Synthesis of TS-1 Films on Porous Supports for Epoxidation of Allyl Chloride by Hydrogen Peroxide
2013-07-31GuLingWangLi
Gu Ling; Wang Li
(1. School of Chemistry and Environmental Engineering, Shanxi Datong University, Datong 037009; 2. Key Laboratory for Green Chemical Technology of the Ministry of Education, School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072)
Synthesis of TS-1 Films on Porous Supports for Epoxidation of Allyl Chloride by Hydrogen Peroxide
Gu Ling1,2; Wang Li2
(1. School of Chemistry and Environmental Engineering, Shanxi Datong University, Datong 037009; 2. Key Laboratory for Green Chemical Technology of the Ministry of Education, School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072)
Titanium silicalite-1 (TS-1) films were synthesized on stainless steel plate, glass slide and monolith supports via anin-situhydrothermal method. Characterization data showed that the formation of TS-1 films was easier on the porous flat support with rough surface such as monolith than on the smooth non-porous supports like glass slide and stainless steel plate. The film on the monolith had the highest uniformity and smallest size of crystals. The catalytic property of monolithsupported film was tested for epoxidation of allyl chloride (ACH) by H2O2in a fixed bed reactor. Under the condition of a methanol (solvent)/ACH (90%)/H2O2(30%) ratio of 12:1:1, a LHSV of 1.35 h-1and a temperature of 318 K, the conversion of allyl chloride and the selectivity to epichlorohydrin reached 79 % and 51 %, respectively.
TS-1; film; epoxidation; epichlorohydrin
1 Introduction
TS-1, a Ti-containing zeolite with MFI structure, is used very often for the selective oxidation of small olefins by hydrogen peroxide in liquid phase under moderate conditions[1-2]. However, the difficulty in separating TS-1 powders from the liquid phase after the reaction prevents the large scale utilization of this technology. Zeolite membranes such as MFI and BEA zeolite membranes have been investigated extensively in catalytic membrane reactors by combining the catalytic process with the separation operation. In the reaction system, the film can serve as both the catalyst and the permselective membrane[3-7]. As regards the TS-1 membranes, however, they are generally used as catalytically inert membrane reactors for selective permeation of products or simply acting as a distributor of reactants, and do not play any direct role in the catalytic reaction. There are a few reports on the use of TS-1 membranes as catalytically active centers. Wu and co-workers[8]used composite membrane (TS-1/ polydimethylsiloxane) as catalytic interphase contactor in the two-phase oxidation of n-hexane by dilute H2O2. Maira and co-workers[9]prepared a catalytic TS-1 membrane for gas-phase photocatalytic oxidation of trichloroethylene, but the activity is very low due to the insufficient active sites. Wang and co-workers[10-13]prepared TS-1 films on porous α-Al2O3tube and obtained their activity that was higher than that of the powders.
In the present work, TS-1 films were prepared on porous and non-porous supports via anin-situhydrothermal method. The morphologies of films were characterized to analyze the effect of supports. Then the catalytic performance of film on porous support was tested using the epoxidation reaction of allyl chloride (ACH) by hydrogen peroxide which was a reaction for the synthesis of important chemical intermediate epichlorohydrin (ECH)[14].
2 Experimental
2.1 Support pretreatment
The nonporous supports included stainless steel plate (SS-316L) and glass slide (15×20 mm). The porous support was monolith (2MgO·2Al2O3·5SiO2) with a specific surface area of 10.54 m2/g and a diameter of 18 mm.
Prior to the synthesis, stainless steel plate and glass slidewere washed sequentially with hydrochloric acid (4.5%), sodium hydroxide solution (2%) and distilled water, followed by drying at 393 K for 12 hours. Monolith was calcined at 823 K for 8 hours.
2.2 Synthesis of TS-1 films
TS-1 films were hydrothermally synthesized with tetrapropylammonium hydroxide (TPAOH, 40%) used as the template. Tetraethyl orthosilicate (TEOS) and tetrabutyl orthotitanate (TBOT) were used as the sources of Si and Ti, respectively. In detail, TEOS and TPAOH were mixed under stirring. TBOT was diluted with 2-propanol and the mixture was then added dropwise into the resulting solution. The final composition of synthetic mixture had a molar ratio of TEOS:TBOT:TPAOH:H2O equating to 1:0.04:0.15:100. A gel was obtained after heating the mixture at 353 K for 3 hours. Then the gel and the support were transferred into a Teflon-lined autoclave, sealed and crystallized at 443 K for 96 hours. Finally, the obtained TS-1 films were washed with distilled water several times, dried at 393 K overnight and calcined at 823 K for 6 h.
2.3 Characterization
X-ray diffraction patterns were recorded by a Rigaku D-max 2 500 V/PC X-ray diffractometer with CuKα radiation (40 kV, 150 mA) to identify the crystalline phase of the as-synthesized TS-1 films. Scanning electron microscopy (SEM) images were obtained on a Philips XL-30 environmental scanning electron microscope. FT-IR spectra were acquired on a MAGNA-IR 560 spectrometer using the KBr wafer technique.29Si MAS NMR measurements were performed on an InfinityPlus (300 MHz) nuclear magnetic resonance spectrometer operating at ambient temperature. The chemical shift was referred to an external standard of sodium 3-(trimethylsilyl)-1-propanesulfonate.
2.4 Catalytic reaction
The epoxidation of ACH by H2O2was carried out in a fixed bed reactor at 318 K under atmospheric pressure. 20 mL of supported TS-1 film catalysts were loaded into the reactor. Methanol (solvent), ACH (90%) and H2O2(30%) were fed to the reactor at a molar ratio of 12:1:1 by a micro-feeding pump at a flow rate of 0.45 mL/min. The liquid hourly space velocity (LHSV) was 1.35 h-1. The products were analyzed by a gas chromatograph (Agilent 6890) equipped with a capillary column (HP-5) and a flame ionization detector. The conversion of ACH (XACH) and selectivity of ECH (SECH) were calculated as follows.

3 Results and Discussion
3.1 Characterization of TS-1 films
The XRD patterns of as-synthesized films on three supports are presented in Figure 1. The peaks at 2θ=7.8, 8.8, 23.2, 23.8, 24.3 and 45° are in good agreement with those of TS-1 reported in the literature[15-16]. It is evident that the films on the porous and non-porous supports both had MFI topology structures. Furthermore, the presence of a single diffractive peak at 2θ=24.3° also indicated a change from monoclinic symmetry (S-1) to orthorhombic symmetry (TS-1)[16-17]. Noticeably, the peaks representing TS-1 were very weak when monolith was used as the support (Figure 1c), which might be attributed to the small amount and size of TS-1 particles on monolith surface.
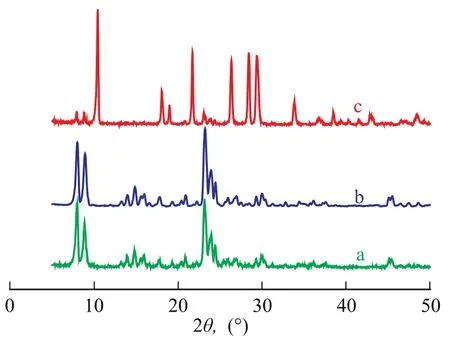
Figure 1 XRD patterns of TS-1 films on different supports
Figure 2 shows the FT-IR spectra of TS-1 films on glass slide and monolith. The absorption bands at 1 220 cm-1, 1 100 cm-1, 960 cm-1, 800 cm-1, 550 cm-1, and 450 cm-1were the same as those of TS-1 powder, indicating that the TS-1 crystals grow on the support. Another important and distinctive feature of FT-IR spectra was the band near 960 cm-1. The presence of this band is a proof for the incorporation of Ti into the framework of zeolites[18-23].
Figure 3 shows the29Si MAS NMR spectra of the TS-1films on monolith and commercial TS-1 powder with a Ti/Si molar ratio of 39. Both of them showed two peaks atδ=-113.3 and -115.3, indicating that the orthorhombic symmetry was formed on the supports. It is reported that S-1 and TS-1 with low titanium content exhibit monoclinic symmetry at room temperature[24-25]. With the increase of the framework titanium content, the symmetry of TS-1 structure gradually changes from monoclinic to orthorhombic. Therefore, on the basis of XRD patterns, FT-IR spectra and29Si MAS NMR spectra, it is concluded that a significant fraction of titanium was found in the framework of TS-1.

Figure 2 FT-IR spectra of TS-1 films on different supports

Figure 329Si MAS NMR of TS-1
The scanning electron microscopy (SEM) images of TS-1 films on different supports are shown in Figure 4. It can be seen that the morphologies of all samples are flat rectangular instead of typical sphere of TS-1 powder. And the size of the crystals is larger than that of TS-1 powder. These results are in agreement with the result described in the reference[26]. It is noted that the uniformity of the crystallites and their size are strongly influenced by the porosity of the supports. The TS-1 film on the monolith support has the highest uniformity and smallest crystal size. The morphology of films supported on the stainless steel and the glass slide is similar. But the latter has larger size of crystals and worse mechanical strength because somecracks are present in the film after calcination at 823 K for 6 hours. It indicates that it is easier to obtain TS-1 film with relatively high mechanical strength on porous support with rough surface, compared with that formed on non-porous support with smooth surface. It can be seen from the intersection (Figure 4) that the crystals grow both on the external surface and inside the pores of the support. And there is a transitional layer between the support and the zeolite layer. This suggests a good interaction between the zeolite layer and the support which can enhance the bonding between the TS-1 film and the support.

Figure 4 SEM images of TS-1 films on different supports
3.2 Catalytic activity
As shown in Scheme 1, the product of epoxidation of ACH by hydrogen peroxide is ECH, but many byproducts, including 3-chloro-1,2-propanediol, 3-chloro-1-methoxy-2-propanol, 3-chloro-2-hydroxy-1-propionic acid as well as polymers of ACH can also be produced[14,27-28]. Since the films on porous supports show more uniform morphology with smaller crystals than those on non-porous supports, TS-1 films on monolith was used to catalyze the reaction. The reaction data over TS-1 films supported on porous SiO2pellets, which had been described in our previous work[14], were also presented for comparison. As shown in Figure 5, steady conversion and selectivity were realized after approximately 110 minutes. And both the conversion of ACH and selectivity to ECH over TS-1/monolith was higher than the case with TS-1/SiO2, which might be attributed to its uniform morphology and small crystallite size. Actually, it can be seen from Figure 4 that the particle size of TS-1 on monolith was about 5 μm whereas that of TS-1 on SiO2was about 20 μm, and the particles on SiO2grew in a less compacted way. As a result, TS-1 films supported on monoliths exhibited a higher conversion of ACH (79%) coupled with a higher selectivity to ECH (51%).

Scheme 1 Reaction equation of epoxidation of ACH with H2O2
4 Conclusions
TS-1 films were prepared on both porous and non-porous supports. The porous support with the rough and flat surface was beneficial to the formation of TS-1 films with uniform surface morphology, small size of crystals and relatively high mechanical strength. In the epoxidation of ACH with H2O2, the film supported on monolith showed a conversion of 79% and a selectivity of 51% under the condition of a methanol (solvent):ACH (90%):H2O2(30%) ratio of 12:1:1, a LHSV of 1.35 h-1and a temperature of 318 K.

Figure 5 Activity of TS-1 films in epoxidation of ACH with H2O2
Acknowledgements:The authors are grateful to the Natural Science Foundation of Shanxi Province, China (No. 2011011023-2) for financial support.
[1] Laufer W, Hoelderich W F. Direct oxidation of propylene and other olefins on precious metal containing Ticatalysts[J]. Appl Catal A: Gen, 2001, 213(2): 163-171
[2] Liu N, Guo H, Wang X, et al. Increasing the propylene epoxidation activity of TS-1 catalysts by hydrothermal treatment of ammonia solution[J]. React Kinet Catal Lett, 2005, 87(1): 77-83
[3] McLeary E E, Jansen J C, Kapteijn F. Zeolite based films,membranes and membrane reactors: Progress and prospects[J]. Micropor Mesopor Mater, 2006, 90(1/3): 198-220
[4] Gopalakrishnan S, Yamaguchi T, Nakao S. Permeation properties of templated and template-free ZSM-5 membranes[J]. J Membr Sci, 2006, 274(1/2): 102-107
[5] Yuan W, Lin Y S, Yang W. Molecular sieving MFI-type zeolite membranes for pervaporation separation of xylene isomers[J]. J Am Chem Soc, 2004, 126(15): 4776-4777
[6] Holmes S M, Markert C, Plaisted R J, et al. A novel method for the growth of silicalite membranes on stainless steel supports[J]. Chem Mater, 1999, 11(11): 3329-3332
[7] Nishiyama N, Ichioka K, Park D H, et al. Reactant- selective hydrogenation over composite silicalite-1-coated Pt/TiO2particles[J]. Ind Eng Chem Res, 2004, 43(5): 1211-1215
[8] Wu S, Gallot J E, Bousmina M, et al. Zeolite containing catalytic membranes as interphase contactors[J]. Catal Today, 2000, 56(1/3): 113-129
[9] Maira A J, Lau W N, Lee C Y, et al. Performance of a membrane-catalyst for photocatalytic oxidation of volatile organic compounds[J]. Chem Eng Sci, 2003, 58(3-6): 959-962
[10] Wang X, Zhang X, Liu H, et al. Preparation of titanium silicalite-1 catalytic films and application as catalytic membrane reactors[J]. Chem Eng J, 2010, 156(3): 562-570
[11] Wang X, Li G, Wang W H, et al. Synthesis, characterization and catalytic performance of hierarchical TS-1 with carbon template from sucrose carbonization[J]. Micropor Mesopor Mater, 2011, 142(2/3): 494-502
[12] Chen P, Chen X, Chen X, et al. Preparation and catalytic activity of titanium silicalite-1 zeolite membrane with TPABr as template[J]. J Membr Sci, 2009, 330(1/2): 369-378
[13] Qiu F, Wang X, Zhang X, et al. Preparation and properties of TS-1 zeolite and film using Sil-1 nanoparticles as seeds[J]. Chem Eng J, 2009, 147(2-3): 316-322
[14] Wang L, Zhou Y, Mi Z. Epoxidation of allyl chloride and hydrogen peroxide over titanium silicalite-1 film on SiO2pellet support[J]. J Chem Techn Biotechn, 2007, 82(4): 414-420
[15] Taramasso M, Perego G, Notari B. Preparation of porous crystalline synthetic material comprised of silicon and titanium oxides: The United States, US 4410501[P]. 1983
[16] Thangaraj A, Eapen M J, Sivasanker S, et al. Studies on the synthesis of titanium silicalite TS-1[J]. Zeolites, 1992, 12(8): 943-950
[17] Phonthammachai N, Krissanasaeranee M, Gulari E, et al. Crystallization and catalytic activity of high titanium loaded TS-1 zeolite[J]. Mater Chem Phys, 2006, 97(2/3): 458-467
[18] Zou J J, Liu Y, Pan L, et al. Photocatalytic isomerization of norbornadiene to quadricyclane over metal (V, Fe and Cr)-incorporated Ti-MCM-41[J]. Appl Catal B, 2010, 95(3/4): 439-445
[19] Zou J J, Zhang M Y, Zhu B, et al. Isomerization of norbornadiene to quadricyclane using Ti-containing MCM-41 as photocatalysts[J]. Catal Lett, 2008, 124(1/2): 139-145
[20] Pan L, Zou J J, Zhang X, et al. Photoisomerization of norbornadiene to quadricyclane using transition metal doped TiO2[J]. Ind Eng Chem Res, 2010, 49(18): 8526-8531
[21] Zou J J, Zhu B, Wang L, et al. Zn- and La-modified TiO2photocatalysts for the isomerization of norbornadiene to quadricyclane[J]. J Mol Catal A, 2008, 286(1/2): 63-69
[22] Pan L, Zou J J, Liu X Y, et al. Visible-light-induced photodegradation of rhodamine B over hierarchical TiO2: Effects of storage period and water-mediated adsorption switch[J]. Ind Eng Chem Res, 2012, 51(39): 12782-12786
[23] Pan L, Zou J J, Wang S, et al. Morphology evolution of TiO2facets and vital influences on photocatalytic activity[J]. ACS Appl Mater Interfaces, 2012, 4(3): 1650-1655
[24] Thangaraj A, Kumar R, Mirajkar S P, et al. Catalytic properties of crystalline titanium silicalites: I. Synthesis and characterization of titanium-rich zeolites with MFI structure[J]. J Catal, 1991, 130(1): 1-8
[25] Li G, Wang X, Guo X, et al. Titanium species in titanium silicalite TS-1 prepared by hydrothermal method[J]. Mater Chem Phys, 2001, 71(2): 195-201
[26] Au L T Y, Chau J L H, Ariso C T, et al. Preparation of supported Sil-1, TS-1 and VS-1 membranes: Effects of Ti and V metal ions on the membrane synthesis and permeation properties[J]. J Membr Sci, 2001, 183(2): 269-291
[27] Wr´oblewska A, Ławro E, Milchert E. Technological parameter optimization for epoxidation of methallyl alcohol by hydrogen peroxide over TS-1 catalyst[J]. Ind Eng Chem Res, 2006, 45(22): 7365-7373
[28] Wr´oblewska A, Milchert E. Optimization of the technological parameters of epoxidation of methallyl chloride by hydrogen peroxide over TS-1 catalyst[J]. Org Proc Res Dev, 2006, 10(3): 525-533
Recieved date: 2013-03-21; Accepted date: 2013-04-23.
Ms. Gu Ling, Telephone: 0352-7624712; E-mail: gulingyidan@163.com.
杂志排行
中国炼油与石油化工的其它文章
- Study on the Synthesis and Properties of PET Using Hydrotalcite as Catalyst
- Kinetic Modeling of Methanol to Olefins (MTO) Process on SAPO-34 Catalyst
- CFD Simulation of Orifice Flow in Orifice-type Liquid Distributor
- Photocatalytic Denitrogenation over Modified Waste FCC Catalyst
- Experimental and Molecular Dynamics Simulations for Investigating the Effect of Fatty Acid and Its Derivatives on Low Sulfur Diesel Lubricity
- Study on Reactive Adsorption Desulfurization of Model Gasoline on Ni/ZnO-HY Adsorbent
