Kinetic Modeling of Methanol to Olefins (MTO) Process on SAPO-34 Catalyst
2013-07-31PuJianglongWengHuixin
Pu Jianglong; Weng Huixin
(East China University of Science and Technology, Shanghai 200237)
Kinetic Modeling of Methanol to Olefins (MTO) Process on SAPO-34 Catalyst
Pu Jianglong; Weng Huixin
(East China University of Science and Technology, Shanghai 200237)
A kinetic model of MTO process over the SAPO-34 catalyst considering the effect of water and coke deposition has been proposed. The model takes into account three steps of the MTO reaction in which the products cover 5 lumped components. The water in the feed not only reduces the concentration of methanol but also alleviates the deactivation of SAPO-34 catalyst. The kinetic parameters have been estimated by the least square method. It has been proved that the calculated values in the kinetic model are in good agreement with the experimental values.
methanol to olefin process (MTO); kinetic modeling; SAPO-34; deactivation
1 Introduction
The rapid development of the national economy leads to a rising demand for ethylene and propylene, which are the basic chemical raw materials. The methanol to olefins (MTO) process provides a new route for the production of ethylene and propylene. Compared with the traditional ethylene and propylene production process (steam cracking of NGL or naphtha), MTO has some advantages, because MTO can provide a wide and flexible range of ethylene and propylene ratios to meet the market needs[1]. The industrial implementation of MTO process at high methanol conversion has been possible thanks to the high activity and selectivity of the SAPO-34 catalyst[2]. The kinetic model for MTO process has been studied by many researchers because of the importance of MTO process in the production of lower olefins. Considering the effect of coke on the MTO process, Bos, et al. proposed a kinetic model consisting of 12 reactions involving 6 component lumps plus coke[3]. The kinetic model agrees well with the carbon pool mechanism proposed by Dahl and Kolboe[4]. Gayubo, et al. simplified the model of Bos by eliminating the slower steps and studied the effect of water in the reaction in order that the model could be easily used in the design of the reactor for optimized production of lower olefins[5].
In this paper, the kinetic model for the MTO process is studied on SAPO-34 catalyst in a fixed bed reactor, which has taken into consideration the effects of catalyst deactivation by water and coke deposition. The new kinetic model, which covers 3 steps for the formation of olefins in the MTO process, has 5 lumped products. The reaction rate equations have been introduced according to the proposed reaction network, and the parameters of the model are estimated by the least square method.
2 MTO Kinetic Modeling
2.1 MTO reaction network
The reaction of MTO process is composed of three steps, viz.: (1) dehydration of methanol to dimethyl ether (DME); (2) formation of intermediate species, [CH2]; and (3) formation of olefins and other paraffins[4]. The overall reaction path may be represented by:
Since the source of the olefins is uncertain, the methanol and DME are regarded as one lumped component called oxygenates[6]. The products ethylene, propylene and butene are lumped as C2, C3, and C4, respectively. Since the amount of methane, CO, and CO2in the products is small, they are lumped as C1. The new proposed kineticmodel for MTO process agrees well with the Hydrocarbon Pool mechanism[4]which is shown in Figure 1.
The kinetic scheme includes 6 first-order and one secondorder reactions. Reaction 6 is the second order one, in which the formation rate of ethylene from propylene depends on the concentration of propylene and methanol in the medium[3]. Upon considering both the accuracy and simplicity of the model, the secondary reaction of butene is ignored because of the small amount of butene compared with propylene in the products. According to the kinetic scheme shown in Figure 1, the reaction rate equations can be obtained easily, which are formulated as a function of the mass fraction (expressed on a water-free basis,Xi) of the corresponding reactants.
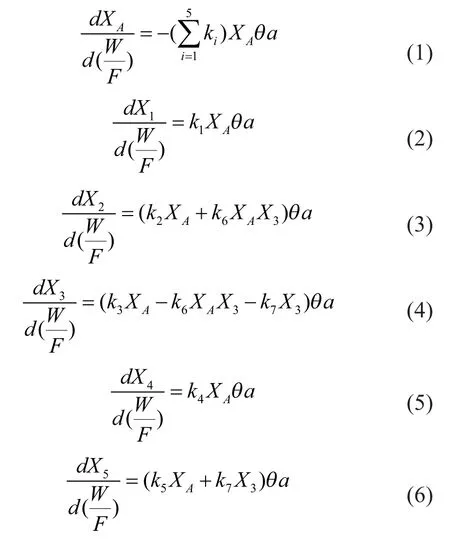
In the model,θis a function that quantifies the effect of water in the reaction medium;ais the activity of SAPO-34 catalyst;kiis the reaction rate constant of each reaction, which follows the relationship of Arrhenius equation:

2.2 Deactivation of SAPO-34 catalyst
Coke deposition is extraordinarily rapid in the MTO process, because under more severe conditions a coke deposition rate equating to 3—4% of the methanol amount in the feed is measured in the first minute of time on stream[5]. The activity of the SAPO-34 catalyst declines with increase of the time on stream. Moreover, the activity of SAPO-34 catalyst changes with the reaction temperature, space velocity, and the degree of methanol dilution by water in the feed. Consequently, the coke induced deactivation of catalyst cannot be overlooked especially with respect to the fresh SAPO-34 catalyst. The activity of SAPO-34 catalyst is defined by the ratio between the reaction rate of catalyst at a given time on stream,r, and the reaction rate that the fresh catalyst would have at that time under the same reaction conditions,rf:

The deactivation rate equation can be expressed by the following relationship, which indicates that the deactivation of SAPO-34 catalyst complies with the parallel deactivation mechanism:
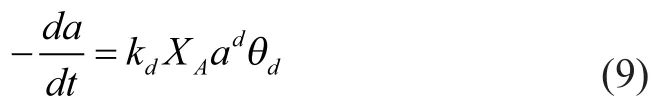
In this equation,kdis the deactivation rate constant which follows the relationship of Arrhenius equation, andθdis a function that quantifies the attenuating effect of water in the reaction medium on coke deposition of SAPO-34 catalyst.
2.3 Effect of water in the kinetic model
The presence of water in feed has a great effect on the reaction rate in the MTO process. Compared with the methanol in the water-free feed, the presence of water decreases the rate of reaction because of the dilution of the methanol concentration in feed. The effect of water resistance can be quantified by the functionθ:

In the equation,Kwis a parameter that quantifies the water resistance for the reaction rate, andXwis the water content in the reaction medium which is the weight fraction (on awater-free basis) of water in the reaction medium.
On the other hand, the presence of water in feed also has a significant effect of attenuating coke induced deactivation of catalyst. This result can be attributed to the competition of water against coke deposition on the acid sites of SAPO-34 catalyst. Water can be adsorbed on the acid sites of SAPO-34 catalyst prior to the coke deposition because water is a polar molecule[7]. The inhibition effect of water on coke deposition alleviates the deactivation of the SAPO-34 catalyst which can be quantified by the functionθd[8]:

whereKwdis a parameter that qualifies the water resistance against the coke deposition on SAPO-34 catalyst.
The water content in the reaction medium,Xw, is a sum of the water in feed,Xw0,and the water formed,Xwf.

The water formed in the reaction medium has a relationship with the oxygenate content,XA, which can be expressed by the following equation[9]:
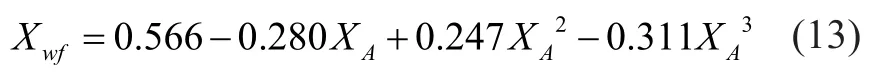
3 Estimation of Kinetic Parameters
3.1 Experimental data on SAPO-34
The experimental data on the parameter estimation were obtained from Qi[10], whose experiments were conducted in a fixed bed reactor using the fresh SAPO-34 catalyst. The temperature of the reaction was 375 ℃, 400 ℃, 425 ℃, 450 ℃, and 475 ℃, respectively, and the space velocity over the catalyst varied from 3.84 h-1to 53.19 h-1(grams of methanol fed into the reactor in an hour versus grams of catalyst), while the total reaction pressure was kept at atmospheric pressure. The ratio of water and methanol in feed was 0 and 1 in order to investigate the effect of water on the reaction. The time on steam of the process was equal to 1.5 minutes.
3.2 Estimation procedure
The procedure for estimating the kinetic model involves non-linear parameter estimation problems. The ordinary differential equations were solved by the fourth-order method of Lung-Kutta, in which the initial conditions were: (W/F)0=0,XA0=1, andXi0=0 (i=1, 2, 3, 4, 5). After the concentration of each component at the outlet was calculated, the parameters in the kinetic model were estimated by the least square method in order to minimize the objective function,EOF.
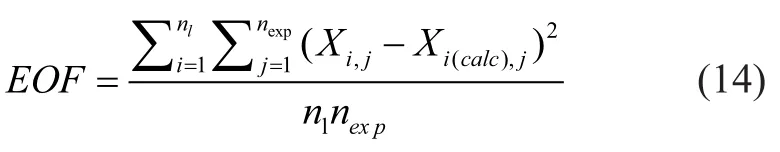
whereXi,jis the experimental value of weight fractions forilump in the experimental pointj, andXi(calc),jis the calculated value, whereasnlis the number of lumps, andnexpis the number of experimental points.
4 Results and Discussion
4.1 Kinetic parameters
The parameters of the kinetic model are estimated and are presented in Table 1, in which the corresponding value of the objective function,EOF, is 1.78×10-4.
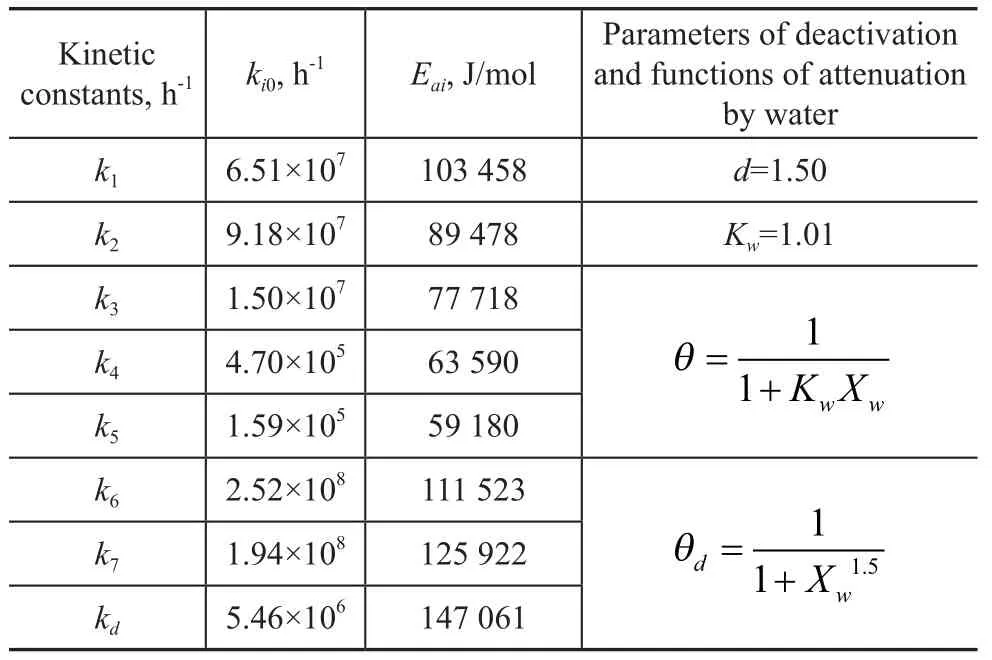
Table 1 Parameters of the kinetic model of MTO over SAPO-34 catalys
The average relative errors and the maximum relative errors of calculated values are listed in Table 2. Since the desired products in the MTO process mainly cover ethylene and propylene, the emphasis of the study is to predict the ethylene and propylene yields. As shown in Table 2, the maximum relative error of ethylene yield is 15.83% and its average relative error is 4.10%. The maximum relative error of propylene yield is 15.81%, with the average relative error reaching 3.54%. The average relative errors of all the lumped components are less than 10%. Consequently, the calculated values of the kinetic modelare reliable.
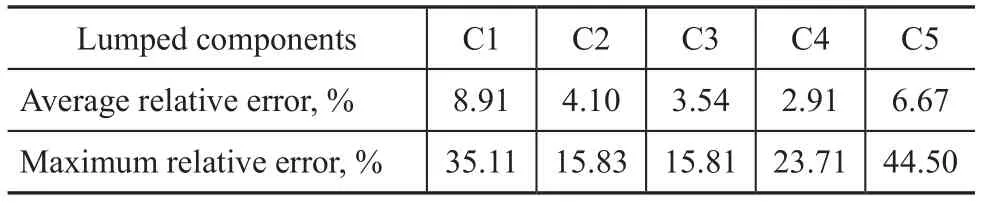
Table 2 Relative errors of each lumped component
4.2 Statistical test of the model
The statistical tests of the kinetic model, including the decisive index, theρ2tests and theFtests, are conducted. The significant level,αin theFtest is 5%, which evaluates the deviation for the mean regression. The results of the statistical tests confirm thatρ2>0.95 andF>10Ft,as shown in Table 3. Consequently, the kinetic model and the estimated parameters are reliable.
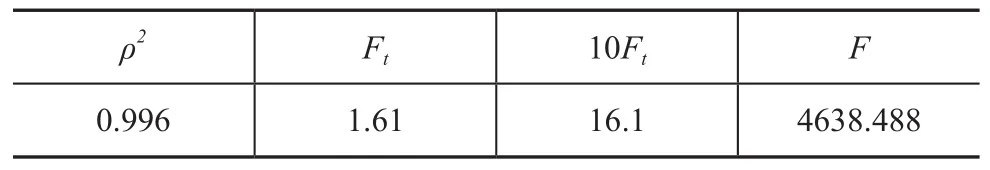
Table 3 Statistical test of the kinetic model
The calculated values of each lumped component are compared with the experimental values as shown in Figure 2. It can be seen from Figure 2 that the data points are almost symmetrically distributed on both sides of the diagonal line. It is proved that the calculated values are in good agreement with the experimental values.
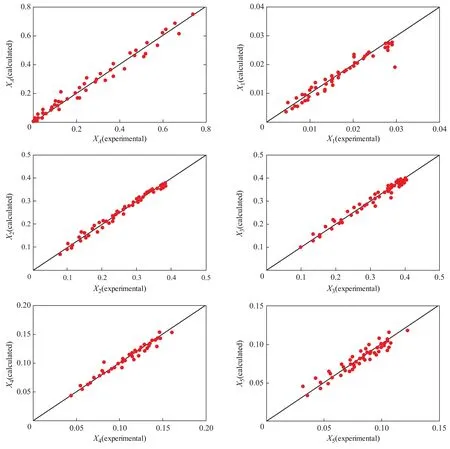
Figure 2 Comparison between calculated values and experimental values
5 Conclusions
In this study, a new kinetic model for MTO process in fixed bed reactor was built by considering the effect of water and coke deposition. The effect of water in the feed on the reaction is related with dilution of methanol and attenuation of the SAPO-34 catalyst deactivation. Since the increase of the coke deposition on fresh SAPO-34 catalyst is rapid at the beginning of the reaction, the deactivation of the SAPO-34 catalyst must be considered. The parameters of the kinetic model are estimated by the least square method and the calculated values are compared with the experimental values. The result has verified that the calculated values are in good agreement with the experimental values, with the average relative errors of all lumped components being less than 10%. So the kinetic model has a good capability to predict the performance of the lumped components in fixed bed reactor.
Nomenclature
a — activity of the SAPO-34 catalyst
d — order of deactivation
W — weight of SAPO-34 catalyst, g
F — mass flow rate of methanol in the feed, g/h
R — molar gas constant, J mol-1K-1
t — time on stream, min
T — thermodynamic temperature, K
θ — function of attenuation by water responsible for the main reaction
[1] Najafabadi A T, Fatemi S, Sohrabi M, et al. Kinetic modeling and optimization of the operating condition of MTO process on SAPO-34 catalyst[J]. Journal of Industrial and Engineering Chemistry, 2012, 18 (1): 29-37
[2] Froment G F, Dehertog W J H, Marchi A J. Zeolite catalysis in the conversion of methanol into olefins[J]. Catalysis, 1992, 9 (1): 1-64
[3] Bos A N R, Tromp P J J, Akse H N. Conversion of methanol to lower olefins kinetic modeling, reactor simulation and selection[J]. Ind Eng Chem Res, 1995, 34(11): 3808-3816
[4] Dahl I M, Kolboe S. On the reaction mechanism for propene formation in the MTO reaction over SAPO-34[J]. Catal Lett, 1993, 20: 329-336
[5] Gayubo A G, Aguayo A T, Sánchez del Campo A E, et al. Kinetic modeling of methanol transformation into olefins on a SAPO-34 catalyst[J]. Ind Eng Chem Res, 2000, 39(2): 292-300
[6] Chen D, Grønvold A, Moljord K, et al. Methanol conversion to light olefins over SAPO-34: reaction network and deactivation kinetics[J]. Ind Eng Chem Res, 2007, 46(12): 4116-4123
[7] Wang Geng, Tang Yu, Xue Zhenxin. Recent progress in methanol to olefins technology[J]. Liaoning Chemical Industry, 2011, 40 (7): 735-738 (in Chinese)
[8] Gayubo A G, Aguayo A T, Alonso A, et al. Reaction scheme and kinetic modelling for the MTO process over a SAPO-18 catalyst[J]. Catalysis Today, 2005, 106: 112-117
[9] Sánchez del Campo A E. Kinetic modelling of the transformation of methanol into light olefins on a SAPO-34 catalyst[D]. University of the Basque Country, Bilbao, Spain, 1997
[10] Qi Guozhen, Xie Zaiku, Chen Qingling. Kinetics of methanol to olefins over fresh SAPO-34 catalyst[J]. Chemical Reaction Engineering and Technology, 2012, 28 (6): 506-512 (in Chinese)
Recieved date: 2013-04-15; Accepted date: 2013-05-09.
Prof. Weng Huixin, Telephone: +86-0 21-64252816; E-mail: hxweng@ecust.edu.cn.
杂志排行
中国炼油与石油化工的其它文章
- Study on the Synthesis and Properties of PET Using Hydrotalcite as Catalyst
- Development and Commercial Application of RSDS-II Technology for Selective Hydrodesulfurization of FCC Naphtha
- CFD Simulation of Orifice Flow in Orifice-type Liquid Distributor
- Photocatalytic Denitrogenation over Modified Waste FCC Catalyst
- Experimental and Molecular Dynamics Simulations for Investigating the Effect of Fatty Acid and Its Derivatives on Low Sulfur Diesel Lubricity
- Study on Reactive Adsorption Desulfurization of Model Gasoline on Ni/ZnO-HY Adsorbent
