基于Ni(Ⅱ)-双三氮唑体系的三维八钼酸盐配位聚合物的合成、晶体结构和性质
2013-09-15王秀丽田爱香刘国成
王秀丽 赵 丹 田爱香 刘国成
(渤海大学化学系,锦州 121000)
0 Introduction
Polyoxometalates(POMs)-based inorganic-organic hybrid materials have attracted extensive attention in the recent years owing to their versatile architectures and potential applications in catalysis,electrochemistry,biochemistry,photochemistry and magnetism[1-8].As a remarkable member of POM family,octamolybdates are good candidates to generate various high dimensional frameworks with novel topologies[9-13].Up to now,eight isomeric forms of octamolybdates(α,β,γ,δ,ε,ζ,η and θisomers)have been reported[14-17],which can be easily captured under hydrothermal conditions.Therefore,octamolybdates have been mostly employed as structural units to construct POM-supported metal-organic frameworks(MOFs)through linking transition metals and organic ligands.Especially,some of these isomeric forms can connect with the pure N-containing organic ligands through Mo-N bonds.For example,theγ-Mo8O264-anion contains six MoO6units (six coordination sites)and two MoO5units (five coordination sites).The MoO5unit exposes the Mo ion with low coordination number and easily coordinates with pure N-containing organic ligands through Mo-N bonds,satisfying its steady six-coordinated mode.Currently,the reports on MOFs containing Mo-N bond are very limited.So it is worth for further studying.
As is known,the organic moiety plays an important role in constructing POM-based hybrid compounds.In previous reports,the exible N-containing ligands become the preferred candidates for the construction of novel intriguing structures and topologies due to their various coordination sites and flexibility,and some POM-based novel topological structures constructed by this kind of ligands have been obtained as expected[18-24].In the earlier work,we introduced the exible bis(triazole)ligands to the Mo8O26-Cu system and successfully obtained a series of organomolybdenum compounds containing Mo-N bonds[25].
In this work,we employed the metal NiIIinstead of CuⅠ/CuⅡfor investigating whether the Mo8O26-Ni compounds also containing Mo-N bond can be obtained as expected.In addition,flexible 1,2-bis(1,2,4-triazol-1-y1)ethane (bte) (Scheme 1)was selected as exible ligand in this Mo8O26-NiIIsystem.It contains four potential coordination sites and a shorter-(CH2)2-spacer,which may induce novel coordination forms. Fortunately, a new octamolybdate-based compound containing relatively rare Mo-N bond,[Ni2(bte)5(γ-Mo8O26)]·5H2O(1),has been synthesized under hydrothermal conditions.The title compound exhibits a novel topology structure and good electrochemical and fluorescence properties.
1 Experimental
1.1 General procedures
All chemicals were of reagent grade and used without further purification.Organic ligand bte was synthesized according to the literature method[26].Elemental analyses(C,H and N)were performed on a Perkin-Elmer 240C elemental analyzer.Thermogravimetric analysis(TGA)was carried out in N2on a Pyris Diamond instrumentwith a heating rate of 10℃·min-1.A CHI 440 electrochemical workstation connected to a Digital-586 personal computer was used for the control of electrochemical measurement and data collection.A conventional three-electrode system was used.A saturated calomel electrode (SCE)was used as the reference electrode and a platinum wire as the counter electrode.Thetitlecompound chemically bulk-modified carbon paste electrode(1-CPE)was used as the working electrode.Fluorescence spectra were performed on a Hitachi F-4500 fluorescence/phosphorescence spectrophotometer at roomtemperature.
1.2 Synthsisof[Ni2(bte)5(γ-Mo8O26)]·5H 2O(1)
A mixture of Na2MoO4·2H2O(0.124 g,0.51 mmol),NiCl2·6H2O (0.12 g,0.50 mmol),bte (0.03 g,0.19 mmol)and H2O(10 mL),wasstirred for 30 min and then sealed into a 20 mL Teflon-lined autoclave,kept at 160℃for 3 d.After the autoclave cooled to room temperature over 12 h,light blue block-shape crystals of 1 were filtered off and washed with distilled water(36%yield based on Mo).Elemental analyses calcd.for C30H50Mo8N30Ni2O31(%):C 16.28,H 2.26,N 18.99.Found (%):C 16.51,H 2.08,N 19.24.IR (solid KBr pellet,cm-1):1 633.6(m),1 533.3(s),1433.0(m),1377.1(m),1288.4(s),1215.1(s),1134.1(s),1058.8(w),997.1(m),941.2(w),889.1(w),852.5(w),669.3(s).
1.3 Preparation of 1-CPE
The bulk-modified carbon paste electrode of compound 1(1-CPE)was fabricated asfollows[27]:0.03 g of compound 1 and 0.5 g of graphitepowder were mixed and grounded together by an agate mortar and pestle to achieve a uniform mixture,and then 0.18 mL of Nujol was added with stirring.The homogenized mixture was packed into a glass tube with a 3 mm inner diameter,and the tube surface was wiped with weighing paper.Electrical contact was established with a copper rod through the back of the electrode.
1.3 X-ray Crystallography
Crystallographic data for compound 1 was collected on a Bruker Smart 1000 CCD diffractometer with Mo Kα (λ =0.071 073 nm)byω and θscan mode at 296 K.The structure of compound 1 was solved by direct methods using the SHELXS program of the SHELXTL package and refined by full-matrix leastsquares methods with SHELXL[28].Metal atoms were located fromthe E-maps,and other non-hydrogen atoms were located in successive difference Fourier syntheses and refined with anisotropic thermal parameters on F2.Positions of the hydrogen atoms on carbon atoms were calculated theoretically.The H atoms of the water molecules were not included in the model because of the weak high-angle diffractions and the disorder of the water molecules[29-30].A summary of the crystallographic data and structure refinements for compound 1 are given in Table 1.Selected bond lengths and angles are given in Table2.
CCDC:881771.

Table 1 Crystal data and structure refinement for title complex
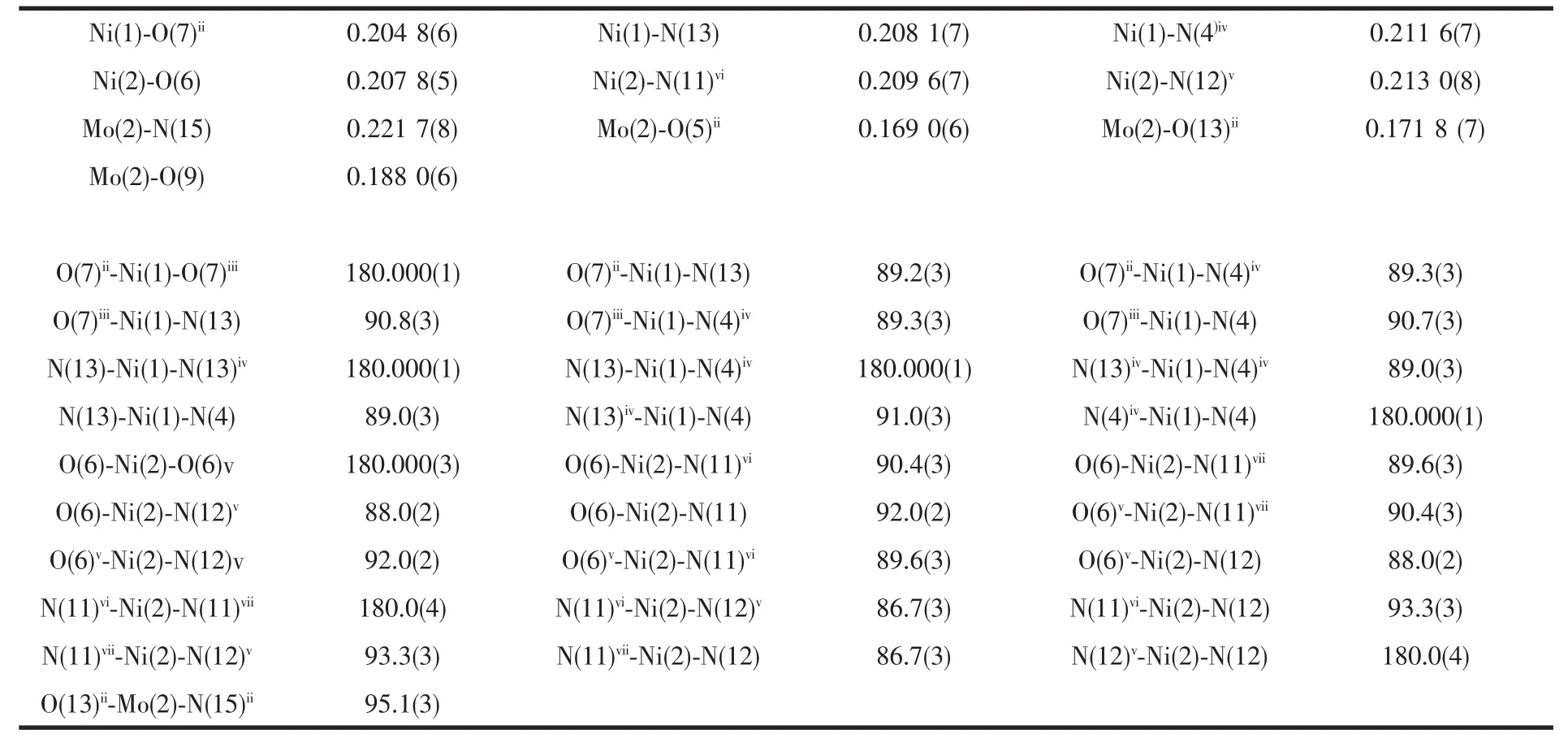
Table 2 Selected bond lengths(nm)and angles(°)for title complex
2 Resultsand discussion
2.1 Description of thestructure
Single crystal X-ray structural analysisrevealsthat the asymmetric structure unit contains aγ-[Mo8O26]4-anion(abbreviated toγ-Mo8),two NiIIions and two types of bte ligands with different coordination modes(bteaand bteb),as shown in Fig.1.The btealigands exhibit“Z”-type conformation,linking two NiIIatoms through two apical N donors,while the btebligands show “U”-type conformation.Moreover,the “U”-type btebligands own two connection forms:i)linking two NiIIatoms through two apical N donors similar to that of“Z”-type btea;ii)linking one NiIIatom and one MoVIatom of the γ-Mo8anion.
Thereare twocrystallographically independent NiIIions in compound 1.The Ni1 ion shows an octahedral coordination geometry{NiN4O2},which is completed by four nitrogen atoms of four btebligands,two terminal oxygen atoms of twoγ-Mo8anions(Ni1-O7ii=0.204 8(6)nm;Ni1-N4iv=0.211 6(7)nm;Ni1-N13=0.208 1(7)nm).The Ni2 ion is also six-coordinated in an octahedron geometry {NiN4O2}by four nitrogen atoms of two btealigands and two btebligands,two terminal oxygen atoms of twoγ-Mo8anions (Ni2-O6=0.207 8(5)nm;Ni2-N11vi=0.209 6(7)nm;Ni2-N12v=0.213 0(8)nm).These bond distances are comparable to those in the sixcoordinated NiIIcompounds[18,20]
In compound 1,two “Z”-type btealigands and four “U”-type btebligands link six NiIIions,inducing a special circle.These special circles link each other by sharing the Ni2 ions and construct a unique 2D metal-organic layer,as shown in Fig.2.Theγ-Mo8anions embedded in these special circles through the Ni-O bonds,acting as a structural directing agent.In addition,theγ-Mo8anions are extended into an infinite 2D inorganic layer bridged by Ni1 and Ni2 atoms along the a axis.
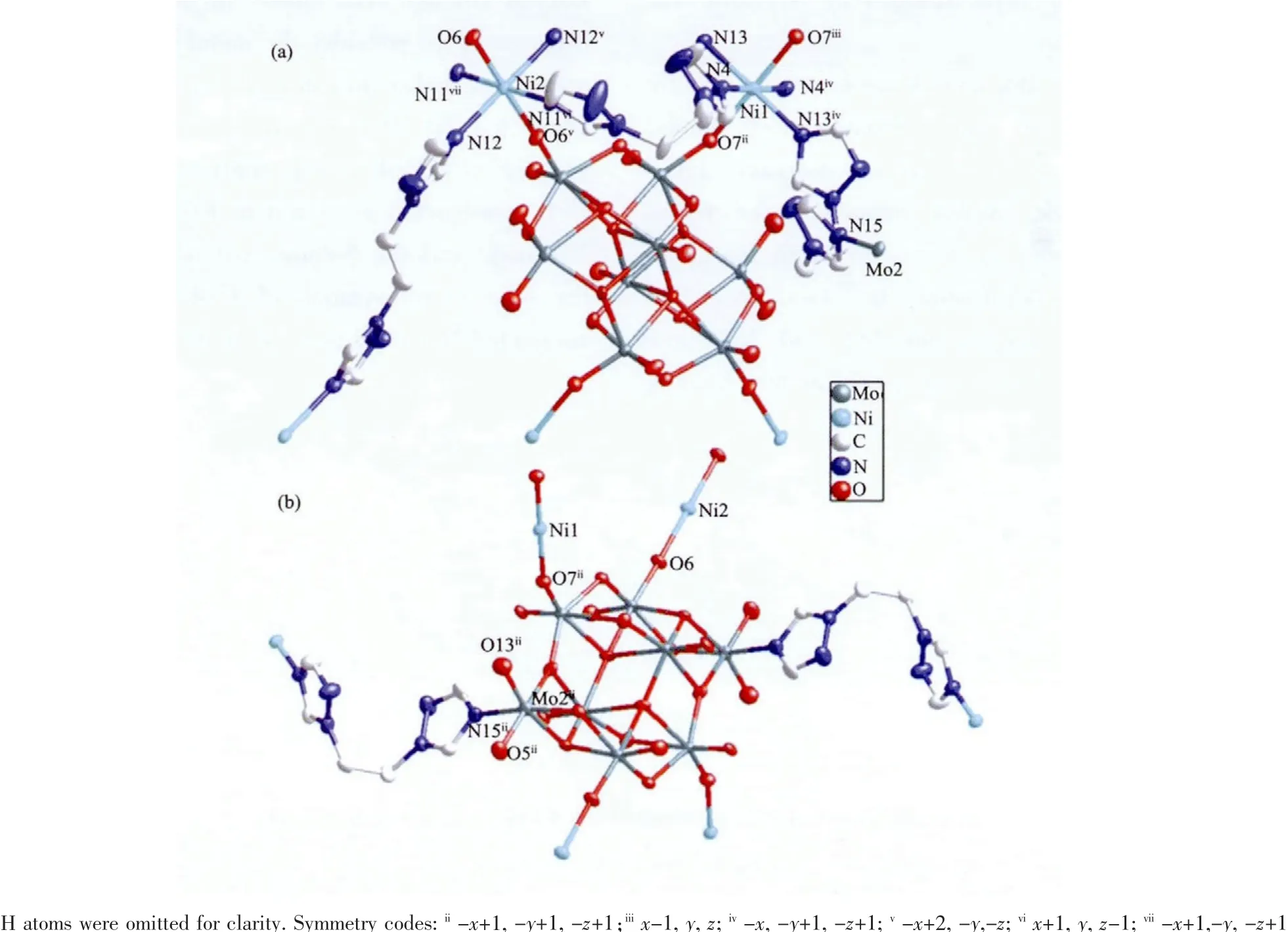
Fig.1 (a)The ball-and-stick representation with 50%probability thermal ellipsoids of the structural unit in compound 1 and(b)coordination mode ofγ-Mo8O26 anion functionalized by bteb ligand through Mo2-N15 bond

Fig.2 Ball/stick(a)and the schematic(b)diagram of the 2D metal-organic layer in compound 1;(c)The γ-Mo8O26 anions are linked by Ni1 and Ni2 ions and embed in the 2D metal-organic framework
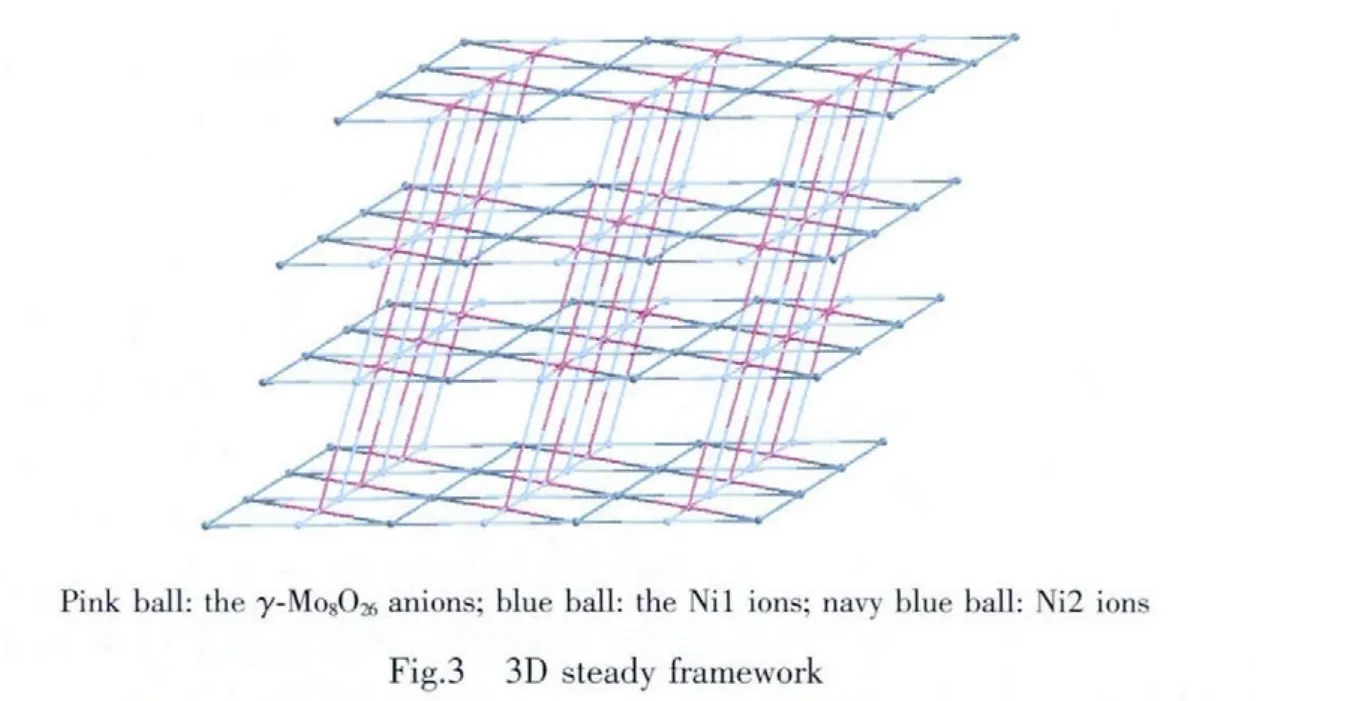
Moreover,theγ-Mo8anions are functionalized by another “U”-type btebligands through Mo-N bonds,which connect the adjacent 2D metal-organic layer into a 3D framework.In this compound,the γ-Mo8anion can be regarded as a six-connected inorganic node, which is coordinated by two pairs of crystallographically independent Ni1 and Ni2 ions through O7 and O6 atoms and a pair of btebligands directly through N15 atoms.In order to simplify this framework,we consider the metal Ni1,Ni2 andγ-Mo8anions as nodes and a total topology of (6,6,6)-connected 3D MOF with the Schläfli symbol of{32·44·54·62·73}{32·46·56·6}2analyzed by OLEX program[31]is constructed as shown in Fig.3.The 6-connected Ni1 node and the 6-connectedγ-Mo8node possesses the same vertex symbols of 32·46·56·6,while the 6-connected Ni2 node is 32·44·54·62·73.
2.2 Thermogravimetric analysis
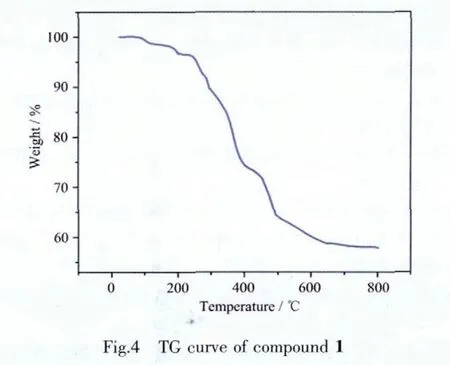
The thermogravimetric analysis (TGA)of 1 was performed under N2atmosphere with a heating rate of 10 °C·min-1in the temperature range of 25~800 ℃(Fig.4).The TGcurve of compound 1 shows two distinct weight losssteps:Thefirst weight lossstep occursbelow 250 ℃,corresponding to the loss of water molecules.The second weight loss step in the range of 250~800 ℃can be attributed tfo the decomposition of bte ligands 37.12%(Calcd.37.07%).
2.3 Electrochemical properties
The voltammetric behaviours of compound 1 bulkmodified carbon paste electrode (1-CPE)were carried out in the 0.1 mol·L-1H2SO4aqueous solution at different scan rates.The cyclic voltammetry for 1-CPE at different scan rates is presented in the potential range of+800 to-210 mV(Fig.5a).There exhibit three reversible redox peaks I-I′,II-II′and III-III′with the mean peak potentials E1/2=(Epa+Epc)/2 of approximate+295,+156,and-82 mV,respectively,which can be attributed to the redox of the [Mo8O26]4-anion[9].With increasing of scan rates,the cathodic peak potentials shift to the negative direction and the corresponding anodic peak potentials shift to the positive direction.It can be seen that the peak currents are proportional to the scan rates up to 500 mV·s-1,which indicates that the redox process of the 1-CPE is surface-controlled(Fig.5b).
It′s well known that direct electroreduction of nitrite requiresa large overpotential[32]at most electrode surfaces and no obvious response was observed for nitrite at the bare CPEin the potential range of+800 to-210 mV in 0.1 mol·L-1H2SO4aqueous solution.In this work,the 1-CPE(scan rate:200 mV·s-1)displayed good electrocatalytic activity toward the reduction of nitrite.At the 1-CPE,with addition of nitrite,all three reduction peak currents gradually increase,while the corresponding oxidation peak currents decrease,indicating that 1-CPE shows good electrocatalytic activity toward the reduction of nitrite.
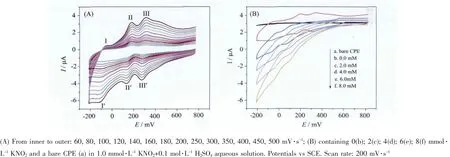
Fig.5 (a)Cyclic voltammograms of the 1-CPE in the 0.1 mol·L-1 H2SO4 aqueous solution at different scan rates;(b)Cyclic voltammograms of the 1-CPE in 0.1 mol·L-1 H2SO4 aqueous solution
2.4 Fluorescence property
The fluorescence properties of compound 1 and thefree ligand bte were investigated in the solid state at room temperature(Fig.6).The maximal emission peak of compound 1 was observed at about 403 nm (λex=270 nm),whereas the bte at 387 nm (λex=300 nm).The significant red shift of the emission observed in 1 might be attributed to a change in the highest occupied molecular orbital and lowest unoccupied molecular orbital energy levels ofγ-Mo8anions and neutral ligands coordinating to metal centers,or assigned to the ligand-to-metal charge-transfer(LMCT)band[33].

Fig.6 Fluorescence spectra for compound 1(blue line)and bte(red line)in the solid state at room temperature
3 Conclusions
In summary,a new 3D MOF with the Schläfli symbol of {32·44·54·62·73}{32·46·56·6}2has been isolated successfully under hydrothermal conditions.In the title compound,theγ-Mo8anion not only acts as a structural directing agent embeding in the 2D metalorganic layer,but also acts as the bridging unit functionalized by the bte ligands through Mo-N bonds.The electrochemical and fluorescence properties indicate that the title compound is an excellent electrocatalytic and luminescent material.
[1]Zhong Y L,Ng W W,Zhang J X,et al.J.Am.Chem.Soc.,2009,131:18293-18298
[2]Yamase T.Chem.Rev.,1998,98:307-325
[3]Qi W,Wang Y Z,Lin W,et al.Chem.Eur.J.,2010,16:1068-1070
[4]Yan X H,Zhu P L,Fei J B,et al.Adv.Mater.,2010,22:1283-1287
[5]Ritchie C,Ferguson A,Nojiri H,et al.Angew.Chem.Int.Ed.,2008,47:5609-5612
[6]Xi ZW,Zhou N,Sun Y,et al.Science,2001,292:1139-1141
[7]Botar B,Geletili Y V,Kgerler P,et al.J.Am Chem.Soc.,2006,128:11268-11277
[8]Kamata K,Yonehara K,Sumida Y,et al.Science,2003,300:964-966
[9]Liu H Y,Wu H,Ma JF,et al.Dalton Trans.,2011,40:602-613
[10]Liu H Y,Bo L,Yang J,et al.Dalton Trans.,2011,40:9782-9788
[11]Zhang C J,Pang H J,Tang Q,et al.New J.Chem.,2011,35:190-196
[12]Lan Y Q,Li SL,Wang X L,et al.Inorg.Chem.,2008,47:8179-8187
[13]Li S L,Lan Y Q,Ma J F,et al.Inorg.Chem.,2007,46:8283-8290
[14]Bridgeman A J.J.Phys.Chem.,A,2002,106:12151-12160
[15]Wu C D,Lu C Z,Zhuang H H,et al.Inorg.Chem.,2002,41:5636-5637
[16]Hageman D,Zapf P J,Zubieta J.Chem.Commun.,1998,1283-1284
[17]Raring R S,Zubieta J.Inorg.Chim.Acta,2001,312:188-196
[18]Wang X L,Li J,Tian A X,et al.CtystEngComm,2011,13:2194-2196
[19]Wang X L,Hu H L,Liu G C,et al.Chem.Commum.,2010,46:6485-6489
[20]Wang X L,Li J,Tian A X,et al.Cryst.Growth Des.,2011,11:3456-3462
[21]Tian A X,Ying J,Peng J,et al.Inorg.Chem.,2008,47:3274-3283
[22]WU Jing-Jing(吴晶晶),HAN Zhan-Gang(韩占刚),WANG Yan-Nan(王彦娜),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2010,26:921-925
[23]WANG Xiao-Lan(王晓兰),LI Yang-Gang(李阳光),MANG Jing-Xi(孟靖昕),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2010,26:391-397
[24]Wang X L,Zhao D,Tian A X,et al.Inorg.Chim.Acta,2012,388:114-119
[25]Tian A X,Liu X J,Ying J,et al.CtystEngComm,2011,13:6680-6687
[26]LIU Xun-Gao(刘训高),ZHANG Yu-Mei(张玉梅),LI Bao-Long(李宝龙),J.Suzhou Univ.:Natural Sci.(Suzhou Daxue Xuebao:Ziran Kexueban),2005,21:59-62
[27]Wang X L,Kang Z H,Wang E B,et al.Mater.Lett.,2002,56:393-396
[28]Sheldrick GM.Acta Crystallogr.A,2008,64:112-122
[29]Gao Y Z,Xu Y Q,Han Z G,et al.J.Solid State Chem.,2010,183:1000-1006
[30]Bi Y F,Du SC,Liao WP.Chem.Commun.,2011,47:4724-4726
[31]Dolomanov O V,Blake A J,Champness N R,et al.J.Appl.Crystallogr.,2003,36:1283-1284
[32]Keita B,Nadjo L.J.Electroanal.Chem.,1987,227:77-98
[33]Liu X G,Wang L Y,Zhu X,et al.Cryst.Growth Des.,2009,9:3997-4005
