Microwave-Assisted Decarboxylation of Sodium Oleate and Renewable Hydrocarbon Fuel Production
2013-07-31WangYunpuLiuYuhuanRuanRongshengWenPingweiWanYiqinZhangJinsheng
Wang Yunpu; Liu Yuhuan; Ruan Rongsheng; Wen Pingwei; Wan Yiqin; Zhang Jinsheng
(1. Nanchang University, State Key Laboratory of Food Science and Technology, Nanchang 330047; 2. Nanchang University, Engineering Research Center for Biomass Conversion, Ministry of Education, Nanchang 330047)
Microwave-Assisted Decarboxylation of Sodium Oleate and Renewable Hydrocarbon Fuel Production
Wang Yunpu1,2; Liu Yuhuan1,2; Ruan Rongsheng1,2; Wen Pingwei2; Wan Yiqin1,2; Zhang Jinsheng1,2
(1. Nanchang University, State Key Laboratory of Food Science and Technology, Nanchang 330047; 2. Nanchang University, Engineering Research Center for Biomass Conversion, Ministry of Education, Nanchang 330047)
The carboxyl terminal of sodium oleate has a stronger polarity than that of oleic acid; this terminal is more likely to be dipole polarized and ionically conductive in a microwave field. Sodium oleate was used as the model compound to study the decarboxylation of oleic acid leading to hydrocarbon formation via microwave-assisted pyrolysis technology. The pyrolysis gas, liquid, and solid products were precisely analyzed to deduce the mechanism for decarboxylation of sodium oleate. Microwave energy was able to selectively heat the carboxyl terminal of sodium oleate. During decarboxylation, the double bond in the long hydrocarbon chain formed a p-π conjugated system with the carbanion intermediate. The resulting p-πconjugated system was more stable and beneficial to the pyrolysis reaction (decarboxylation, terminal allylation, isomerization, and aromatization). The physical properties of pyrolysis liquid were generally similar to those of diesel fuel, thereby demonstrating the possible use of microwaves for controlling the decarboxylation of sodium oleate in order to manufacture renewable hydrocarbon fuels.
microwave radiation; sodium oleate; conjugated system; decarboxylation; hydrocarbon
1 Introduction
The development of renewable hydrocarbon fuels has attracted considerable attention. The kerosene fraction obtained from crude oil refining is the major jet fuel used in military and civil aviation turbine engines. The total yearly consumption of aviation turbine fuel was approximately 6.8 million barrels in 2007, and this number has been estimated to increase to 8.6 million barrels by 2013[1]. Renewable hydrocarbon fuel or fuel additives have gained popularity because of their environmental benefits and the crude oil shortage problem. To date, the renewable hydrocarbon fuel produced from vegetable oil (fatty glyceride) is the renewable material that is most similar to diesel fuel[2–5].
Vegetable oil mainly consists of triglycerides (about 90%), a small amount of mono- and diglycerides, and some free fatty acids. Palmitic acid (C16:0) and stearic acid (C18:0) are the most common saturated fatty acids. The main unsaturated fatty acids cover oleic acid (C18:1) and linoleic acids (C18:2). Vegetable oil may contain few other types of fatty acids, such as lauric acid (C12:0), myristic acid (C14:0), and erucic acid (C22:1)[6]. The unsaturated oleic acid content of olive oil, rapeseed oil, and theSwida wilsonianafruit oil ranges from 55% to 85%, 55% to 65%, and 35% to 45%, respectively.S. wilsonianais mainly distributed in Jiangxi Province. The flesh and nucleolus ofS. wilsonianafruit contain fat. Under normal circumstances, the oil yield of the dried fruit ofS. wilsonianaranges from 25% to 30%. Therefore,S. wilsonianais an ideal material for the biodiesel production.
Pyrolysis of vegetable oil has a promising potential for renewable fuel production, particularly in industries that engage in hydrocracking process. Hydrocracking is a conventional petroleum refining process[7–10]. The pyrolysis of triglycerides has existed for over 100 years in history, especially in areas which are deficient in oil supply. Rel-evant studies in 1947 showed that a certain amount of fuel with its property similar to diesel could be obtained via the large-scale thermal cracking of calcium soap of tung oil (Chinese wood oil)[11]. The Forster pyrolysis of calcium soap of Macauba oil can produce a mixture similar to diesel fuel, but its pyrolysis liquid contains a large quantity of aldehydes and ketones[12].
The pyrolysis reaction mechanism of organic substances usually follows the free radical path[13]. However, given the complexity of this reaction, the carbonium ion theory has been considered as a rational explanation for the mechanism of traditional catalytic pyrolysis[14]. The carbonium ion produced in the reaction is an unstable intermediate product, which is a transition state in the reaction. According to relevant literature information, the dominant reaction during the pyrolysis of biomass-containing triglycerides is the decarboxylation (decarbonylation) of oxygen-containing compounds such as carboxyl esters, acids, ketones, and aldehydes. The following reaction paths are possible: (1) the decarboxylation and decarbonylation of oxygen-containing compounds followed by the breaking of carbon–carbon (C—C) single bonds in the hydroxyl group; (2) the broken carbon–carbon single bond in unsaturated oxygen-containing compounds produces shortchain hydrocarbons coupled with decarboxylation and decarbonylation; and intermediates such as free radicals or carbon ions are formed during this process and would participate in different reactions, including disproportionation, cracking, isomerization, and aromatization. Longchain heavy hydrocarbons are noticeably formed through polymerization or polycondensation reaction[15–20].
Satisfactory results were obtained in previous studies when the saponified substance fromS. wilsonianafruit oil underwent the microwave-assisted decarboxylation of the polarized carboxyl terminal[21]. However, these studies did not elucidate the mechanism of microwave-assisted polarization. In the current study, sodium oleate (a main component of the saponified substance fromS. wilsonianafruit oil) was used to further study the microwaveassisted pyrolysis of sodium stearate[22]. The production of renewable hydrocarbon fuel was investigated with the assistance of microwaves. Microwave-assisted pyrolysis demonstrated a unique advantage during pyrolysis and decarboxylation of sodium oleate to produce renewable hydrocarbon fuels.
2 Test Materials and Methods
2.1 Materials
Sodium oleate (AR grade) and propanetriol (AR grade) were provided by the Sinopharm Chemical Reagent Co., Ltd. and Nanjing Runbang Chemical Co., Ltd., respectively. All other reagents were manufactured in China and analytically pure. The intelligent microwave-thermogravimetric pyrolysis device was self-designed and manufactured by the Nanjing Cemu Microwave Technology Co., Ltd. The Agilent 6890N/5973 inert gas chromatography–mass spectrometry (GC-MS) system with the chromatographic column (HP-5ms; 30 m×0.25 mm×0.25 μm) was purchased from Agilent U.S. Fourier transform infrared spectrometry (FT-IR) was conducted using a Nicolet 5700 apparatus made by the Thermo Fisher Scientific Corp. The GC9310 system was purchased from the Shanghai Chromatographic Instrument Company. A condensate water system, a SYD-265B kinematic viscometer for petroleum products, and a DM-100 digital density meter were also used in experiments.
2.2 Microwave-assisted polarization and pyrolysis of sodium oleate
The equipment used in the experiments included a microwave-thermogravimetric pyrolysis device, a programmable logic controller real-time data collection and control system, a PC configuration software collection and control system, a quartz reactor, a condensation system, and a collection system. The schematic of the experimental system is shown in Figure 1. The system could be adjusted in the stepless mode within the range of 0 to 2 000 W. The temperature was controlled by an infrared probe. The following control programs were available: (1) the microwave power could automatically decrease with a decreasing weight, and (2) the microwave power could decrease with prolonged working hours. A 250-ml heat-resistant flat-bottomed quartz reactor was used. Before the reaction, the quartz reactor was filled with nitrogen. All liquid pyrolysis products were quantitatively collected and analyzed by GC-MS. The characteristic functional groups of the solid pyrolysis residue were analyzed by FT-IR. The gas components CO, CO2, H2, CH4, C2H4, and C2H6were determined by GC. The weight of solid pyrolysis residue was then measured. The total volume of non-condensablegases was calculated by using the difference method. The above experiments were repeated thrice.
The effect of microwave power on pyrolysis and decarboxylation of sodium oleate was studied. Sodium oleate (30 g) and glycerol (10%, functioning as the hydrogen donor) were mixed in a quartz reactor. The microwave power necessary for conducting the reaction was set at 400 W, 600 W, and 800 W, respectively, and the reaction time was fixed at 10 min. The temperature change was recorded by a computer. The temperature of the condensate water recycling system was set at 5 ℃. The liquid, solid, and gas products were then collected and weighed. The weight of the liquid and solid products was directly measured, whereas the weight of the gas product was determined through calculation. The same amount of distilled water was added into another quartz flask to clean the entire system under the same test conditions. The liquid product was then condensed, collected, and dried in a rotary evaporator. The volume of the residual liquid was then calculated. Approximately 30 g of oleic acid was weighed and treated according to the same procedure functioning as the control. Under the same test conditions, the effects of different temperature (350 ℃ to 600 ℃) and different hydrogen donor concentrations (5%, 10%, and 20%) on decarboxylation of sodium oleate were analyzed using the same microwave power (600 W).

Figure 1 Schematic of microwave-assisted pyrolysis system
2.3 Analysis of liquid hydrocarbons, solids, and gases obtained during the pyrolysis reaction
After membrane filtration, the pyrolysis liquid was analyzed by GC-MS. The following conditions were followed: the column temperature was kept at 60 ℃ for 2 min and then raised to 280 ℃ at a rate of 10 ℃/min; the vaporizer temperature was 250 ℃; the sample size was 0.2 μL; the split ratio was 20:1; and the carrier gas was He which was introduced at a flow rate of 1.0 mL/min. For conducting MS analyses, the electron multiplier voltage was 1941 V; the ion source temperature was 230 ℃; the interface temperature was 280 ℃; and the analysis mode was the scan mode. The complex components of the pyrolysis products could not be differentiated, although the analysis of components was based on the retention time of certain internal standards (for example, adamantane). Therefore, the mass spectrograms of these pyrolysis products should be used for analysis. A mass ratio greater than 85% was required. One mg of the pyrolysis carbon residue sample was mixed with KBr, and pressed into pellets. The Nicolet 5700 FT-IR spectrometer was then used to scan the components in the range from 400 cm-1to 4 000 cm-1. The pyrolysis gases were analyzed by GC.
3 Results and Discussion
3.1 Effects of microwave power and temperature on decarboxylation of sodium oleate
The yield of liquid products obtained from microwaveassisted pyrolysis of sodium oleate (73%) was significantly improved (see Figure 2), as compared with the previous experiments on pyrolysis of sodium stearate (55%), and no wax was identified. The double bond of sodium oleate may have enhanced the activity of specific areas of the carbon chain under microwave-assisted polarization, which could be beneficial to the formation of stable p-π conjugates and π-π conjugate intermediates. This phenomenon enabled the decarboxylation reaction to proceed smoothly. Meanwhile, the breaking of the C—C single bond at the β position is dominant because of the existence of the allyl group.
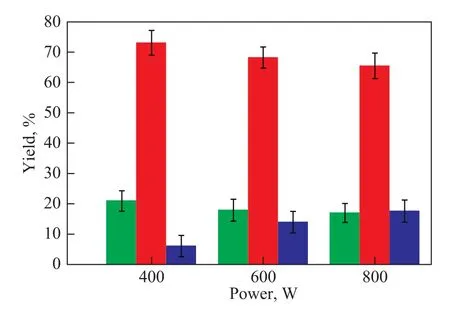
Figure 2 Yield of each component under different microwave power ratings
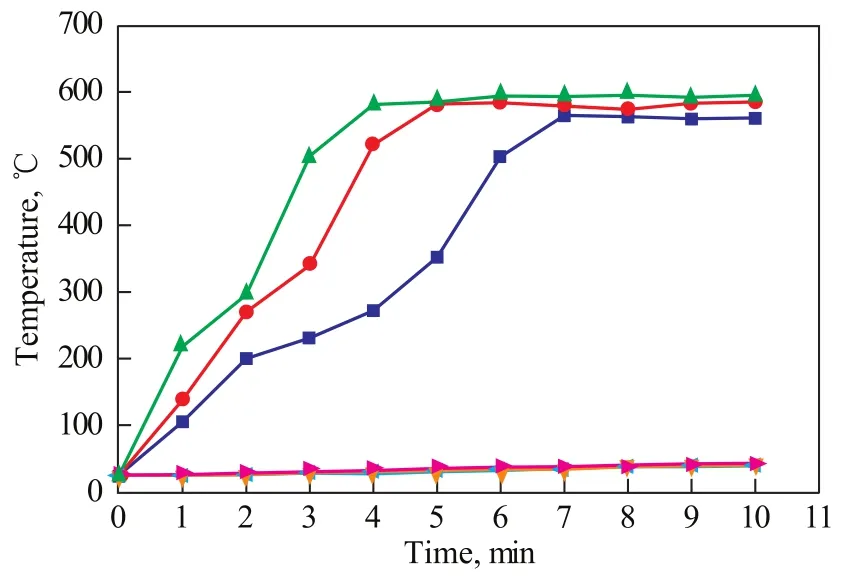
Figure 3 Temperature changes of reactants under different microwave power ratings
Almost no heat was absorbed by oleic acid under microwave-assisted polarization (Figure 3). Therefore, pyrolysis did not occur. This phenomenon is caused by the fact that the oleic acid has a relatively weak polarity, although the metal ions on the carboxyl terminal of sodium oleate may have significantly improved the molecular polarity. Thus, the pyrolysis reaction was more likely to occur with the assistance of microwaves. In addition, the yield of liquid hydrocarbons under the present test conditions was gradually reduced with an increasing microwave power. This decrease in yield is caused by the faster rise in the temperature of sodium oleate as a result of the higher microwave power. The hydrocarbons produced thereby have a relatively weak microwave-absorbing capacity coupled with a minimal chance of secondary cracking. A trace amount of hydrocarbon gases was produced (such as methane, ethane, ethylene, and acetylene). Only 4 min was required at a microwave power of 800 W to increase the temperature of sodium oleate to 600 ℃, and 5 min to achieve full pyrolysis.
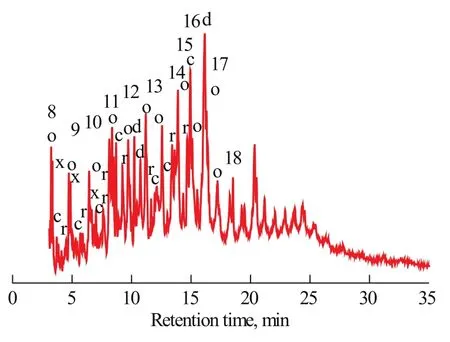
Figure 4 Main components of liquid products of microwaveassisted pyrolysis of sodium oleate.
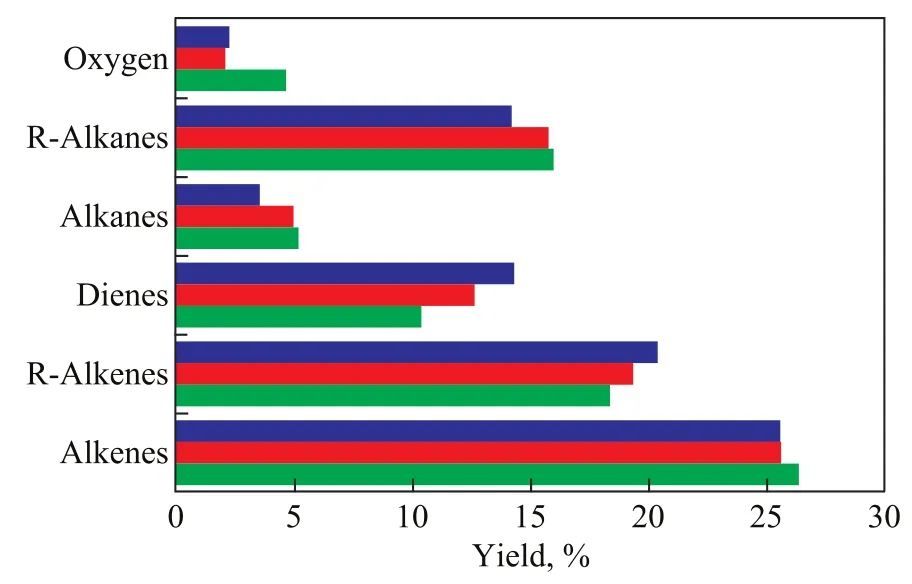
Figure 5 Relative contents of hydrocarbons in pyrolysis liquid products under different microwave power ratings.
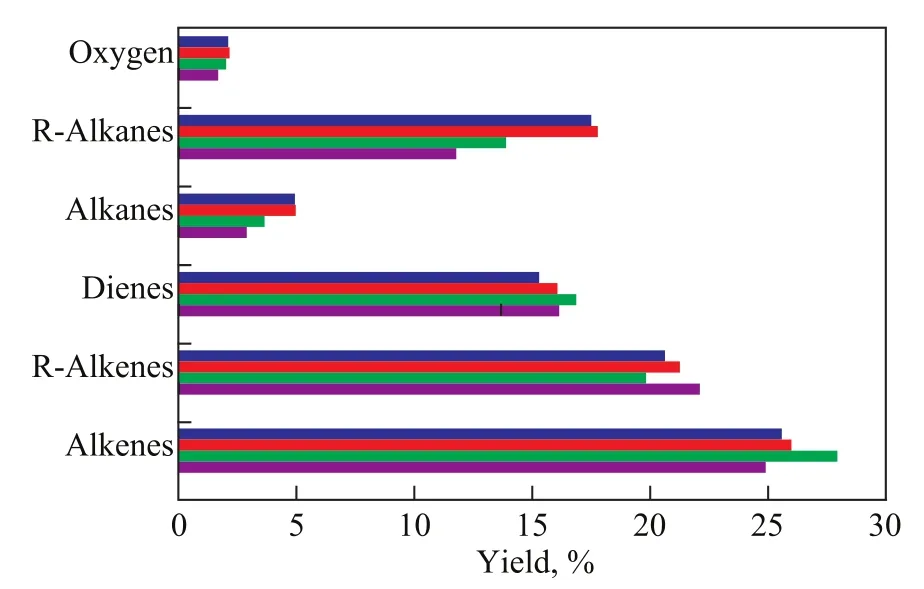
Figure 6 Relative contents of hydrocarbons in pyrolysis liquid products obtained under different reaction temperatures

Table 1 Main components and relative content of the liquid products of microwave-assisted pyrolysis of sodium oleate under different microwave power ratings
A main characteristic of microwave-assisted pyrolysis of sodium oleate was the regular distribution of linear chain monoolefins (C8—C19), alkanes (C8—C17), and dienes (C13to C18; see Figure 4 and Table 1). All the double bonds were found at different locations, except the olefins with double bonds at the terminal locations. The highest peak corresponded to heptadecadiene, which is believed to be formed via breaking of the weakest C–C single bond and the decarboxylation reaction of sodium oleate (C18:1). The rest of the compounds with relatively large contents were generally monoolefins and dienes, which are the typical pyrolysis products of sodium oleate (C18:1). Furthermore, a large number of cycloolefins and cycloalkanes were detected in the pyrolysis products (see Figure 5 and Table 1). The presence of these components accelerates the ring closing reaction of compounds containing double bonds under microwave-assisted polarization. The carboxyl terminal of sodium oleate is a sodium ion, which has a stronger polarity, as compared with oleic acid. The medium is heated in the microwave field through dipole polarization and interface polarization. The polarity of the carboxyl terminal is the strongest. Therefore, this terminal is the first to be heated. The irregular dipole movement and arrangement could lead to entropy reduction, which is beneficial to the decarboxylation reaction. In addition, the content of oxygen-containing compounds was signif icantly decreased during the microwave-assisted polarization and decarboxylation of sodium oleate (see Figure 5). Over 70% of the compounds were hydrocarbon compounds. Therefore, the utilization of the pyrolysis liquid could be greatly improved.
No pyrolysis of sodium oleate occurred at 350 ℃ and 400 ℃(Figure 6). The relative contents of alkanes and cycloalkanes were gradually decreased. The relative contents of olefins, cycloolefins, and diolefins were at first increased and then decreased with an increasing pyrolysis temperature. The low temperature was not favorable for the formation of the back-end allylation and the breaking of the carbon chain after the decarboxylation of sodium oleate. Therefore, high-quality renewable hydrocarbon fuels can be produced at lower temperatures with the assistance of microwaves.
3.2 Deduction of the sodium oleate decarboxylation process under microwave radiation
Among the molecules of sodium oleate, the oxygen atoms on the carbonyl and hydroxyl groups form the p-π conjugated system. The electronic cloud around the oxygen atoms on hydroxyl group is deflected to the carbonyl group. This deflection may decrease the bond length difference and enhance the carboxyl terminal polarity, which both favor dipole polarization, ionic conduction, and decarboxylation in the microwave field. The following reaction mechanism is deduced:
(1) Formation of the carbanion intermediate by microwave polarization
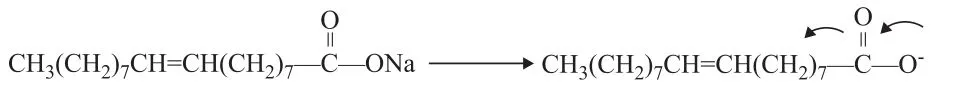
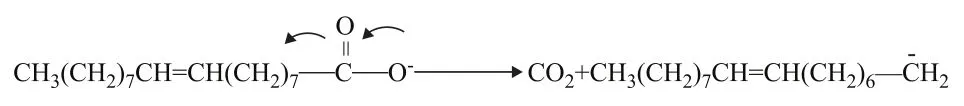
(2) Decarboxylation
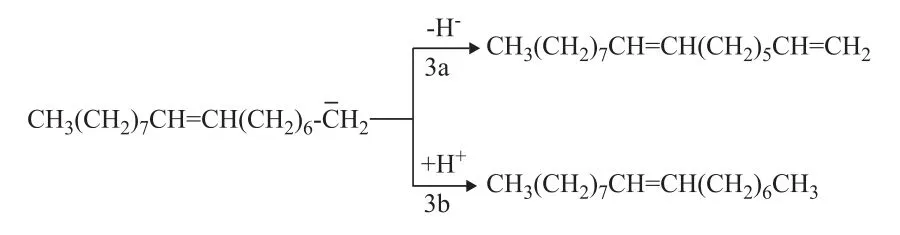
(3) Terminal allylation
(4) Cracking of unsaturated hydrocarbons (shown for the predominant allyl position)
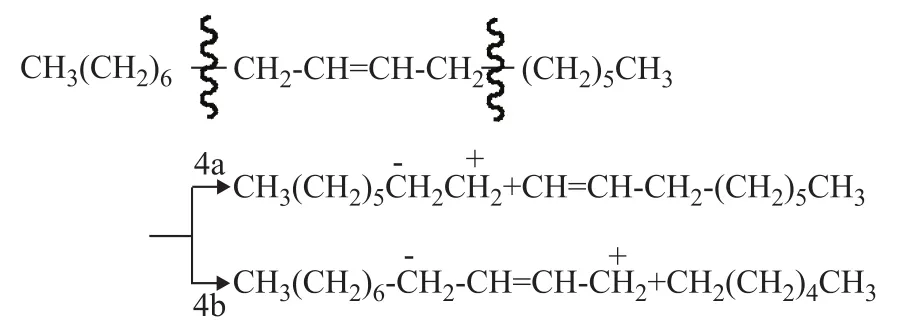
(5) Isomerization
a) Moving the double bond
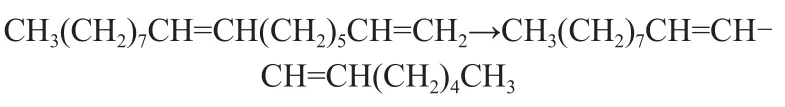
b) Forming more stable radicals

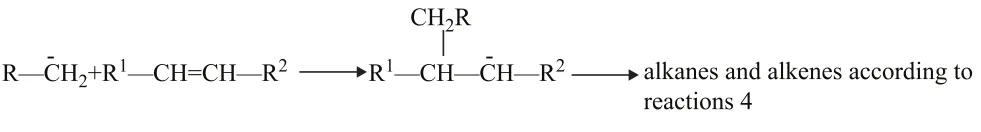
(6) Formation of branched radicals
a) Direct isomerization to form more stable radicals

b) Reactions of carbanion intermediate with double bonds
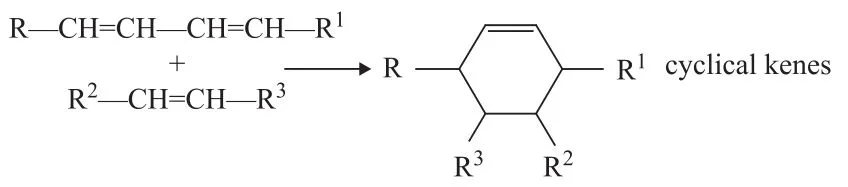
(7) Diels-Alder reaction (one of the possible pathwaystoward the formation of rings)
(8) Hydrogen abstraction and dehydrogenation
(9) Fatty acid salt cracking (previously unknown or uncommon reaction)

The interface and dipole polarization is the primary reactions involving sodium oleate in the microwave field. The existence of sodium ions on the carboxyl terminal may increase the polarity of the carboxyl terminal. Thus, the movement and arrangement of the carboxyl terminal dipole are random and disordered. The positive charges tend to move toward the negative pole, whereas the negative charges move toward the positive pole. The Lorentz force of ions or polar molecules would act in a similar manner as that of the electromagnetic wave, thereby reducing the entropy. The probability of the effective collision of molecules is increased by hundreds of millions times, as compared with the case without microwave radiation. Glycerol was added to the reaction as hydrogen donor; its dielectric constant is 56.2, which is smaller than that of water. Therefore, glycerol is a strong microwave absorber. The decarboxylation reaction of fatty acid salts occurs after it is “strengthened and activated” near the glycerol molecules. The hydrocarbon products would then rapidly leave the high-temperature area after decarboxylation. After the dissociation of sodium ions on the carboxyl terminal, the intramolecular p-π conjugated system would become more stable. Consequently, the length of two C—O bonds would be less different. Thus, the resulted negative ion intermediate formed thereby should be more stable. The breaking off of the hydroxyl and carboxyl groups would cause the removal of CO2, thereby forming carbanion intermediate at the high thermal potential point of glycerol. The double bond in sodium oleate forms a p-π conjugated system with carbanion. The intermediate is relatively stable, which would allow for the occurrence of decarboxylation reaction (see Figure 7). In addition, the π orbital of the double bond of sodium oleate and that σ orbital of the adjacent carbon atom both participate in the electron delocalization reaction to form the σ-π hyperconjugation (on the allyl group). This hyperconjugation is a precondition for the subsequent isomerization reaction. The double bond in sodium oleate forms the π-π conjugated system after migration and causes diene synthesis with other double bonds (Diels-Alder reaction). This reaction could result in the production of different series of cycloolefins and cyclanes. The cycloolefins formed accordingly would further react with the hydrogen donor (glycerol), thereby forming the aromatic hydrocarbon series.
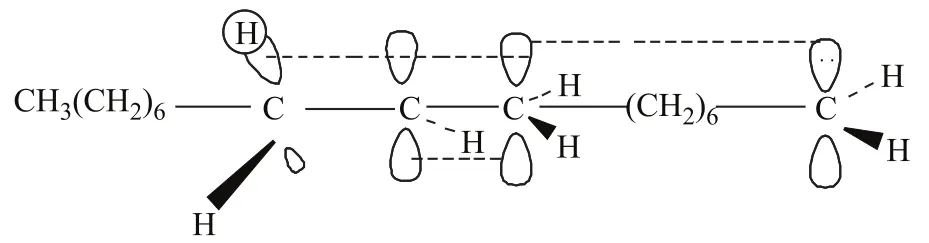
Figure 7σ-πhyperconjugated system andp-πconjugated system of pyrolysis intermediates

Figure 8 Samples of liquid products of microwave-assisted pyrolysis under different microwave power ratings (from leftto right: 400 W, 600 W, and 800 W)
The viscosity (2.40 mm2/s to 2.55 mm2/s) and density (0.870 g/cm3to 0.885 g/cm3) of the sodium oleate pyrolysis liquid product were measured using a SYD-265B petroleum product kinematic viscometer and a DM-100 digital density meter, respectively. The physical properties of this liquid were similar to those of diesel, thereby demonstrating the feasibility of using the microwave-assisted pyrolysis of sodium oleate to produce renewable hydrocarbon fuels.
4 Conclusions
In this study, the decarboxylation reaction was performed under the assistance of microwaves using sodium oleate as the model compound and glycerol as the hydrogen donor. Through GC-MS analysis, a series of alkanes, olefins, and aromatic hydrocarbon compounds were obtained from pyrolysis liquids. This experiment has proved the feasibility of using the microwave-assisted polarization of sodium oleate to produce renewable hydrocarbon fuels. The following main conclusions were made:
(1) The carboxyl terminal of sodium oleate has a stronger polarity than that of oleic acid. Therefore, dipole polarization and ionic conduction are more likely to occur in the microwave field. The microwave could selectively heat the carboxyl terminal, which is favorable for the decarboxylation reaction.
(2) The double bond in sodium oleate forms a p-π conjugated system with a carbanion intermediate during the decarboxylation process. Such a system is more stable, which is beneficial to the occurrence of pyrolysis reactions (e. g., decarboxylation, terminal allylation, isomerization, and aromatization).
(3) The viscosity (2.40 mm2/s to 2.55 mm2/s) and density (0.870 g/cm3to 0.885 g/cm3) of the sodium oleate pyrolysis liquid obtained from this experiment were similar to those of diesel, which demonstrates the feasibility of using the microwave-assisted polarization of sodium oleate to produce renewable hydrocarbon fuels.
Acknowledgements:This work was supported by the following projects: the National Natural Science Foundation of China (No. 21266022); the National High Technology Research and Development Program 863 (2012AA101800-03; 2012AA021205-6; 2012AA021704); the International Cooperation of Jiangxi Province (No. 20101208); the International Science & Technology Cooperation Program of China (No. 2010DFB63750); and the Natural Science Foundation of Jiangxi Province (No. 2008GZH0047).
[1] Kick T, Herbst J, Kathrotia T, et al. An experimental and modeling study of burning velocities of possible future synthetic jet fuels[J]. Energy, 2012, 43(1): 111-123
[2] Wang H W, Matthew A. Autoignition studies of conventional and Fischer–Tropsch jet fuels[J]. Fuel, 2012, 98(8): 249-258
[3] Xu J M, Jiang J C, Lu Y J, et al. Liquid hydrocarbon fuels obtained by the pyrolysis of soybean oils[J]. Bioresource Technol, 2009, 100(20): 4867-4870
[4] Hilten R, Speir R, Kastner J, et al. Production of aromatic green gasoline additives via catalytic pyrolysis of acidulated peanut oil soap stock [J] Bioresource Technol, 2011, 102(17): 8288-8294
[5] Maher K D, Bressler D C. Pyrolysis of triglyceride materials for the production of renewable fuels and chemicals[J]. Bioresource Technol, 2007, 98(12): 2351-2368
[6] Lappi H, Alén R. Pyrolysis of vegetable oil soaps—Palm, olive, rapeseed and castor oils[J]. J Anal Appl Pyrol, 2011, 91(1): 154-158
[7] Barreto C C K, Oliveira C C, Souza G G, et al. Evaluation of the stability during storage of a diesel-like fuel obtained by the pyrolysis of soybean oil[J]. Biomass Bioenerg, 2012, 37(2): 42-48
[8] Hu Z F, Ma X Q, Chen C X. A study on experimental characteristic of microwave-assisted pyrolysis of microalgae[J]. Bioresource Technol, 2012, 107(3): 487-493
[9] Lam S S, Russell A D, Lee C L, et al. Microwave-heated pyrolysis of waste automotive engine oil: Influence of operation parameters on the yield, composition, and fuel properties of pyrolysis oil[J]. Fuel, 2012, 92(1): 327-339
[10] Arshad A S, Farid N A. Microwave induced pyrolysis of oil palm biomass[J]. Bioresource Technol, 2011, 102(3): 3388-3395
[11] Chang C C, Wan S W. China’s motor fuels from Tung oil[J]. Ind Eng Chem, 1947, 39(2): 1543-1548
[12] Fortes I C P, Baugh P J. Study of calcium soap pyrolysates derived from Macauba fruit (Acrocomia sclerocarpa M.). Derivatization and analysis by GC/MS and CI-MS[J]. J Anal Appl Pyrol, 1994, 29(2): 153-167
[13] Ito T, Sakurai Y, Kakuta Y, et al. Biodiesel production fromwaste animal fats using pyrolysis method[J]. Fuel Process Technol, 2012, 94(1): 47-52
[14] Cumming K A, Wojciechowski B W. Hydrogen transfer, coke formation, and catalyst decay and their role in the chain mechanism of catalytic cracking[J]. Catal Rev Sei Eng, 1996, 38(1): 109-135
[15] Sanna A, Andresen J M. Bio-oil deoxygenation by catalytic pyrolysis: New catalysts for the conversion of biomass into densified and deoxygenated bio-oil[J]. Chem Sus Chem, 2012, 5(10): 1944-1957
[16] Changi S, Zhu M H, Savage P E. Hydrothermal Reaction Kinetics and Pathways of Phenylalanine Alone and in Binary Mixtures[J]. Chem Sus Chem, 2012, 5(9): 1743-1757
[17] Garciano L O, Tran N H, Kannangara G S K, et al. Pyrolysis of a Naturally Dried Botryococcus braunii Residue[J]. Energ Fuel, 2012, 26(6): 3874-3881
[18] Na J G, Han J K, Oh Y K, et al. Decarboxylation of microalgal oil without hydrogen into hydrocarbon for the production of transportation fuel[J]. Catal Today, 2012, 185(1): 313-317
[19] Ben H X, Ragauskas A J. One step thermal conversion of lignin to the gasoline range liquid products by using zeolites as additives[J]. Rsc Adv, 2012, 2(11): 12892-12898
[20] Hollak S A W, Bitter J H, van Haveren J, et al. Selective deoxygenation of stearic acid via an anhydride pathway[J]. Rsc Adv, 2012, 2(8): 9387-9391
[21] Liu Y H, Wang Y P; Wang Y K, et al. Microwaveassisted pyrolysis of Swida wilsoniana fruit oil soap for preparing renewable hydrocarbon fuel via selective decarboxylation[J]. Transactions of the Chinese Society of Agricultural Machinery (China), 2012: 43(2): 106-111 (in Chinese)
[22] Wang Y P, Liu Y H, Ruan R, et al. Mechanism of Hydrocarbon Generation from Sodium Stearate Decarboxylation by Microwave Assisted Pyrolysis[J]. Acta Chim Sin, 2012, 70(2): 114-120 (in Chinese)
Recieved date: 2013-02-26; Accepted date: 2013-03-18.
Professor Liu Yuhuan, Telephone: +86-13755621329; E-mail: liuyuhuan@ncu.edu.cn.
杂志排行
中国炼油与石油化工的其它文章
- Study on the Synthesis and Properties of PET Using Hydrotalcite as Catalyst
- Kinetic Modeling of Methanol to Olefins (MTO) Process on SAPO-34 Catalyst
- CFD Simulation of Orifice Flow in Orifice-type Liquid Distributor
- Photocatalytic Denitrogenation over Modified Waste FCC Catalyst
- Experimental and Molecular Dynamics Simulations for Investigating the Effect of Fatty Acid and Its Derivatives on Low Sulfur Diesel Lubricity
- Study on Reactive Adsorption Desulfurization of Model Gasoline on Ni/ZnO-HY Adsorbent
