on negative plates under constant current and voltage charge-discharge condition.Our present work mainly focuses on the capacity performance of synthetic materials under high rate charging and discharging. The average discharging specific capacity of pure PbSO4(based on negative active materials composed of PbSO4/GNS composites)is 49,5,4.7 and 0.5 mAh·g-1,respectively,at current density of 100 mA·g-1,200 mA·g-1,100 mA·g-1and 300 mA·g-1showing low capacity all the time and terrible acceptability of recharging after high rate charging and discharging.In contrast,the average discharging specific capacity of synthetic composites reaches 87,94,110 and 69 mAh·g-1,respectively,at the same series of current density revealing distinguished increment on capacity and rechargeable performance after high rate charging and discharging,thereinto,initial relative low capacities could be caused by insufficient activation of negative active materials.Capacities at the current density of 100 mA·g-1and 200 mA·g-1are high and steady while those at 300 mA·g-1start to decline gradually with the cycles indicating capacity of negative active materials can be improved in a certain range by forming PbSO4/GNScomposites.The results indicate that lead sulfate has formed massive agglomerates when it is used as active material directly,and the agglomerates cannot be converted to lead efficiently again[1].However,synthetic composites can solve the problem by combining graphene nano sheets.
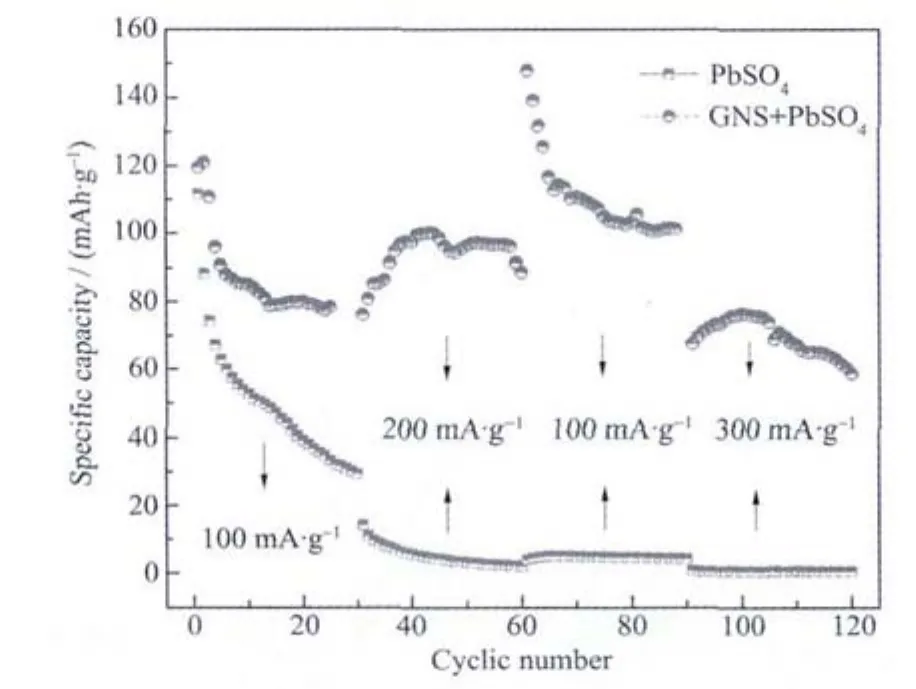
Fig.5 Cycle performance plot of PbSO4/GNSand PbSO4 at a current density of 100 mA·g-1,200 mA·g-1 and 300 mA·g-1
Considering that graphene nano sheets have large surface area,high electronic mobility and other excellent functions,graphene nano sheets in the composites on negative plate can provide an electronically conducting pathway through regions occupied by PbSO4insulator,disperse the PbSO4to restrain growth of lead sulfate crystals,form mixed potential on negative plates to convert more lead sulfate back to metallic lead,and the large surface may store H+and HSO4-to improve conductivity and speed up mass transfer process.If the PbSO4/GNS composite works well due to reasons above,decline of capacities at 300 mA·g-1may be caused by oxidation of graphene nano sheets and large amount of hydrogen emission;still,this should be evaluated by more studies and suitable inhibitant of hydrogen evolution also needs to be developed.
3 Conclusions
In conclusion,lead sulfate combined with graphene nano sheets can be used as negative active materials and a simple way of impregnation method to synthesize PbSO4/GNS composites is developed.Synthetic composites can enhance the capacity and rechargeable performance of lead sulfate.An average discharging specific capacity of PbSO4/GNScomposites reaches 110,94 and 69 mAh·g-1,respectively,at current density of 100 mA·g-1,200 mA·g-1and 300 mA·g-1,respectively,exhibiting exciting high rates and rechargeable performance of composites compared with that of pure lead sulfate.According to cyclic voltammetry results,synthetic composites show obvious capacitive effect especially at high scanning rate.Existence of PbSO4can be confirmed by XRD test,and it can be observed in SEM images that lead sulfate particles are well dispersed on graphene nano sheets.
The improvement on performance of lead sulfate by combing graphene nano sheets can be explained by capacitive contribution for forming mixed potential on negative plates,conductivity of graphene nano sheets for providing an electronically conducting pathway through PbSO4regions,larger surface area for dispersing lead sulfate particles and storing electrolyte ions to speed up mass transfer.
[1]Lam T,Haigh P,Phyland G,et al.J.Power Sources,2004,133(1):126-134
[2]Aidman G.J.Power Sources,1996,59(1):25-30
[3]Vermesan H,Hirai N,Shiota M,et al.J.Power Sources,2004,133(1):52-58
[4]Petkova G,Nikolov P,Pavlov D.J.Power Sources,2006,158(2):841-845
[5]Pavlov D,Nikolov P,Rogachev T.J.Power Sources,2011,196(11):5155-5167
[6]Lam T,Louey R.J.Power Sources,2006,158(2):1140-1148
[7]Lam T,Louey R,Haigh P,et al.J.Power Sources,2007,174(1):16-29
[8]Cooper A,Furakawa J,Lam T,et al.J.Power Sources,2009,188(2):642-649
[9]Furukawa J,Takada T,Monma D,et al J.Power Sources,2010,195(4):1241-1245
[10]Novoselov S,Geim K,Morozov V,et al.Science,2004,306(5696):666-669
[11]Chou S L,Wang J Z,Choucair M,et al.Electrochem.Commun.,2010,12(2):303-306
[12]Zhu N,Liu W,Xue M,et al.Electrochim.Acta,2010,55(20):5813-5818
[13]Li Y,LüX,Lu J,et al.J.Phys.Chem.C,2010,114(49):21770-21774
[14]Yan Z,Hu X.Mater.Chem.Phys.,2003,77(2):402-405
[15]Zhang B,Zhong J,Li W,et al.J.Power Sources,2010,195(13):4338-4343
[16]Chen F,Hu X,Ren A,et al.CN Patent,102201575.2011-9-28.
[17]Hummers S,Offeman E.J.Am.Chem.Soc.,1958,80(6):1339-1339
[18]Du Q,Zheng M,Zhang L,et al.Electrochim.Acta,2010,55(12):3897-3903
[19]Moseley T.J.Power Sources,2009,191(1):134-138
