Synthesis of 2-Methyl-4-methoxyaniline from o-Nitrotoluene Using Pt/C and Acidic Ionic Liquid as Catalyst System*
2013-06-07LIUYingxin刘迎新LIXiying李喜英FANGYanyan方艳艳andZHANGLin张琳
LIU Yingxin (刘迎新)**, LI Xiying (李喜英), FANG Yanyan (方艳艳) and ZHANG Lin (张琳)
Research and Development Base of Catalytic Hydrogenation, College of Pharmaceutical Science, Zhejiang University of Technology, Hangzhou 310032, China
Synthesis of 2-Methyl-4-methoxyaniline from o-Nitrotoluene Using Pt/C and Acidic Ionic Liquid as Catalyst System*
LIU Yingxin (刘迎新)**, LI Xiying (李喜英), FANG Yanyan (方艳艳) and ZHANG Lin (张琳)
Research and Development Base of Catalytic Hydrogenation, College of Pharmaceutical Science, Zhejiang University of Technology, Hangzhou 310032, China
2-Methyl-4-methoxyaniline (MMA) was synthesized by one-pot method through the hydrogenation and Bamberger rearrangement of o-nitrotoluene in methanol using acidic ionic liquid and 3% Pt/C as catalyst system. The effects of ionic liquid type, dosage of ionic liquid and 3% Pt/C, reaction temperature and reaction pressure on o-nitrotoluene conversion and MMA selectivity were investigated. The results indicated that the imidazolium-based acidic ionic liquid which contains SO3H-functionalized cation showed higher selectivity to MMA than other acidic ionic liquids used in this work. Using 1-(propyl-3-sulfonate)-3-methylimidazolium hydrosulfate ([HSO3-pmim][HSO4]) as the acid catalyst, the selectivity to MMA was as high as 67.6% at 97.8% of o-nitrotoluene conversion. As 3% Pt/C increased from 0.01 g to 0.025 g, the selectivity to MMA decreased from 73.4% to 62.5%, because of the hydrogenation of intermediate o-methyl-phenylhydroxylamine to o-toluidine becoming more dominant. An increase in hydrogen pressure also had obviously dramatic effect in lowering the MMA selectivity. After easy separation from the products, the catalyst system could be reused at least 3 times.
acidic ionic liquid, Bamberger rearrangement, 2-methyl-4-methoxyaniline, o-nitrotoluene, hydrogenation

Figure 1 Simplified reaction scheme of o-nitrotoluene to MMA

Figure 2 Structures of the acidic ionic liquids used in this work
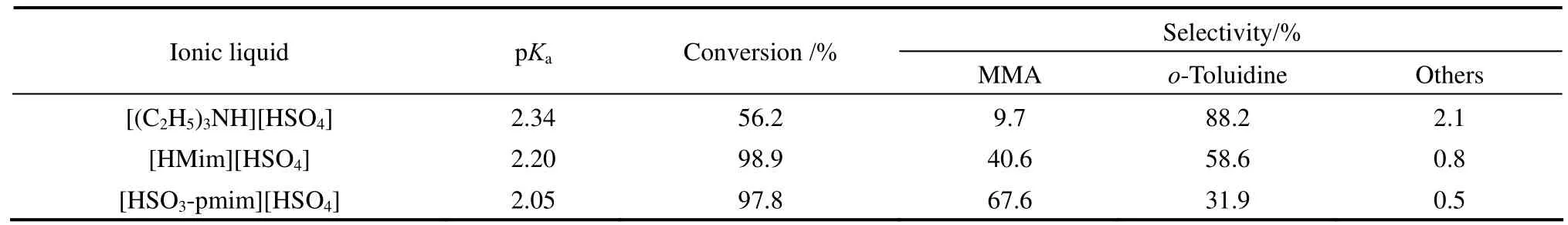
Table 1 Effect of ionic liquid type on the hydrogenation of o-nitrotoluene to MMA①
2 EX PERIMENTAL
2.1 Preparation of ionic liquids
Three ionic liquids, triethylamine hydrosulfate ([(C2H5)3NH][HSO4]), N-methylimidazolium hydrosulfate ([Hmim][HSO4]) and 1-(propyl-3-sulfonate)-3-methylimidazolium hydrosulfate ([HSO3-pmim][HSO4]) used in this work were synthesized according to the procedures described in the literatures [5,12,13]. The structures of the three ionic liquids are shown in Fig. 2, which were confirmed by1HNMR and FTIR.1HNMR spectra were run on a Bruker Avance III 500 MHz spectrometer in D2O using tetramethylsilane (TMS) as an internal reference (δ). FTIR measurements were obtained on a Nicolet 5700 FTIR absorption spectrometer using KBr.
IR and1HNMR spectra data for the ionic liquids are shown as follows:
[Hmim][HSO4]: IR (KBr, υ/cm−1): 3420, 3130, 2958, 2866, 1173, 1072, 1006, 886, 580, 451.1HNMR (500 MHz, D2O): δ 3.75 (s, 3H), 7.20 (s, 2H), 8.42 (s, 1H).
[(C2H5)3NH][HSO4]: IR (KBr, υ/cm−1): 2931, 2800-2250, 1173, 1071, 850, 581.1HNMR (500 MHz, D2O): δ 1.21 (t, 3H), 3.17 (q, 2H).
[HSO3-pmim][HSO4]: IR (KBr, υ/cm−1): 3420, 3137, 2958, 2596, 1629, 1460, 1173, 1072, 990, 580.1HNMR (500 MHz, D2O): δ 2.21 (m, 2H), 2.85 (t, 2H), 3.80 (s, 3H), 4.31 (t, 2H), 7.38 (s, 1H), 7.45 (s, 1H), 8.67 (s, lH).
2.2 Acidity characterization of ionic liquids
The acidity of the ionic liquids was characterized by pKameasured according to the method in [14]. A solution (0.005 mol·L−1) of ionic liquid was prepared in degassed water. The solution was then titrated with aqueous KOH solution (0.1 mol·L−1). The pH of the solution was measured using a composite electrode on a PHS-2F pH meter at room temperature. pKafor each ionic liquid was calculated by the procedure described by Albert and Serjeant [15].
2.3 General procedure for synthesis of MMA
The hydrogenation of o-nitrotoluene to MMA was carried out in a 100 ml stainless steel autoclave with a magnetic stirrer. An amount of 3%Pt/C (Shanxi Rock New Materials Co., China) and acidic ionic liquid, 2 ml o-nitrotoluene and 50 ml CH3OH were added into the autoclave. The autoclave was then purged for 4 times with hydrogen. The reaction products were analyzed using Agilent GC6820 chromatograph equipped with flame ionization detector (FID) and DB-5 capillary column.
3 RESUL TS AND DISCUSSION
3.1 Acidity of ionic liquids
The acidic catalytic property of ionic liquid is closely related to its acidity strength. pKais an important indicator to reflect the acidity strength. The smaller the pKavalue is, the greater the acidity of the sample. In this work, we used the method of acid-base titration to measure the acidity of the three ionic liquids. The results are shown in Table 1. It can be seen that with the same anion, the acidity of the ionic liquid is greatly influenced by the cation type. The acidities of the two imidazolium-based ionic liquids were larger than the triethylamine ionic liquid. In addition, as the sulfonic acid group can provide acid sites, the acidity of the ionic liquid containing sulfonic acid group on cation ([HSO3-pmim][HSO4]) was greater than the one without sulfonic acid group on cation ([Hmim][HSO4]). The order of acidity is as follows: [HSO3-pmim][HSO4]> [Hmim][HSO4]>[(C2H5)3NH][HSO4].
3.2 Effect of ionic liquid type
As shown in Fig. 1, the synthesis of MMA from o-nitrotoluene involves the hydrogenation of o-nitrotoluene to intermediate o-methyl-phenylhydroxylamine and the Bamberger rearrangement of o-methyl-phenylhydroxylamine to MMA. The first step must be carried out in the presence of acid, otherwise only o-toluidine is observed. Meanwhile, the Bamberger rearrangement will proceed only in the presence of acidic catalyst. Therefore, it is necessary to study the effects of the acidic ionic liquid type and its dosage on the selectivity to MMA. Table 1 shows the results of o-nitrotoluene hydrogenation to MMA using three different acidic ionic liquids as acid catalysts, keeping other reaction conditions constant.
It can be seen that the conversion of o-nitrotoluene and the selectivity to MMA were obviously affected by the type of the ionic liquid. Using [(C2H5)3NH][HSO4] as the acid catalyst, the conversion of o-nitrotoluene was 56.2%, whereas an appreciable amount of o-toluidine was formed with only 9.7% of MMA selectivity. Higher conversion and selectivity to MMA were obtained when the two imidazolium-based acidic ionic liquids were used as acid catalysts. Specially, using the imidazoliumbased acidic ionic liquid [HSO3-pmim][HSO4], which contains SO3H-functionalized cation, the selectivity to MMA was up to 67.6% at 97.8% of o-nitrotoluene conversion. From Table 1, it can be seen that the selectivity to MMA was in good agreement with the acidity order of ionic liquids. The results suggested that the strong acidic ionic liquid favors the conversion of o-nitrotoluene to intermediate o-methyl-phenylhydroxylamine and the Bamberger rearrangement of o-methyl-phenylhydroxylamine to MMA. In the following work, ionic liquid [HSO3-pmim][HSO4] was used as the acid catalyst.
3.3 Effect of the dosage of ionic liquid
The effect of the ionic liquid [HSO3-pmim][HSO4] dosage on hydrogenation of o-nitrotoluene to MMA was studied in the range of 2 ml to 6 ml by keeping the other reaction parameters constant, and the results are shown in Table 2. The conversion of o-nitrotoluene increased from 59.9% to 97.8% and the selectivity to MMA increased from 45.1% to 67.6% as the dosage of [HSO3-pmim][HSO4] increased from 2 ml to 4 ml. Further increase in the dosage of [HSO3-pmim][HSO4] from 4 ml to 6 ml, the conversion of o-nitrotoluene and the selectivity to MMA did not increase significantly. From the point of view of economy, the dosage of [HSO3-pmim][HSO4] was controlled to 4 ml.
3.4 Effect of the dosage of 3% Pt/C
The effect of 3% Pt/C dosage in the range of 0.01-0.025 g on the synthesis of MMA was studied. As shown in Table 3, at lower 3% Pt/C catalyst dosage (0.01 g), the selectivity to MMA was 73.4%, whereas the conversion of o-nitrotoluene was only 12.1%. This indicated that lower Pt/C catalyst can not provide enough active sites for the hydrogenation of o-nitrotoluene to intermediate o-methyl-phenylhydroxylamine. With increasing the catalyst dosage from 0.015 g to 0.025 g, the conversion of o-nitrotoluene increased from 37.4% to 98.2%, whereas the selectivity to MMA decreased from 70.1% to 62.5%. This is because that more hydrogenation active sites is available for further hydrogenation of o-methyl-phenylhydroxylamine to o-toluidine, with a obvious loss of selectivity to MMA.
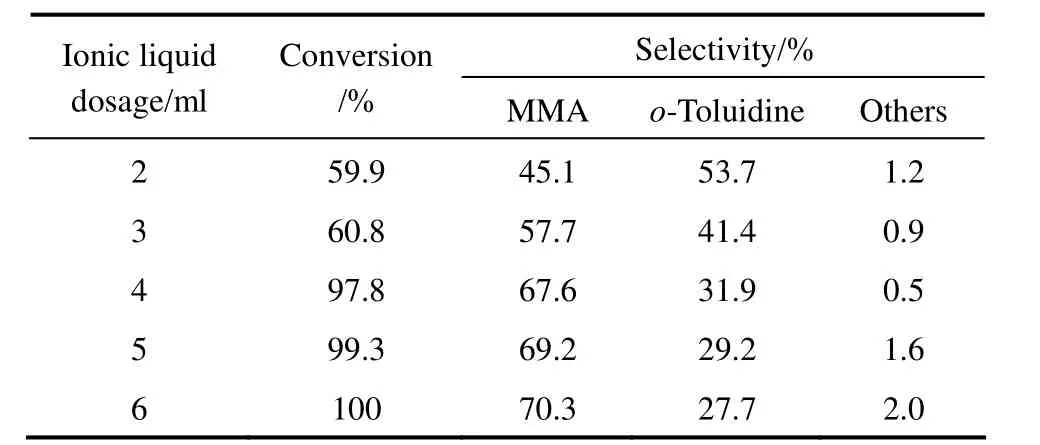
Table 2 Effect of the dosage of [HSO3-pmim][HSO4] on o-nitrotoluene hydrogenation to MMA①

Table 3 Effect of the dosage of 3% Pt/C on o-nitrotoluene hydrogenation to MMA①
3.5 Effect of reaction temperature
Considering the low boiling point of the solvent methanol, the effect of reaction temperature in the range of 40-60 °C on the conversion of o-nitrotoluene and the selectivity to MMA was investigated, and the results are shown in Table 4. From the results it can be noted that the reaction temperature has little effect on both o-nitrotoluene conversion and MMA selectivity as the reaction temperature increased from 40 °C to 60 °C. Since low temperature is not easy to control, we chose 50 °C as the optimum reaction temperature.

Table 4 Effect of the reaction temperature on o-nitrotoluene hydrogenation to MMA①
3.6 Effect of hydrogen pressure
Since the first step involves the hydrogenation of o-nitrotoluene to o-methyl-phenylhydroxylamine, the effect of hydrogen pressure on the conversion of o-nitrotoluene and the selectivity to MMA was also studied, and the results are shown in Table 5. As the pressure increased from 0.02 MPa to 0.04 MPa, the conversion of o-nitrotoluene remained almost constant (>97%) while the selectivity to MMA increased from 61.6% to 67.6%. With the increase in the pressure from 0.04 MPa to 0.10 MPa, the conversion of o-nitrotoluene increased slightly, while the selectivity to MMA decreased from 67.6% to 55.1%. It is because that with the increase in hydrogen pressure, a greater concentration of surface hydrogen becomes available on the active catalyst sites, facilitating the hydrogenation of o-methyl-phenylhydroxylamine to o-toluidine, with a large loss of selectivity to MMA.

Table 5 Effect of hydrogen pressure on o-nitrotoluene hydrogenation to MMA①
3.7 Recycling of catalyst system
Compared with the traditional inorganic acid, easy recycling is an attractive property for the acidic ionic liquids [7]. Consequently, the recycling of 3% Pt/C-[HSO3-pmim][HSO4] catalyst system was investigated. After reaction, 3% Pt/C was firstly filtered. The solvent methanol was removed by a rotary evaporator at 40 °C. Then [HSO3-pmim][HSO4] was extracted with ethyl acetate for 5 times at room temperature and was dried under vacuum at 100 °C. The catalyst system was then directly reused in the next run. The results are shown in Table 6. The conversion of o-nitrotoluene and the selectivity to MMA decreased with the recycling of the catalyst system, which might be ascribed to the leakage of 3% Pt/C-[HSO3-pmim] [HSO4] and the deactivation of the catalyst during the recycling. It can be seen that the catalyst system still showed high catalytic performance after reused two times, with 84.8% of o-nitrotoluene conversion and 47.4% selectivity to MMA.

Table 6 Recycling of 3% Pt/C-[HSO3-pmim][HSO4] catalyst system①
REFERENCES
1 Wang, S.F., Ma, Y.H., Wang, Y.J. Xue, W., Zhao, X.Q., “Synthesis of p-aminophenol from the hydrogenation of nitrobenzene over metal-solid acid bifunctional catalyst”, J. Chem. Tech. Biotech.,83, 1466-1471 (2008).
2 Deshpande, A., Figueras, F., Kantam, M.L., Ratnam, K.J., Reddy, R.S., Sekhar, N.S., “Environmentally friendly hydrogenation of nitrobenzene to p-aminophenol using heterogeneous catalysts”, J. Catal.,275, 250-256 (2010).
3 Nadgeri, J.M., Biradar, N.S., Patil, P.B., Jadkar, S.T., Garade, A.C., Rode, C.V., “Control of competing hydrogenation of phenylhydroxylamine to aniline in a single-step hydrogenation of nitrobenzene to p-aminophenol”, Ind. Eng. Chem. Res.,50, 5478-5484 (2011).
4 Qiu, X., Jiang, J.J., Wang, X.Y., Shen, Y.J., “Synthesis of N-phenyl-2-methyl-4-methoxyanline”, Org. Chem.,25, 561-566 (2005).
5 Li, H.L., Yu, S.T., Liu, F.S., Xie, C.X., Li, L., “Synthesis of dioctyl phthalate using acid functionalized ionic liquid as catalyst”, Catal. Commun,8, 1759-1762 (2007).
6 Zhao, D.B., Wu, M., Kou, Y., Min, E., “Ionic liquids: Applications in catalysis”, Catal. Today,74, 157-189 (2002).
7 Parvulescu, V.I., Hardacre, C., “Catalysis in ionic liquids”, Chem. Rev.,107, 2615-2665 (2007).
8 Chiappe, C., Pieraccini, D.J., “Ionic liquids: Solvent properties and organic reactivity”, J. Phys. Org. Chem.,18, 275-297 (2005).
9 Wei, Z.J., Li, Y., Li, F.J., Chen, C.J., Liu, Y.X., Ren, Q.L., “Catalytic esterification reactions over immobilized Brönsted ionic liquid”, CIESC J.,60, 1452-1458 (2009). (in Chinese)
10 Wei, Z.J., Li, Y., Thushara, D., Liu, Y.X., Ren, Q.L., “Novel dehydration of carbohydrates to 5-hydroxymethylfurfural catalyzed by Ir and Au chlorides in ionic liquids”, J. Taiwan Inst. Chem. Eng.,42, 363-370 (2011).
11 Wei, Z.J., Liu, Y.X., Thushara, D., Ren, Q.L., “Entrainer-intensified vacuum reactive distillation process for the separation of 5-hydroxylmethylfurfural from the dehydration of carbohydrates catalyzed by a metal salt-ionic liquid”, Green Chem.,14, 1220-1226 (2012).
12 Li, J., Jiang, J.C., Xu, J.M., Li, X.Y., “Synthesis of acidic ionic liquids and their application in activity for catalytic synthesis of triethyl citrate”, Biochem. Eng.,43, 15-20 (2009).
13 Xu, Z.J., Wan, H., Miao, J.M., Han, M.J., Yang, C., Guan, G.F.,“Reusable and efficient polystyrene-supported acidic ionic liquid catalyst for esterifications”, J. Mol. Catal. A Chem.,332, 152-157 (2010).
14 Fei, Z.F., Zhao, D.B., Geldbach, T.J., Scopelliti, R., Dyson, P.J., “Bronsted acidic ionic liquids and their zwitterions: Synthesis, characterization and pK(a) determination”, Chem. Eur. J.,10, 4886-4893 (2004).
15 Albert, A., Serjeant, E.P., The Determination of Ionization Constants: A Laboratory Manual, 3rd edition, Chapman and Hall, New York (1984).
1 INTRODUCTION
uids have
intensive attention in recent years, which have been successfully applied to many organic synthesis reactions as new green solvents and catalysts [6, 7]. The acidic ionic liquids have the potential to substitute industrial acid catalysts, because they have the characters of non-volatility of solid acid, the mobility of inorganic liquid acid and the feasibility of structural and functional tunability [8]. In our previous work, we synthesized several acidic ionic liquids and used them as solvents and acid catalysts in esterification, alcoholysis of isoflavone and dehydration of carbohydrates, and found that they showed high performance in the acidic reactions [9-11]. In this work, we prepared three acidic ionic liquids and used them as Bamberger rearrangement catalysts instead of sulfuric acid to synthesize MMA from o-nitrotoluene hydrogenation by one-pot method. The effects of ionic liquid type, dosage of ionic liquid and 3%Pt/C, and other factors on o-nitrotoluene conversionand MMA selectivity were investigated.
Received 2012-03-21, accepted 2012-11-03.
* Supported by the National Natural Science Foundation of China (21106134) and the Natural Science Foundation of Zhejiang Province (Y4100671).
** To whom correspondence should be addressed. E-mail: yxliu@zjut.edu.cn
2-Methyl-4-methoxyaniline (MMA) is an important intermediate in the synthesis of N-phenyl-2-methyl-4-methoxyanline, which is wildly used in the synthesis of hot (pressure)-sensitive dyes, pharmaceuticals, rubbers, agricultural chemicals, and so on. However, there are few reports about the synthesis of MMA. MMA can be synthesized by one-pot method through the hydrogenation and Bamberger rearrangement of o-nitrotoluene in methanol, similar to the synthesis of p-aminophenol from nitrobenzene hydrogenation [1-3]. The simplified reaction scheme is shown in Fig. 1. Qiu et al. [4] report the one-pot synthesis of MMA through the hydrogenation of o-nitrotoluene in methanol, using Pt/C as hydrogenation catalyst and acetic acid and sulfuric acid as Bamberger rearrangement catalyst. However, sulfuric acid can’t be reused and has other disadvantages such as equipment corrosion, more byproducts, tedious workup procedure and environmental problem [5].
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Effect of Hydrogen Reduction of Silver Ions on the Performance and Structure of New Solid Polymer Electrolyte PEI/Pebax2533/AgBF4Composite Membranes*
- Separation of Recombinant Geranylgeranyl Diphosphate Synthase of Deinococcus radiodurans from Expressed Strain Cell Homogenate by Immobilized Metal Affinity Chromatography on a Characterized Monolithic Cryogel Column*
- Immobilization of Papain in Biosilica Matrix and Its Catalytic Property*
- Comparison on Thermal Conductivity and Permeability of Granular and Consolidated Activated Carbon for Refrigeration*
- Supercritical Fluid Extraction of a Novel Template from Mesoporous Zirconia and the Effect on Porous Structure*
- Adsorption and Desorption Behavior of Tannic Acid in Aqueous Solution on Polyaniline Adsorbent*
