Study on the Synthesis and Properties of PET Using Hydrotalcite as Catalyst
2013-07-31LiGuiheFuZhifengCaoDing
Li Guihe; Fu Zhifeng; Cao Ding
(State Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing 100029)
Study on the Synthesis and Properties of PET Using Hydrotalcite as Catalyst
Li Guihe; Fu Zhifeng; Cao Ding
(State Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing 100029)
Poly(ethylene terephthalate) (PET) was synthesized by the in-situ polymerization method using layered double hydrotalcite (LDH) as the catalyst, and the thermal and flame retardation properties of PET were investigated as required. As identified by differential scanning calorimetry (DSC) and thermogravimetric (TGA) analysis, the crystallization rate and thermal degradation temperature of the as-prepared PET sample were enhanced compared with commercial PET sample. It was confirmed from the fire-resistant property study that the LDH can be used as an efficient flame-retardant besides functioning as a catalyst in the transesterification /polycondensation process for PET synthesis.
poly(ethylene terephthalate); layered double hydrotalcite; transesterification; polycondensation; catalyst
1 Introduction
Poly(ethylene terephthalate) (PET) featuring good mechanical strength, toughness, fatigue resistance, and high crystal melting temperature is widely used in the production of films, plastic objects and fibres[1]. The production of PET in an industrial scale is normally carried out through two stages, namely: one is the prepolymer synthesis which is mainly related with bis(2-hydroxyethyl) terephthalate (BHET) and its oligomer, and another is associated with a polycondensation step of the prepolymers. The prepolymer is synthesized either through esterification of terephthalic acid (TPA) with ethylene glycol (EG) or through transesterification of dimethyl terephthalate (DMT) with EG. Although all the catalysts used for transesterification can catalyze the polycondensation reaction, but these catalysts do not provide satisfactory products because they are also active in ester decomposition[2]. Therefore, a different catalyst, which is mainly an antimony compound, has been applied to catalyze the polycondensation step in most commercial plants since the invention of PET[3]. However, there are intensive research activities to find a replacement for antimony compounds because of not only the negative impact of antimony on both health and environment[4]but also its very low activity in the transesterification reaction[5]that needs to use two different catalysts in PET production via the DMT process, which is one of the drawbacks of this process with respect to the TPA process. Recently, the hydrotalcite-like (HT) compounds, the naturally occurring layered double hydroxides (LDH) with carbonate anion, a representative example of which has the formula of Mg6Al2(OH)16(CO3)·4H2O, have been patented as safer, cheaper, and more efficient catalysts in both stages of PET production[6].
In a previous paper[7], the activity, selectivity and polycondensation reaction kinetics of LDH were studied. It has been proposed that the hydroxide group of LDH is necessary for its activity in polycondensation of BHET. The catalytic activity is not related to its specific surface area, which is common in other heterogeneous catalysts. In addition, the activity of LDH decreases with increasing calcination time and temperature. Like the inorganic hydroxides, LDH has flame-proof properties and has been used for preparation of flame-retardant polymer composites. LDH/PET nanocomposites were also prepared by direct melt compounding[8]. Our aim in this study is tosynthesize and characterize PET via in-situ polymerization method by using hydrotalcite as a catalyst in both DMT transesterification and successive polycondensation. Five kinds of PET nanocomposites had been synthesized, with the mass ratio of LDHs:DMT equating to 0.25% (PET-0.25), 0.5% (PET-0.5), 0.75% (PET-0.75), 3% (PET-3), and 5% (PET-5), respectively. The thermal and flame retardation properties of the PET samples were investigated.
2 Experimental
2.1 Materials
Commercially available DMT (manufactured by the Liaohua Petrochemical Co.), EG (analytical reagent grade, manufactured by the Beijing Chemical Plant), hydrotalcite (Mg6Al2(OH)16(CO3)·4H2O, prepared by the Beijing University of Chemical Technology), were used as received. Irganox® 1010, which was used as the stabilizer, was generously provided by the Research Institute of Liaoyang Petrochemical Corporation. All other chemicals were used without further treatment.
2.2 Transesterification and polycondensation reaction
Transesterification was carried out in a 3-L jacketed stainless steel reactor, which was equipped with a transesterification distillation system for removing the released methanol and was also fitted with a polycondensation system. As a typical method for preparation of PET, the starting materials DMT, EG, hydrotalcite and Irganox® 1010 were charged into the reactor under gentle agitation. The esterification reaction was carried out at about 190 ℃ and the temperature in the fractionation column was controlled at 65 ℃ under a nitrogen flow with continuous removal of the released methanol. When the volume of the collected methanol was around 95% of the theoretical value, the esterification was considered to be over.
The reaction mixture was heated slowly up to 220 ℃ and remained at this temperature for one hour to finish the transesterificaton reaction. The temperature was then raised to 255±5 ℃, and the pressure of the reaction system was gradually reduced to 60 Pa, at which the reaction temperature was raised to the final value of 290±5 ℃.
The polymerization was carried out isothermally at this temperature for about 2 hours with simultaneous removal of EG and other volatiles by distillation to acquire an expected molecular weight of the product. Finally, the pressure in the system was regulated to the atmospheric pressure using nitrogen purging to prevent degradation of the product by oxidation.
2.3 Measurements
Intrinsic viscosity of the product was measured at 30 ℃ by an Ubbelohde viscometer using a polymer solution of 0.1 g/dL in phenol/tetrachloroethane (mass ratio of 60/40) and calculated according to the “one point” method[8].1H NMR spectra of samples were recorded on a Bruker AV600-MHz NMR spectrometer, using tetramethylsilane (TMS) as the internal standard. Either CDCl3or CD3COCD3was used as the solvent. The thermal transition process was carried out by a Perkin-Elmer DSC-7 differential scanning calorimeter under nitrogen purging according to the following procedure. The PET samples were heated at a rate of 40℃/min to the desired temperature to ensure complete melting, and were then kept at this temperature for 3 min. Samples were then cooled down to -100 ℃ at a cooling rate of 100 ℃/min before being reheated to 230 ℃ at a heating rate of 20 ℃/min. Thermogravimetric analysis (TGA) was carried out using a TG 209 thermogravimetric analyzer manufactured by Netzsch GmbH, Germany. Each PET sample weighing about 10 mg was heated at a rate of 10 ℃/min and investigated in the temperature range from 20 ℃ to 600 ℃ under the nitrogen flow at a flow rate of 50 mL/min.
3 Results and Discussion
3.1 Synthesis of PET
PET was synthesized via a typical two-step transesterif ication and polycondensation procedure involving DMT and EG catalyzed by LDH. The influence of polycondensation time on the intrinsic viscosity ([η]) of PET namocomposite (PET-3) is shown in Table 1. It can be seen from Table 1 that the optimum polycondesation time was about 2 hours when the [η] of the formed PET was the same as the commercially available PET ([η]=0.67). The generated PET would degrade when reaction time exceed-ed 2 hours. Therefore, the polycondensation time for all the PET samples was determined. The reaction conditions and results of the prepared PET samples are summarized in Table 2.
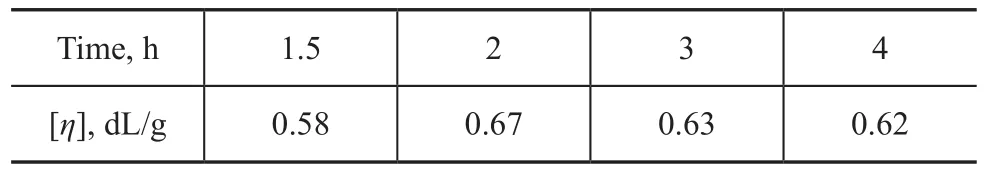
Table 1 The influence of polycondensation time on the [η] of PET-3
As shown in Table 2, an increase of LDH content would slightly decrease the transesterification time (tt) and initial transesterification temperature (Tini), when the transesterification reaction by-product, methanol, began to be distilled off. The reason for the decline ofttandTiniis that the overall activation energy of the transesterification decreases with an increasing catalyst concentration[9]. These results were similar to that reported by M. Di Serio in the reference[10], which indicated that the active LDH catalysts showed nearly the same activity of those classical catalysts.

Table 2 Reaction conditions and results (at a polycondensation time of 1.5 h)
It also can be seen from Table 2 that as more LDH was added (exceeding 3%), no clear reduction inTiniandttwas observed in the transesterification process. This might be attributed to the aggregation of LDH to retain its layered structure and the unsuccessful exfoliation of LHD by EG, oligomer or PET, when the LDH content was higher. At the same time, the decrease of [η] in the PET nanocomposites was observed, which might be attributed to the increase in rigidity of the PET matrix in the presence of LDH mineral. Moreover, the thermal properties as well as the flame-retardant property, which would be discussed later, were effectively influenced by LDH.
3.2 Polymer composition
The composition of the samples was characterized by1H NMR spectroscopy as a conventionally adopted approach in polymer science research nowadays[11]. As shown in Figure 1, in addition to the large absorbance of the repeated terephthalate units at 8.41, the peak at 5.09 as been assigned to the EG units. There was no difference between the as-prepared PET sample and commercial PET sample, which confirmed that the LDH could be used as a single catalyst in PET synthesis from DMT with EG to reveal its excellent activity and selectivity.

Figure 1 Calculated1HNMR results and relative difference of PET samples
3.3 Thermal properties of PET catalyzed by LDH
The thermal properties of the synthesized PET samples were measured using DSC (Table 3), and the thermal stability of samples was characterized by means of thermogravimetric analysis (TGA). The results are summarized in Table 3. The TGA thermograms of samples are shown in Figure 2 (a), while the DSC thermograms are illustrated in Figure 2 (b). During the DSC experiments, allsamples were heated well beyond the melting temperature and then cooled down to -100 ℃ before the second run test. Figure 3 shows that the degree of super heating ΔT(ΔT=Tch-Tg) on the heating scans decreased with an increasing mass ratio of LDH in the PET samples. It can be concluded that LDH fillers acted as the nucleating agent of crystallization and could accelerate the crystallization rate of PET matrix[12]. This behavior is similar to the results relating to the general nanocomposites prepared by direct melt-compounding of PET and LDH[8,12-13]. In comparison with the pristine PET, the PET that contains LDH fillers used as catalyst shows similar melting temperature (Tm) and glass transition temperature (Tg), so it is supposed that LDH nanofillers cannot affect PET crystal perfection during the experiment, but can only affect the crystallization rate of PET by acting as a nucleating agent. The thermal degradation temperatures (Td) curves show that an increase of LDH content in the PET composites slightly raises the degradation start temperature (Td,on) from 403.8 ℃ to 413.5 ℃ and the degradation end temperature (Td,end) from 446.1 ℃ to 453.5 ℃, respectively, along with forming the similar weight loss curves and slopes. As it is expected, the thermal degradation temperature is enhanced when the LDH ratio increases, which can be ascribed to the prevention of out-diffusion of the volatile gas from the thermally decomposed products, because the LDH layers act as gas barriers, which can reduce the permeability of the volatile gas[14].

Table 3 DSC and TGA analysis of PET samples

Figure 2 TGA (a) and DSC (b) thermograms of PET samples

Figure 3 The relationship between the degree of super heating and LDH content
3.4 Fire-resistant properties of PET catalyzed by LDH
The effect of LDH on the limited oxygen index of PET can be seen clearly from Table 5. The limited oxygen index increased obviously from 23 for pure PET to 31 for PET-3%. The results indicate that the LDH can be used as an efficient flame-retardant, because the magnesium/ aluminum layered double hydroxides can absorb the heat and deliver H2O, CO2upon burning, which can lower the temperature of substrate and enhance the foaming char structure. In addition, the porous thermally decomposed products of LDH with large specific surface area can result in smoke suppression effect by absorbing the smoke and gases produced in the course of combustion[15-16]. The fireresistant property of PET/LDH nanocomposites prepared during in-situ polymerization in this work was higher than that prepared via direct melt compounding of PET and LDH. This phenomenon may be ascribed to the exfoliation of LHD by EG, oligomer or PET in-situ reaction system[8].

Table 5 The limited oxygen index of new PET
4 Conclusions
PET was synthesized by means of a two-step in-situ po-lymerization method using LDH as a catalyst and was characterized by various methods. The transesterification time and initial transesterification temperature decreased with the increase of LDH content. Based on the thermal analysis, the crystallization rate and degradation temperatures of PET samples were improved simultaneously as compared to that of pristine PET sample. The fire-resistant experiments showed that LDH could be used as an efficient flame-retardant. With the growth of the packing industry, which largely uses PET to make bottles and other food packing items, the LDH can be used as a replacement for the traditional antimony catalysts.
[1] Scheirs J, Long T E. Modern Polyesters: Chemistry and Technology of Polyesters and Copolyesters[M]. WILEY, 2003.
[2] MacDonald W A. New advances in poly(ethylene terephthalate) polymerization and degradation[J]. Polym Int, 2002, 51 (10): 923-930.
[3] Meyer U, Hoelderich W F. Transesterification of methyl benzoate and dimethyl terephthalate with ethylene glycol over basic zeolites[J]. Appl Catal A: Gen, 1999, 178 (2): 159-166.
[4] Gorzawski H, Hoelderich W F. Transesterification of methyl benzoate and dimethyl terephthalate with ethylene glycol over superbases[J]. Appl Catal A: Gen., 1999, 179 (1): 131-137.
[5] Thiele U K. The current status of catalysis and catalyst development for the industrial process of poly(ethylene terephthalate) polycondensation[J]. Int J Polym Mater, 2001, 50(3): 387-394.
[6] Di Serio M, Tesser R, Ferrara A, et al. Heterogeneous basic catalysts for the transesterification and the polycondensation reactions in PET production from DMT[J]. J Mol Catal A: Chemical, 2004, 212(1/2): 251-257
[7] El-Toufaili F A. Feix G, Reichert K H. Kinetics and mechanistic investigation of hydrotalcite-catalyzed melt synthesis of poly(ethylene terephthalate)[J]. Macromol Mater Eng, 2006, 291(9): 1144-1154.
[8] Wang M, Zhu M F, Sun B. A New nano-structured flameretardant poly(ethylene terephthalate)[J]. Journal of Macromolecular Science, Part A: Pure and Applied Chemistry, 2006, 43(11): 1867-1875.
[9] (a) El-Toufaili F A, Feix G, Reichert K H. Optimization of simultaneous thermal analysis for fast screening of polycondensation catalysts[J]. Thermochim Acta, 2005, 432 (1): 99-105. (b)El-Toufaili F A, Feix G, Reichert K H. Mechanistic investigations of antimony-catalyzed polycondensation in the synthesis of poly(ethylene terephthalate)[J]. J Polym Sci, Part A: Polym Chem, 2006, 44 (3): 1049-1059.
[10] Di Serio M, Tesser R, Trulli F, et al. Kinetic and catalytic aspects in melt transesteri fication of dimethyl terephthalate with ethylene glycol in the presence of different catalytic systems[J]. J Appl Polym Sci, 1996, 62 (2): 409-415.
[11] Saint-Loup R, Jeanmaire T, Robin J, et al. Synthesis of (polyethylene terephthalate/ poly e-caprolactone) copolyesters[J]. Polymer, 2003, 44 (12): 3437-3449.
[12] Lee W D, Im S S. Preparation and properties of layered double hydroxide/poly(ethylene terephthalate) nanocomposites by direct melt compounding[J]. Polymer, 2006, 47 (4): 1364-1371.
[13] Xu K L, Chen G M, Shen J Q. Exfoliation and dispersion of micrometer-sized LDH particles in poly(ethylene terephthalate) and their nanocomposite thermal stability[J]. Applied Clay Science, 2013, 75-76: 114-119
[14] Wang G A, Wang C C, Chen C Y. Preparation and characterization of layered double hydroxides-PMMA nanocoposites by solution polymerization[J]. J Inorg Organomet Polym, 2005, 15(2): 239-251.
[15] Wang H Y, Han E H, Ke W. In fluence of nano-LDHs on char formation and fire-resistant properties of flame-retardant coating[J]. Progress in Organic Coatings, 2005, 53(1): 29-37.
[16] Kim S. Flame retardancy and smoke suppression of magnesium hydroxide filled polyethylene[J]. Journal of Polymer Science, Part B: Polymer Physics, 2003, 41(9): 936-944.
Recieved date: 2013-05-03; Accepted date: 2013-05-25.
Prof. Cao Ding, Telephone: +86-010-64423811; E-mail: carlcao2008@gmail.com.
杂志排行
中国炼油与石油化工的其它文章
- CFD Simulation of Orifice Flow in Orifice-type Liquid Distributor
- Development and Commercial Application of RSDS-II Technology for Selective Hydrodesulfurization of FCC Naphtha
- Study on Reactive Adsorption Desulfurization of Model Gasoline on Ni/ZnO-HY Adsorbent
- Photocatalytic Denitrogenation over Modified Waste FCC Catalyst
- Experimental and Molecular Dynamics Simulations for Investigating the Effect of Fatty Acid and Its Derivatives on Low Sulfur Diesel Lubricity
- Kinetic Modeling of Methanol to Olefins (MTO) Process on SAPO-34 Catalyst
