Comparison on Thermal Conductivity and Permeability of Granular and Consolidated Activated Carbon for Refrigeration*
2013-06-07JINZhequan金哲权TIANBo田波WANGLiwei王丽伟andWANGRuzhu王如竹
JIN Zhequan (金哲权), TIAN Bo (田波), WANG Liwei (王丽伟)** and WANG Ruzhu (王如竹)
Institute of Refrigeration and Cryogenics, Key Laboratory for Power Machinery and Engineering of M.O.E, Shanghai Jiao Tong University, Shanghai 200240, China
Comparison on Thermal Conductivity and Permeability of Granular and Consolidated Activated Carbon for Refrigeration*
JIN Zhequan (金哲权), TIAN Bo (田波), WANG Liwei (王丽伟)** and WANG Ruzhu (王如竹)
Institute of Refrigeration and Cryogenics, Key Laboratory for Power Machinery and Engineering of M.O.E, Shanghai Jiao Tong University, Shanghai 200240, China
This paper focuses on the development of three types of activated carbon (AC) adsorbents, i.e. granular AC, consolidated AC with chemical binder, and consolidated AC with expanded natural graphite (ENG). Their thermal conductivity was investigated with the steady-state heat source method and the permeability was tested with nitrogen as the gas source. Results show that the thermal conductivity of granular AC with different sizes almost maintains a constant at 0.36 W·(m⋅K)−1, while the value modestly increases to 0.40 W·(m⋅K)−1for the consolidated AC with chemical binder. The consolidated AC with ENG at the density of 600 kg·m−3shows the best heat transfer performance and their thermal conductivity vary from 2.08 W·(m⋅K)−1to 2.61 W·(m⋅K)−1according to its fraction of AC. However, the granular AC and consolidated AC with chemical binder show the better permeability performance than consolidated AC with ENG binder whose permeability changes from 6.98×10−13m2to 5.16×10−11m2and the maximum occurs when the content of AC reaches 71.4% (by mass). According to the different thermal properties, the refrigeration application of three types of adsorbents is analyzed.
adsorbent, activated carbon, expanded natural graphite, thermal conductivity, permeability
1 INTRODUCTION
As a green refrigeration technology, adsorption refrigeration is a good choice for harmonious development of energy and environment [1] because it is driven by the low grade heat and utilizes green refrigerant that has zero Ozone Depletion Potential (ODP) and Global Warming Potentials (GWP). Activated carbon (AC) is a type of widely utilized physical adsorbent with the advantages of high mass transfer performance, stable adsorption performance, and no corrosion to the metal materials, comparing with the chemical adsorbents such as chlorides. However, it has disadvantage of poor heat transfer performance, which will lead to low refrigeration efficiency for the reason of long cycle time it required. In order to improve the heat transfer performance, the consolidated adsorbents, which have higher thermal conductivity and lower thermal contact resistance, are researched by different researchers. Tamainot-Telto and Critoph [2] introduced monolithic carbon, which is developed by solidifying AC with organic binder so as to increase the density and thermal conductivity. However, the organic binder coated on the surface of AC influenced the permeability of adsorbent seriously. For example, the permeability of consolidated AC developed by Tamainot-Telto and Critoph is about 100 times lower than the permeability of pure compact graphite [2-4]. Such a result also leads to long cycle time, and in turn low refrigeration efficiency. Wang et al. manufactured the carbon blocks by solidifying the granular AC with the chemical binder and water, and the thermal conductivity had been improved by about two times as compared with granular AC [3], but they didn’t study the permeability of such type of adsorbent. Expanded natural graphite (ENG), as a type of matrix with high heat and mass transfer performances, is utilized by many researchers for heat and mass transfer intensification [5-10]. Eun et al. introduced silica gel composite blocks utilizing ENG as a matrix, and the blocks showed good thermal conductivity and permeability performance [11, 12]. Wang et al. developed a new type of composite adsorbent made by CaCl2and ENG, which greatly improved the thermal conductivity of CaCl2by about 36 times [13, 14].
Nowadays the research on the composite adsorbent with the ENG binder mainly focuses on the chemical adsorbents, but there are few researches on the compact adsorbent of AC binded with ENG. There are also few researches considering the anisotropy of heat and mass transfer performances of compact ENG, which had been reported recently [4].
For adsorption refrigeration cycles, the heat transfer performance always contradicts with the mass transfer performance, i.e. the heat transfer intensification will influence the mass transfer process. In order to develop a type of AC adsorbent with optimal heat transfer performance as well as the reasonable mass transfer performance, two binders were utilized for developing the solidified AC for heat transfer intensification: the chemical binder and expanded nature graphite (ENG) binder. Meanwhile, the thermal conductivity and permeability of different compositeswere compared to investigate the optimal adsorbents for different application occasions concerning the heat and mass transfer performance. The previous research work demonstrated the anisotropic thermal conductivity and permeability of solidified adsorbent, for which there is an optimum direction for heat and mass transfer process, namely the direction perpendicular to the compression direction of the adsorbent [4]. Considering the anisotropic property and the resistance between the testing metal surface and the surface of adsorbents, we designed the test units for which the adsorbents can be solidified into the testing chamber directly. For such a method, the adsorbent can tightly contact with the metal wall and there is less thermal resistance as well as no gas leakage in the testing process; secondly, the adsorbent can be tested directly by the optimal direction for heat and mass transfer. The steady-state heat source method and Ergun model that were proved suitable for the research on the anisotropic thermal conductivity and permeability [4] are utilized.
2 EX PERIMENTAL
2.1 Development of composite adsorbents
The activated carbon (AC) utilized in the experiments is manufactured from coconut shell (Type XY-20, Shanghai Xinhuoli Activated Carbon Co., China). In order to find a type of optimal adsorbent with higher thermal conductivity and permeability, 6 AC particles with different size, which are shown in Table 1, were tested.
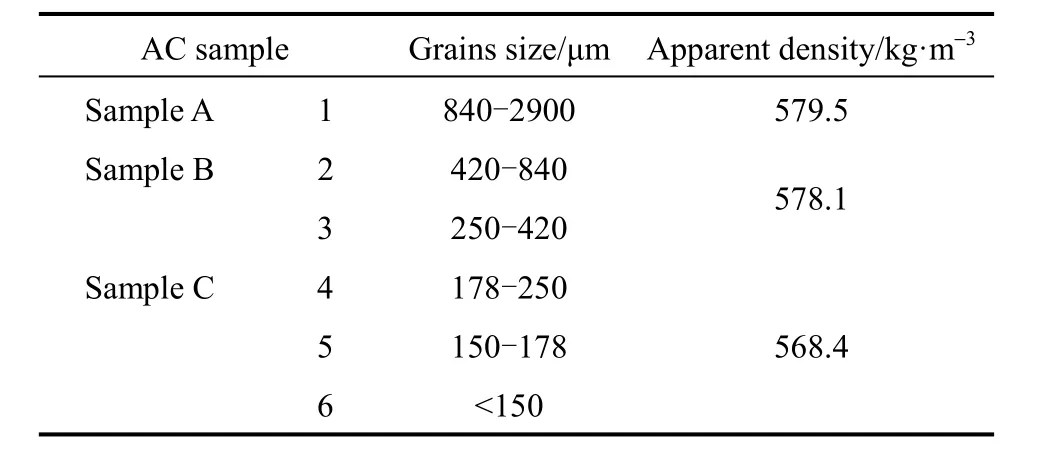
Table 1 Physical properties of AC grains of different size
Consolidated adsorbent of AC with chemical binder, which is shown in Fig. 1 (a), was developed by three steps. Firstly the granular AC was mixed with the binder which is made from vulcanizing silicone rubber (Type 705 binder, Wuxi Forward Chemical Plant, China) in the mass ratio of 5∶1, which is determined by comprehensive consideration to the consolidation and mass transfer performances [15]. Secondly the adsorbent with relative density at about 600 kg·m−3was directly compressed into the specimen chamber of the test units, and finally the consolidated adsorbent was heated at 120-130 °C for 2 h in order to desorb the water or other contaminants from the sample. Three kinds of consolidated absorbents were developed, respectively, using the granular AC Samples A, B, and C.
In order to get the optimal results of thermal conductivity and permeability, 6 different AC samples were tested firstly, and then the optimal AC was selected for the development of consolidated AC/ENG composite adsorbents whose densities are about 600 kg·m−3and the respective mass ratios (AC∶ENG) are 1∶1.5, 1∶1, 1.5∶1, 2∶1, and 2.5∶1. Expanded nature graphite (ENG) is a product of natural graphite, produced by Shanghai Yifan Graphite Co. in China, with size of 178-280 μm and the purity better than 99%. Natural graphite is expanded in an oven at 600 °C for 10 min. The thermal conductivity and the density of the granular ENG were tested, and the values are 16.2 kg·m−3and 0.095 W·m·K−1respectively. The photo of the consolidated AC/ENG is shown in Fig. 1 (b). The procedures of developing the consolidated composite adsorbents are as follows: firstly expands the natural graphite under the temperature of 600 °C for about 10 min, then mix a certain amount of water into the quantified ENG and AC together, and at last, put the ENG-water-AC mixtures into the drying oven and heat them at 120 °C-130 °C for 12 h.
2.2 Thermal conductivity test
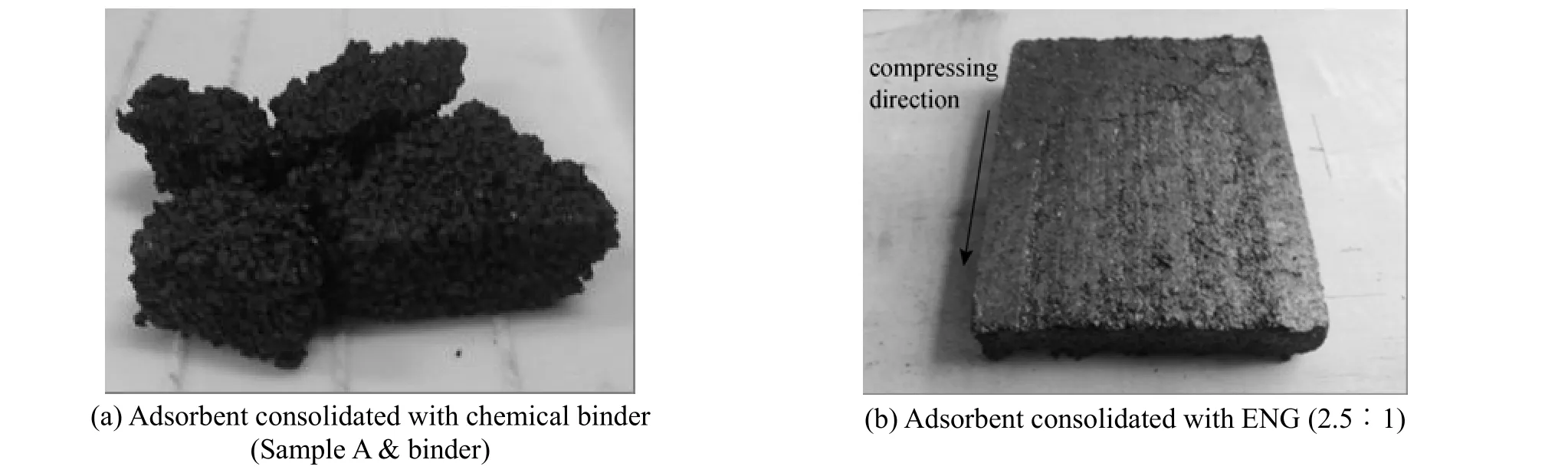
Figure 1 Photos of consolidated AC adsorbents
The thermal conductivity of the adsorbents was investigated by steady-state heat source method which is based on British Standard BS-874 [2]. The schematic design of the test unit is shown in Fig. 2. The maincomponents of the unit include: a center plate heater sandwiched between two specimen chambers, two water coolers fixed symmetrically left and right, a guard heater radially distributed beyond the central plate heater, pieces of thermal insulation materials, and two specimen chambers. The consolidated adsorbents were developed by compressing the samples into the chambers in an optimal direction for heat transfer, which was vertical to the compressing direction [4]. Compared with the thermal conductivity testing method mentioned in Refs. [2] and [4], the samples don’t need to be taken out of the pressing vessel before the thermal conductivity are tested, so that the contact thermal resistance is lowered.
The thermal conductivity λ is determined by measuring the average temperature gradient ΔT through the compacted samples and a known axial heat flux Q under steady-state conditions. When the working conditions are set up and the steady state is reached, the thermal conductivity λ (W−1·m−1⋅K−1) is calculated by the following expression:
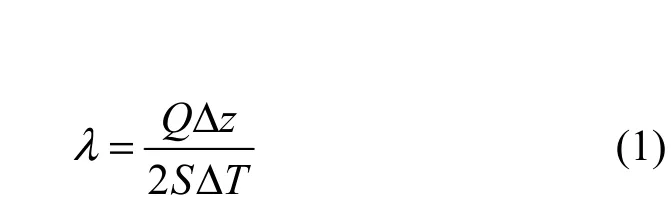
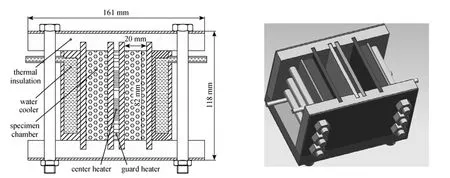
Figure 2 Schematic design of the test unit for thermal conductivity (top & 3D views)
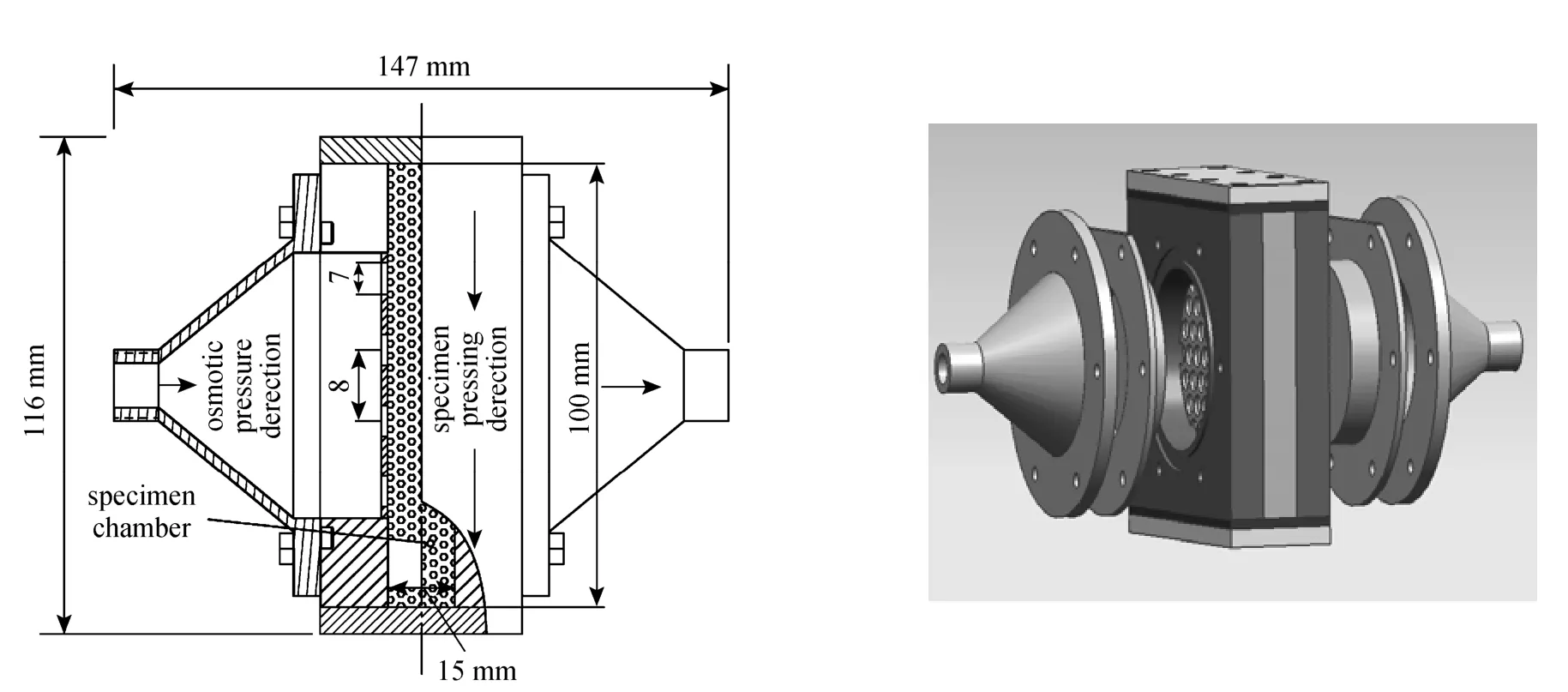
Figure 3 Schematic design of experimental unit for permeability (side & 3D views)
where Q is the power (W) of central square plate heater, Δz is the thickness of the compacted sample, ΔT is the temperature difference across the sample and S is the effective heating area of the central plate heater (m2).
2.3 Permeability test
The permeability test equipment is shown in Fig. 3. The main components are the specimen holder, a differential manometer, a rote meter, and gas source. Before experiments, the composite adsorbent will be compressed into the chamber in an optimal direction for the permeability. The anisotropic thermal conductivity and permeability were addressed in [4] in detail, showing that in the direction perpendicular to the compression are the optimal thermal conductivity and the optimal permeability.
The permeability test comprises measurements of the pressure drop Δp through the sample and the flow rate qvwhen the nitrogen flows across the sample. Ergun model is applicable in the calculation since the samples to be tested are porous media with very low gas velocity, and the intrinsic characteristics of the material obey the following expression [2]:
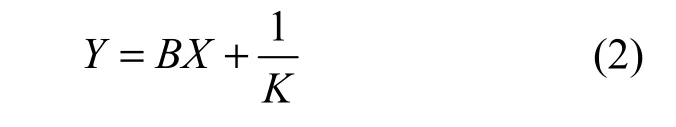
in which
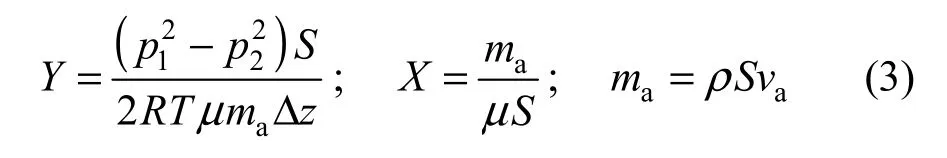
where K is the permeability of the samples (m2), B is the shape factor of the samples, p1and p2are inlet pressure and outlet pressure of the pressure air, S is the effective area of the sample cross section (m2), R is the gas constant (J·kg−1·K−1), T is the sample temperature (K), μ and ρ are the gas viscosity (Pa·s) and density (kg·m−3), respectively; mais gas mass flow rate (kg·s−1), vais axial velocity of the sample (m·s−1).
2.4 Experimental error analysis
The error transfer function of thermal conductivity is
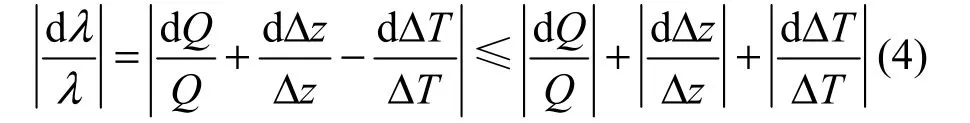
Temperatures are tested by the thermal couples with the measuring error of ±0.5 °C. The heating flow is calculated by the electric current and electric resistance, in which the electric resistance has the relative error of ±1% after calibration and the electric current is controlled by the stabilized power supply which has the relative error of ±1%.The thickness of the sample is measured by the micrometer with the error of ±0.01 mm, and the thickness of the sample in the experiments is 20 mm, the minimum value of temperature difference is 5.31 °C, the electric current is 1 A, the electric resistance is 14.1 Ω. According to these parameters the maximal relative error of the thermal conductivity in the experiments is 13.8%.
The error transfer function of permeability is
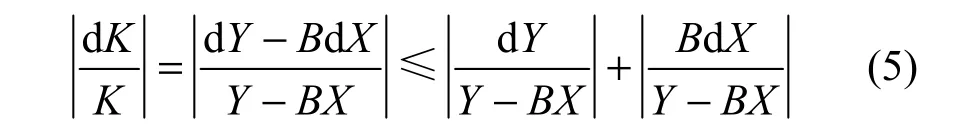
The pressure is calculated by the environmental pressure and the differential pressure meter (Dwyer477A-7, Dwyer Instruments, USA) with the error of ±0.1%, and the gas mass flow rate is tested by the flow meter (Dwyer MMA-23, Dwyer Instruments, USA) with the error of ±4%, the maximal calculated error of K is 4.6%. Considering that the error can also be caused by the gas leakage between the adsorbent and the wall although the value is very small for that the adsorbent contact with the wall tightly by pressing it into the testing slot directly, the error is verified by the repeat experiments. The relative difference for the repeat experiments is generally lower than 12%.
3 RESUL TS AND DISCUSSION
3.1 Choice of optimal granular AC
The thermal conductivity of different AC grains shown in Table 1 were tested firstly, and the relations between thermal conductivity and heating power were shown in Fig. 4. Results revealed that the thermal conductivity changed little with the supplied heating power from 2.252 W to 5.076 W, but when the heating power was too small such as at 1.727 W, the thermal conductivity of AC turned out to be much different from other states. It was mainly caused by the low heating power of that point, which made the states need a very long time for approaching the equilibrium state. In order to make the results more precisely only the results for reasonable values, i.e. the values for the heating power larger than 2.252 W were considered in the following analysis.
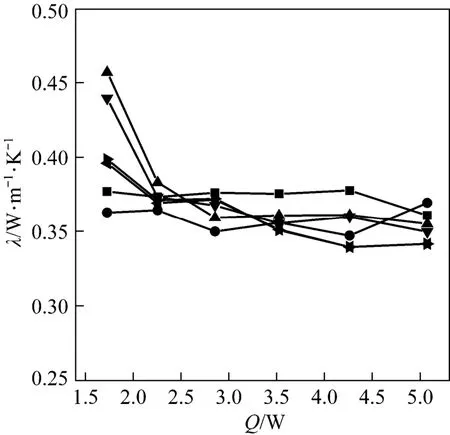
Figure 4 Thermal conductivity of granular AC vs. heating power of central heater■ 6-20; ● 20-40; ▲ 40-60; ▼ 60-80; 80-100; >100
3.2 Thermal conductivity of consoli dated adsorbents
Considering that the thermal conductivity of consolidated adsorbent will be improved for the granular AC with smaller size because of more compact inter-particle contact when the chemical binder exists, three consolidated adsorbents with different sizes of AC and chemical binder are developed. The relations between thermal conductivity and electric current are shown in Fig. 5. The thermal conductivity almost maintain at a constant at 0.40 W·(m⋅K)−1for different electric currents and different consolidated samples, and it is improved about 10% if compared with the non-consolidated granular AC as is shown later in Fig. 7.
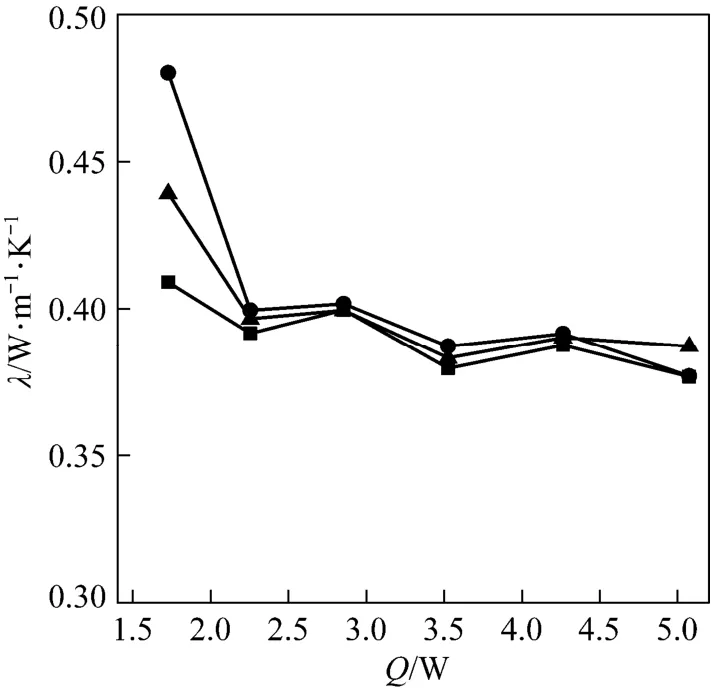
Figure 5 Thermal conductivity of consolidated AC/chemical binder adsorbent vs. heating power of central heater■ Sample 1A; ● Sample 2A; ▲ Sample 3A
Because the size of AC won’t influence the thermal conductivity very much, in order to acquire better mass transfer and uniform mixing performances, Sample B (250-840 μm) is selected for the development of consolidated composite adsorbents with ENG as the binder. Five types of samples, which have ratios between AC and ENG of 1∶1.5, 1∶1, 1.5∶1, 2∶1, and, 2.5∶1 are prepared, and the relations between thermal conductivity and electric current are shown in Fig. 6. The values of the thermal conductivity change little with the electric current, but vary considerably with different AC ratios as is also shown in Fig. 7. The value of thermal conductivity is as high as 2.61 W·m−1⋅K−1when the ratio between AC and ENG is 1∶1.5 [AC 40% (by mass)], and the value decreases gradually to 2.08 W·m−1⋅K−1as the AC ratio increases up to 71.4% (2.5∶1). The optimal values improved as high as about 7 times if compared with the results of granular AC, which could attribute to the high thermal conductivity of ENG whose value is 3.64 W·m−1⋅K−1at the density of 600 kg·m−3.
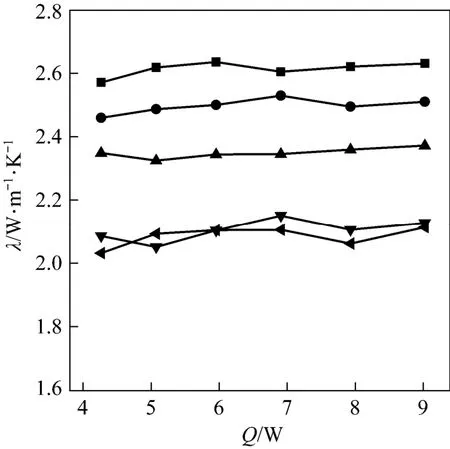
Figure 6 Thermal conductivity of consolidated AC/ENG adsorbent vs. heating power of central heater■ 1∶1.5; ● 1∶1; ▲ 1.5∶1; ▼ 2∶1; 2.5∶1
3.3 Experimental results of permeability
The permeability are tested for different samples, and the results are shown in Table 2. In experiments the data for the consolidated adsorbent with chemical binder and granular AC of larger size cannot be gotten because the pressure difference between two sides of the testing unit almost zero while the permeability is well above the upper measurement limit. For the granular AC with smaller size, which is Sample B, the permeability gotten from experiments is 4.69×10−9m2, which is much higher than the values of consolidatedAC and ENG binder whose values vary from 6.98×10−13m2to 5.16×10−11m2. For the consolidated AC with ENG, the largest value of permeability can be gotten when the ratio of AC reaches 71.4% (ratio 2.5∶1).
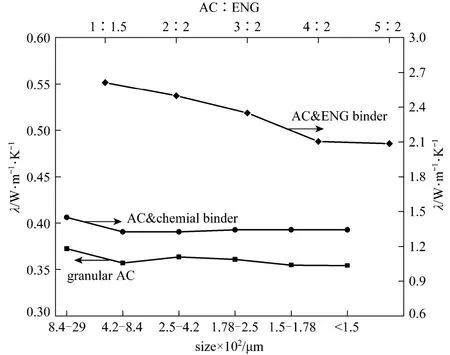
Figure 7 The comparison of thermal conductivity of different consolidated adsorbent

Table 2 Values of permeability test result for different adsorbents
3.4 Discussions for the application of different samples
The thermal conductivity and permeability are all important for the adsorption refrigeration process because they will influence the cycle time. But for different refrigerants, the influences will be a little different for the reason that the mass transfer performance is also related with the refrigerant pressure. For example, for the refrigerant working under the vacuum condition, such as AC-methanol working pair, the mass transfer is very critical, and then the mass transfer is a main factor for the refrigeration efficiency, and the consolidated AC with chemical binder is an optimal choice because it has higher thermal conductivity than granular AC and similar permeability with granular AC. For the refrigerant working pair under the pressure condition, such as AC-ammonia pair, because the mass transfer performances improved significantly by the pressurized refrigerant vapor, the consolidated adsorbent with ENG binder is an optimal choice for the reason of much higher conductivity. For the application of consolidated AC with ENG, the mass and heat transfer direction must keep vertical to the solidifying (sample pressing) direction to maximize the rate of heat and mass transfer; otherwise the optimal results cannot be gotten for the reason of anisotropic heat and mass transfer characteristics of ENG. Compared with the granular AC, the composite AC improved the thermal conductivity effectively, and the thermal conductivity is increased by up to 7.2 times. If comparing the solidified AC developed in [2] the highest thermal conductivity obtained here is improved by about 5 times.
4 CONCLU SIONS
Three different types of adsorbents for the AC adsorption refrigeration system, i.e. granular AC, consolidated AC by chemical binder, and consolidated AC with ENG were tested, and their thermal conductivity and permeability were tested respectively. The main conclusions are as follows:
(1) AC grains of different size show almost the same performance of heat transfer, and the value of the thermal conductivity maintain a constant at 0.36 W·(m⋅K)−1. The permeability of AC with larger size is higher than that of smaller size.
(2) Two kinds of consolidated composite adsorbents show quite different performance of heat transfer. Comparing with the granular AC the thermal conductivity of consolidated AC/chemical binder adsorbents only increases modestly to 0.40 W·(m⋅K)−1, while that of consolidated AC/ENG composite adsorbents can reach as high as 2.61 W·m−1⋅K−1when the ratio between AC and ENG is 1∶1.5. However, The granular AC and consolidated AC/chemical binder adsorbents have better permeability performance than consolidated AC and ENG composite adsorbents.
NOMENCLATURE
B shape factor of sample
K permeability, m2
magas mass flow rate, kg·s−1
p1inlet pressure of air, Pa
p2outlet pressure of air, Pa
Q heating power, W
qvgas volume flow rate, L·min−1
R gas constant, J·kg−1·K−1
S effective heating area of the central square plate heater, m2
T sample temperature, K
ΔT average temperature gradient, K
vaaxial velocity, m·s−1
Δz thickness of the samples, m
λ thermal conductivity, W·m−1·K−1
μ gas viscosity, Pa·s
ρ density, kg·m−3
REFERENCES
1 Wang, R.Z., Wang, L.W., Wu, J.Y., Theory and Application of Adsorption Refrigeration, Science Press, Beijing, China (2007). (in Chinese)
2 Tamainot-Telto, Z., Critoph, R.E., “Monolithic carbon for sorption refrigeration and heat pump applications”, Appl. Therm. Eng., 21, 37-52 (2001).
3 Wang, S.G., Wu, J.Y., Li, X.R., Wang, R.Z., “Developments of consolidated adsorbent and experimental study of consolidated activated carbon blocks”, Acta Energy Sol. Sinica, 25 (3), 283-287 (2004). (in Chinese)
4 Wang, L.W., Tamainot-Telto, Z., Metcalf, S.J., Critoph, R.E., Wang, R.Z., “Anisotropic thermal conductivity and permeability of compacted expanded natural graphite”, Appl. Therm. Eng., 30 (13), 1805-1811 (2010).
5 Fujioka, K., Hatanaka, K., Hirata, Y., “Composite reactants of calcium chloride combined with functional carbon materials for chemical heat pumps”, Appl. Therm. Eng., 28, 304-310 (2008).
6 Mauran, S., Lahmidi, H., Goetz, V., “Solar heating and cooling by a thermochemical process, first experiments of a prototype storing 60 kW·h by a solid/gas reaction”, Sol. Energy, 82, 623-636 (2008).
7 Klein, H.P., Groll, M., “Heat transfer characteristics of expanded graphite matrices in metal hydride beds”, Int. J. Hydro Energy, 29, 1503-1511 (2004).
8 Kim, K.J., Montoya, B., Razani, A., Lee, K.H., “Metal hydride compacts of improved thermal conductivity”, Int. J. Hydro Energy., 26, 609-613 (2001).
9 Menard, D., Py, X., Mazet, N., “Activated carbon monolith of high thermal conductivity for adsorption processes improvement, Part A: Adsorption step”, Chem. Eng. Process., 44, 1029-1038 (2005).
10 Ahmet, S., Ali, K., “Thermal conductivity and latent heat thermal energy storage characteristics of paraffin/expanded graphite composite as phase change material”, Appl. Therm. Eng., 27, 1271-1277 (2007).
11 Eun, T.H., Song, H.K., Han, J.H., “Enhancement of heat and mass transfer in silica-expanded graphite composite blocks for adsorption heat pumps: Part I. Characterization of the composite blocks”, Int. J. Refrig., 23 (1), 64-73 (2000).
12 Eun, T.H., Song, H.K., Han, J.H., “Enhancement of heat and mass transfer in silica-expanded graphite composite blocks for adsorption heat pumps. II. Cooling system using the composite blocks”, Int. J. Refrig., 23 (1), 74-81 (2000).
13 Wang, K., Wu, J.Y., Wang, R.Z., Wang, L.W., “Composite adsorbent of CaCl2and expanded graphite for adsorption ice maker on fishing boats”, Int. J. Refrig., 29, 199-210 (2006).
14 Wang, K., Wu, J.Y., Wang, R.Z., “Thermal conductivity measurement of the CaCl2/expanded graphite compound adsorbent”, J. Shanghai Jiaotong University, 42 (1), 106-109 (2008). (in Chinese)
15 Li, X.R., Wu, J.Y., Lu, Y.Z., “Experimental analysis of adsorbent properties of consolidated composite active carbons with methanol”, J. Eng. Therm. Phys., 24 (3), 382-384 (2003). (in Chinese)
2011-03-03, accepted 2012-11-27.
* Supported by the National Science Foundation for Excellent Young Scholars (51222601), the International Collaborating Project Funded by the Foundation of Science and Technology Commission of Shanghai Municipality (11160706000), the Program for New Century Excellent Talents in University by the Ministry of Education of China and the Shanghai Pujiang Program of China.
** To whom correspondence should be addressed. E-mail: lwwang@sjtu.edu.cn
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Preparation of Mesoporous Carbons from Acrylonitrile-methyl Methacrylate Copolymer/Silica Nanocomposites Synthesized by in-situ Emulsion Polymerization*
- Separation of Recombinant Geranylgeranyl Diphosphate Synthase of Deinococcus radiodurans from Expressed Strain Cell Homogenate by Immobilized Metal Affinity Chromatography on a Characterized Monolithic Cryogel Column*
- Immobilization of Papain in Biosilica Matrix and Its Catalytic Property*
- Effect of Hydrogen Reduction of Silver Ions on the Performance and Structure of New Solid Polymer Electrolyte PEI/Pebax2533/AgBF4Composite Membranes*
- Synthesis of 2-Methyl-4-methoxyaniline from o-Nitrotoluene Using Pt/C and Acidic Ionic Liquid as Catalyst System*
- Adsorption and Desorption Behavior of Tannic Acid in Aqueous Solution on Polyaniline Adsorbent*
