Effect of Hydrogen Reduction of Silver Ions on the Performance and Structure of New Solid Polymer Electrolyte PEI/Pebax2533/AgBF4Composite Membranes*
2013-06-07WANGYanbei王雁北RENJizhong任吉中LIHui李晖andDENGMaicun邓麦村
WANG Yanbei (王雁北), REN Jizhong (任吉中)**, LI Hui (李晖) and DENG Maicun (邓麦村)
National Engineering Research Center of Membrane Technology, Dalian Institute of Chemical Physics, CAS, Dalian 116023, China
Effect of Hydrogen Reduction of Silver Ions on the Performance and Structure of New Solid Polymer Electrolyte PEI/Pebax2533/AgBF4Composite Membranes*
WANG Yanbei (王雁北), REN Jizhong (任吉中)**, LI Hui (李晖) and DENG Maicun (邓麦村)
National Engineering Research Center of Membrane Technology, Dalian Institute of Chemical Physics, CAS, Dalian 116023, China
In this paper, the effect of hydrogen reduction of silver ions on the performance and structure of new solid polymer electrolyte polyetherimide (PEI)/Pebax2533 (Polynylon12/tetramethylene oxide block copolymer, PA12-PTMO)/AgBF4composite membranes is investigated. For PEI/Pebax2533/AgBF4composite membranes prepared with different AgBF4concentration, the permeances of propylene and ethylene increase with the increase of AgBF4concentration due to the carrier-facilitated transport, resulting in a high selectivity. But for propylene/propane mixture, the mixed-gas selectivity is lower than its ideal selectivity. The hydrogen reduction strongly influences the membrane performance, which causes the decrease of propylene permeance and the increase of propane permeance. With the increase of hydrogen reduction time, the membranes show a clearly color change from white to brown, yielding a great selectivity loss. The data of X-ray diffraction and FT-IR prove that silver ions are reduced to Ag0after hydrogen reduction, and aggregated on the surface of PEI/Pebax2533/AgBF4composite membranes.
solid polymer electrolyte membrane, hydrogen reduction of silver ions, facilitated transport, olefin/ paraffin separation
1 INTRODUCTION
Ethylene and propylene are the most important organic materials in the petrochemical industry. Currently, olefins and paraffins (low relative volatilities) are mainly separated by cryogenic distillation processes, which require enormous capital investment and high operational costs [1]. Usually the polymeric membranes are not attractive for industrial purposes due to the plasticization effect induced by the olefins and their low selectivity [2-4]. The separation of olefins from paraffins by carrier-facilitated transport process is mainly studied according to reversible complexation between olefins and carriers [5, 6], which concerns ion-exchange membranes, supported liquid membranes, solid polymer electrolyte membranes, etc [6-14]. The solid polymer electrolyte membranes have attracted extensive attention because of their remarkable selectivity for separation of olefin/paraffin [5, 6]. Generally, solid polymer electrolyte membranes are complexes of polar polymer with metal salts, which are formed by dissolving the salts in polymer hosts. Pinnau et al. [9] found that the properties of the PEO/AgBF4membrane were strongly dependent on the silver salt content. Provided that the AgBF4concentration is high enough (>50%, by mass), the silver ions dissolved in the PEO matrix simultaneously facilitate ethylene transport and hinder ethane transport. This phenomenon suggests that threshold carrier content exists, below which no facilitated transport process occurs for olefins. Because of high carrier concentration, some defects usually arise on the surface of the solid polymer electrolyte membranes. In order to prepare a defect-free thin solid polymer electrolyte layer, the membranes are usually coated with a sealing layer such as polydimethylsiloxane [6].
Because of the plasticization and selectivity loss caused by silver carrier poisoning compounds present in the industrial gas streams, the application of solid polymer electrolyte membranes is facing challenges from commercialized purposes. In previous works, it is well known that silver salt-based solid polymer electrolyte membranes are not stable in the presence of carrier poisoning materials such as hydrogen, light, hydrogen sulfide and acetylenic compounds [9, 10]. However, there are scarce literatures describing the effect of poisoning compounds on the performance and structure of facilitated transport membranes. The mechanisms of carrier reduction under the exposure to these compounds and light are indeterminate. These poisoning compounds affect the lifetime and performance of solid polymer electrolyte membranes through reacting with silver ions. As an olefin carrier, Ag (I) is more active than Ag (0) [11, 12]. In order to develop membranes with lifetime satisfactory for industrial implementation, the effect of hydrogen on the performance and structure of PEI/Pebax2533/AgBF4composite membranes is studied. If the stability problem could be mainly circumvented by investigation of the carrier poisoning mechanisms, it would provide a breakthrough toward finally commercial application of facilitated transport membranes.
In this paper, defect-free PEI/Pebax2533 composite membranes (PEI: polyetherimide; Pebax2533: Polynylon12/tetramethylene oxide block copolymer, PA12-PTMO) are firstly prepared with Pebax2533 as a coating layer on the microporous PEI supporting membranes. And new solid polymer electrolyte PEI/ Pebax2533/AgBF4composite membranes are prepared with complexing method. The effect of reduction of silver ions on the performance and structure of PEI/ Pebax2533/AgBF4composite membranes is investigated. The surface morphology of PEI/Pebax2533/AgBF4composite membranes (before and after reduction) is also analyzed with X-ray diffraction and FT-IR.
2 EX PERIMENTAL
2.1 Materials
The microporous polyetherimide (PEI, Ultem®1000, General Electric) supporting membranes were supplied by National Engineering Research Center of Membrane Technology, Dalian Institute of Chemical Physics. The average pore size of the microporous PEI membranes was about 65 nm. Pebax®2533 (Polynylon12/ tetramethylene oxide block copolymer, PA12-PTMO) was purchased from Arkema, which comprised 80% (by mass) of poly(tetramethylene oxide) and 20% (by mass) of nylon-12. The 1-butanol (Tianjin Bodi Chemical Engineering Co., Ltd.) was used for preparing Pebax2533 solutions. Silver tetrafluoroborate (AgBF4) was purchased from Alfa Aesar. Milli-Q ultrapure water was used to prepare AgBF4solution. All chemicals were used without further purification. All gases used in the permeation experiments were of research grade (purity >99.9%) from Dalian Gases Company.
2.2 Preparation of PEI/Pebax2533/AgBF4composite membranes
PEI/Pebax2533 composite membrane was prepared by dip-coating a thin layer of Pebax2533 on the microporous PEI flat sheet supporting membrane. A predetermined amount of Pebax2533 was dissolved in 1-butanol to form a 2.0%-6.0% (by mass) homogeneous Pebax2533/1-butanol solution with vigorous stirring at 80 °C for 12 h. The prepared Pebax2533/1-butanol solution was kept still for 24 h to remove gas bubbles dissolved in the solution. Then, it was cast on the treated microporous PEI supporting membrane, and the nascent composite membrane coated with Pebax2533 was dried at ambient conditions for 24 h. The prepared PEI/Pebax2533 composite membranes were further dried under vacuum at 50 °C for 24 h before permeation experiments. The thickness of dense Pebax2533 layer for PEI/Pebax2533 composite membranes was about 3-7 μm.
The preparation of PEI/Pebax2533/AgBF4composite membranes was described in the previous work [15]. AgBF4was dissolved in ultrapure water to prepare AgBF4aqueous solution with different AgBF4concentration. The external surface of PEI/Pebax2533 composite membrane was immersed into the AgBF4aqueous solution for 2 h and the other side of the composite membrane was not contacted with the AgBF4aqueous solution. Due to the reversible complexation between silver salt and ether oxygen in Pebax2533 backbone, AgBF4was transferred into the external surface of Pebax2533 layer and formed a solid polymer electrolyte layer. After PEI/Pebax2533/AgBF4composite membrane was removed from AgBF4aqueous solution, the external surface of the prepared membrane was washed quickly with ultrapure water to remove the resident AgBF4solution. The prepared PEI/Pebax2533/ AgBF4composite membranes were dried and then kept into a desiccator away from light before the permeation and reduction experiments. The AgBF4content incorporated into the membranes can be controlled by AgBF4solution with different concentration.
2.3 Hydrogen reduction of silver ions in PEI/ Pebax2533/AgBF4composite membranes
For the hydrogen reduction, the prepared PEI/ Pebax2533/AgBF4composite membranes with different AgBF4content were reduced by continuous hydrogen permeation. The main chemical reaction discussed in this paper is believed to occur as follows:
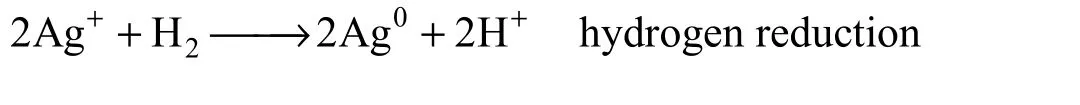
2.4 Membrane permeation properties
The membrane permeation properties for pure gases and 50/50 (vol/vol) propylene/propane gas mixture were determined according to the permeation testing apparatus at 25 °C. For a new PEI/Pebax2533/ AgBF4composite membrane, its permeance and selectivity were not stable, which changed with time. In order to obtain steady membrane performance, the new PEI/Pebax2533/AgBF4composite membrane was usually conditioned by permeating pure propane for 8-24 h before use. In the hydrogen reduction test, pure hydrogen continuously permeated through the membrane except for periodic interruptions where the permeances of propylene and propane were measured without conditioning.
For the pure gases, the upstream pressure was controlled from 0.2 to 0.8 MPa, and the downstream pressure was atmospheric pressure (0.1 MPa). For the gas mixtures, the feed pressure was 0.2 to 0.7 MPa and the permeate pressure was 0.02 MPa. The composition of feed and permeate stream was determined by a gas chromatograph equipped with an aluminum oxide packed column. The ratio of permeate flow rate to total feed flow rate (stage-cut) was always less than 1%, The pure gas permeance (J) was measured by the volume displacement using a gas bubble flow meter and determined by the following Eq. (1).

where J is the gas permeance of PEI/Pebax2533/AgBF4composite membranes [1GPU=1×10−6cm3·cm−2·s−1· (cmHg)−1], ΔV is the gas flux reading (cm3·s−1), ΔP is the pressure difference between feed and permeate (cmHg), and A is the membrane effective area (cm2).
The mixed-gas permeance of each gas component is calculated from Eq. (2):

whereiJ′ is the permeance of each component, p1and p2are the feed and permeate pressures, respectively, x1iand x2iare the mole fraction of the gas components in the feed and permeate streams, respectively.
The ideal selectivity (α) for pure components A and B is calculated by Eq. (3):
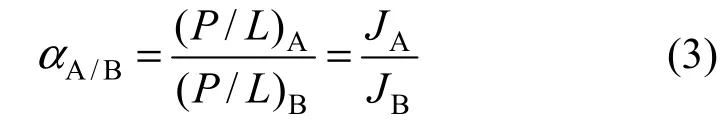
For gas mixture A and B, the mixed-gas selectivity is obtained from Eq. (4):

2.5 X-ray diffraction
X-ray diffraction (XRD) data were acquired using a Philips X’Pert Pro diffractometer fitted with CuKαradiation (λ=0.154 nm) operating at 40 mA and 40 kV.
2.6 Fourier transform infrared spectroscopy
Fourier transform infrared (FT-IR) spectra characterizations of the membrane samples were obtained in ATR (attenuated total reflectance) mode on an AVATAR 370 (Thero Nicolet) instrument and IR spectra presented absorbance from 4000 to 650 cm−1.
2.7 Atomic force microscopy
Tapping mode atomic force microscopy (AFM) was utilized to study the morphology of the hydrogen reduced PEI/Pebax2533/AgBF4samples. The AFM analysis was carried out with Veeco-di310 equipment.
3 RESUL TS AND DISCUSSION
3.1 Pur e-gas perm eation pr operties of PEI/P ebax2533/AgBF4composite membranes
AgBF4is an efficient carrier for olefins. It is believed that non-coordinating anion4BF−bound loosely with silver ion Ag+can be easily complexed with olefin molecules to achieve facilitated transport. Pebax2533 is a thermoplastic elastomer comprising of soft polyether segments (polytetramethylene oxide) and hard polyamide segments (nylon-12). Due to weak complexation of its ether oxygen with silver ions and its good permeability for olefins and paraffins, PEI/Pebax2533 composite membranes are used to prepare new solid polymer electrolyte PEI/Pebax2533/AgBF4composite membranes. When the external surface of PEI/ Pebax2533 composite membrane is immersed into the AgBF4aqueous solution, AgBF4will transfer into the Pebax2533 layer through the complexation between silver salt and ether oxygen in Pebax2533 backbone. And then, new solid polymer electrolyte PEI/Pebax2533/ AgBF4composite membranes are prepared.
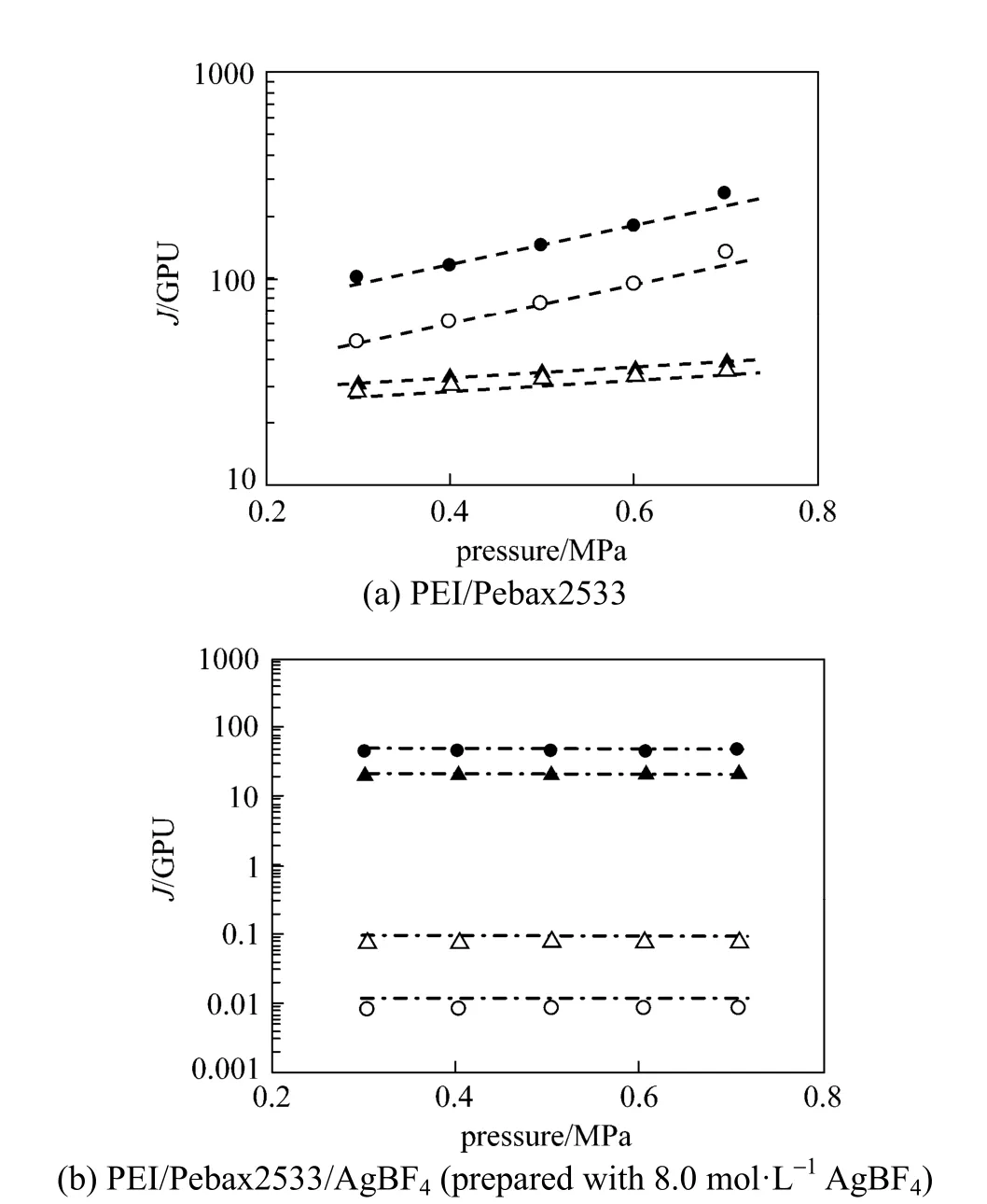
Figure 1 Effect of testing pressure difference on the permeances of e thylene, ethane, propylene and propane for PEI/Pebax2533 and PEI/Pebax2533/AgBF4composite membranes(testing temperature: 25 °C )● C3H6; ○ C3H8; ▲ C2H4; △ C2H6
The pure-gas performance of PEI/Pebax2533 and PEI/Pebax2533/AgBF4composite membranes are illustrated in Fig. 1. For PEI/Pebax2533 composite membrane shown in Fig. 1 (a), the permeances of ethylene, ethane, propylene and propane increase with the increase of testing pressure difference, resulting in a strong plasticization effect. For the PEI/Pebax2533/ AgBF4composite membrane shown in Fig. 1 (b), the permeances of ethylene, ethane, propylene and propane are almost not influenced by the testing pressure difference, showing a suppressed plasticization effect. From these results, it is evident that the permeances of ethane and propane for PEI/Pebax2533/AgBF4composite membrane are roughly one to two orders ofmagnitude lower than those for PEI/Pebax2533 composite membrane. It is mainly caused by the poor solubility of ethane and propane in the solid polymer electrolyte films [16, 17]. With the presence of carrier, the olefin permeance decreases slowly because of the facilitated olefin transport, but the olefin-induced plasticization effect is partially suppressed. Fig. 2 shows the effect of testing pressure difference on the ideal selectivities of ethylene/ethane and propylene/ propane for PEI/Pebax2533 and PEI/Pebax2533/ AgBF4(prepared with 8.0 mol·L−1AgBF4solution) composite membranes. From Fig. 2, PEI/Pebax2533 composite membrane exhibits poor selectivity for ethylene/ethane and propylene/propane separations and the ideal selectivities of ethylene/ethane and propylene/propane are about 1 and 2, respectively. But for PEI/Pebax2533/AgBF4composite membrane, the defect-free solid polymer electrolyte layer can be a barrier to prevent the diffusion of paraffins and facilitate the transport of olefins, which leads to high selectivities of ethylene/ethane and propylene/propane.
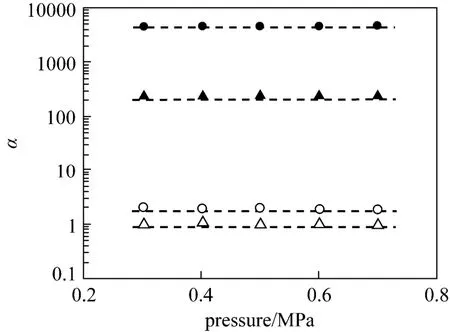
Figure 2 Effect of testing pressure difference on the ideal selectivities of ethylene/ethane and pr opylene/propane for PEI/Pebax2533 and PEI/Pebax2533/AgBF4composite membranes(testing temperature: 25 °C ) [PEI/Pebax2533: △ ethylene/ethane; ○ propylene/propane. PEI/ Pebax2533/AgBF4(prepared with 8.0 mol·L−1AgBF4solution):▲ ethylene/ethane; ● propylene/propane]
Figures 3 and 4 illustrate the pure-gas performance and the ideal selectivity of propylene/propane for PEI/Pebax2533/AgBF4composite membranes prepared with different AgBF4concentration (5.0 mol·L−1, 6.0 mol·L−1and 8.0 mol·L−1). For facilitated gas, propylene, its permeance increases significantly with increasing AgBF4concentration. Conversely, for propane, the non-facilitated gas, its permeance decreases sharply with increasing silver carrier concentration. Consequently, the ideal selectivity of propylene/propane increases quickly with increasing AgBF4content. When AgBF4concentration increases from 5.0 to 8.0 mol·L−1, the percolation channels are formed and carrierfacilitated transport process occurs, resulting in the increase of propylene permeance [15]. Meanwhile, the formation of percolation channels strongly retards the transport process of propane, leading to a sharp decrease of propane permeance and a rapid increase of ideal selectivity (shown in Figs. 3 and 4).
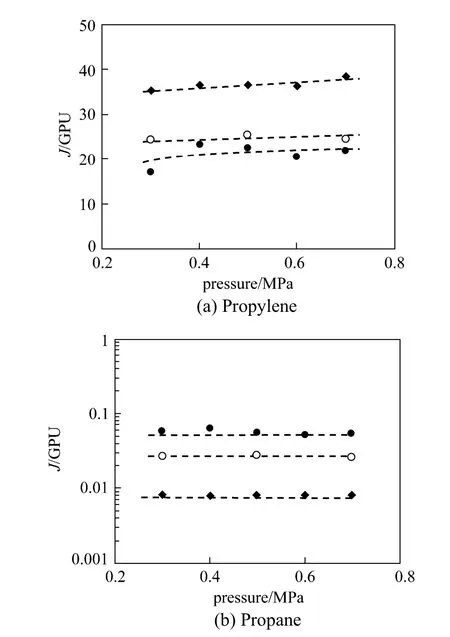
Figure 3 The p ermeances of propylene, p ropane for PEI/Pebax2533/AgBF4composite membranes prepared with different AgBF4concentrations(testing temperature: 25 °C)● 5.0 mol·L−1AgBF4; ○ 6.0 mol·L−1AgBF4; ◆ 8.0 mol·L−1AgBF4
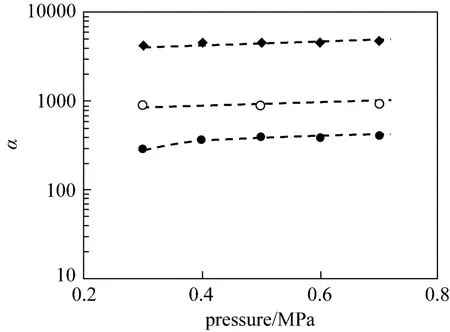
Figure 4 Ideal selectivities of propylene/propane for PEI/ Pebax2533/AgBF4comp osite membranes p repared with different AgBF4concentration(testing temperature: 25 °C)● 5.0 mol·L−1AgBF4; ○ 6.0 mol·L−1AgBF4; ◆ 8.0 mol·L−1AgBF4
3.2 Mix ed-gas per meation pr operties of PEI/ Pebax2533/AgBF4composite membranes
The mixed-gas permeation properties of PEI/ Pebax2533/AgBF4composite membranes were characterized with a 50/50 (vol/vol) propylene/propane mixture. Fig. 5 represents mixed-gas permeances of PEI/Pebax2533/AgBF4composite membranes (prepared with 5.0 and 8.0 mol·L−1AgBF4solutions) as a function of testing pressure difference. It shows anincrease in propane permeance and a simultaneous decrease in propylene permeance. In Fig. 5 (a) and 5 (b), the upper and lower straight lines describe the mean permeances of pure propylene and propane in the scope of testing pressure difference. Fig. 6 shows that the mixed-gas selectivity of propylene/propane for PEI/Pebax2533/AgBF4composite membranes decreases as the testing pressure increases. With an increase in the testing pressure difference from 0.2 to 0.7 MPa, the mixed-gas selectivity of propylene/propane decreases from ~130 to ~30 for PEI/Pebax2533/AgBF4composite membrane prepared with 8.0 mol·L−1AgBF4solution. For membrane prepared with 5.0 mol·L−1AgBF4solution, its mixed-gas selectivity decreases from ~70 to ~20. From Fig. 5, the obvious increase in propane permeance results from swelling of Pebax2533 matrix caused by the high sorption of propylene and the co-permeance of propane and propylene. For propylene/propane mixture, the plasticization and swelling caused by propylene increase the permeance of propane in gas mixture. The presence of propane in the mixture will compete with propylene for the sorption sites in Pebax2533, which decreases the permeance of propylene in gas mixture. Partial carrier saturation may be responsible for the decrease in propylene permeance too [9]. At a given testing pressure difference, the permeance of propylene in the gas mixture is lower than that of pure propylene, while it is higher for propane in the gas mixture than that of pure propane. For PEI/Pebax2533/AgBF4composite membranes, their mixed-gas selectivity of olefin/paraffin is lower than the ideal selectivity.
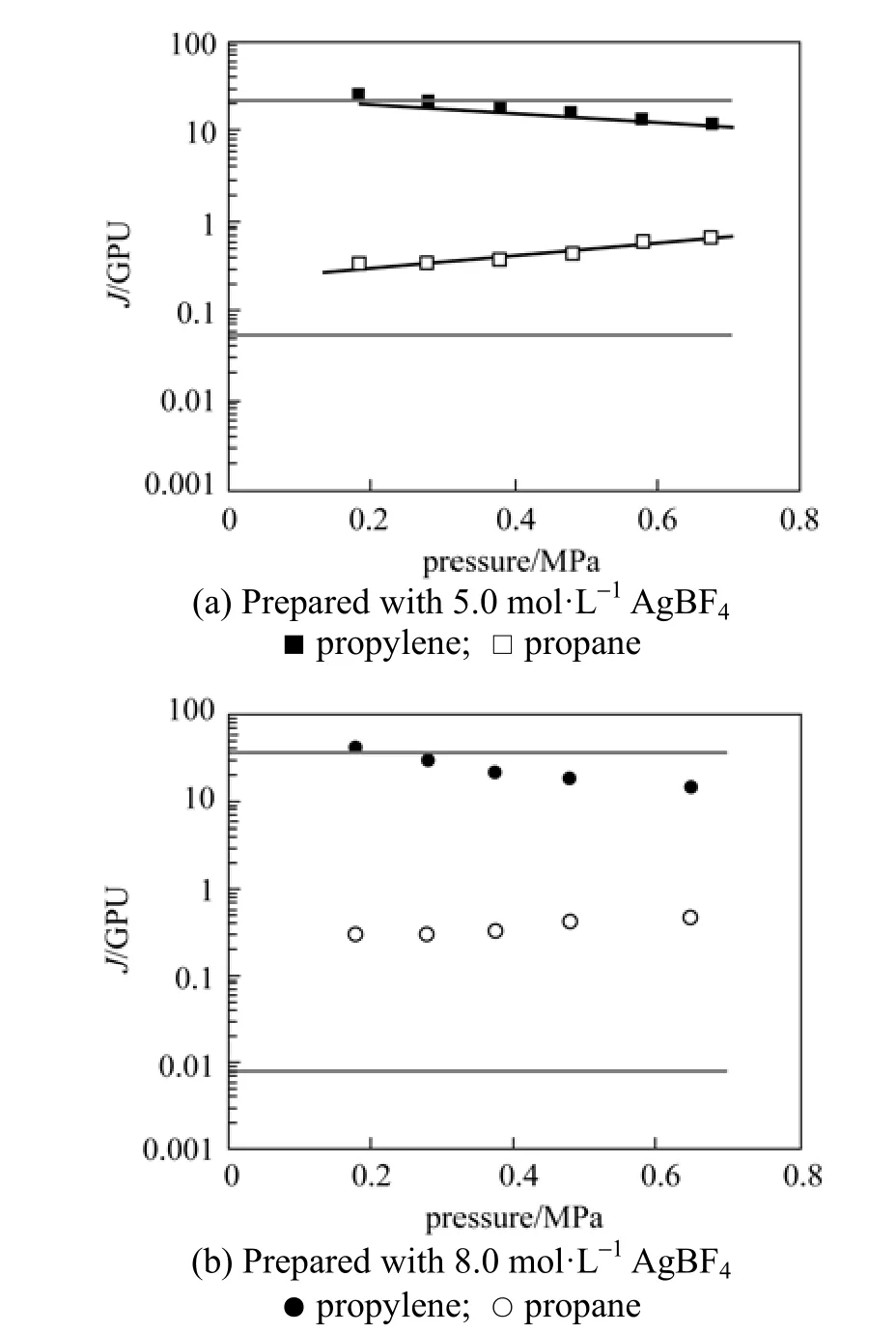
Figure 5 Effect of testing pressure difference on mixed-gas permeances of propylene and propane for PEI/Pebax2533/ AgBF4composite membranes[feed: 50/50 (vol/vol) propylene/propane mixture; testing temperature: 25 °C]
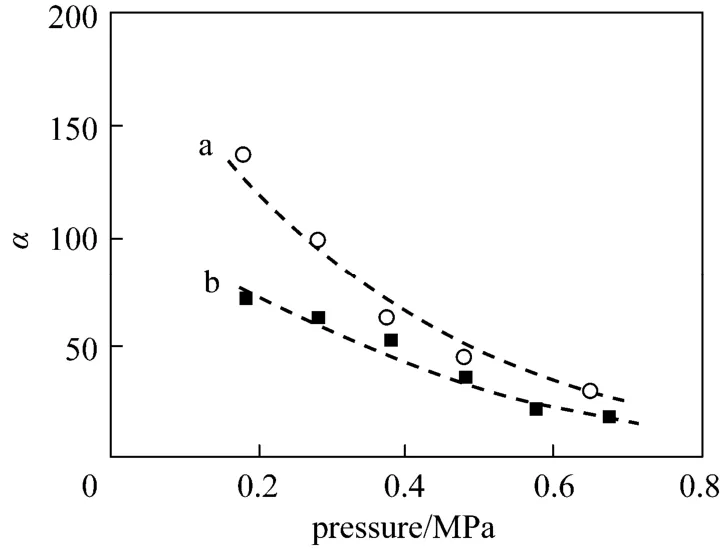
Figure 6 Effect of testing pressure difference on the selectivities of propylene/propane for PEI/Pebax2533/AgBF4composite m embranes[feed: 50/50 (vol/vol) propylene/ propane mixture; testing temperature: 25 °C] a—prepared with 5.0 mol·L−1AgBF4; b—prepared with 8.0 mol·L−1AgBF4
3.3 Effect of hydrogen reduction on the perme ation properties of PEI/Pebax2533/AgBF4composite membranes
Solid polymer electrolyte membrane process failed to achieve commercial success under industrial operating conditions mainly due to the lack of carrier stability [9]. Carriers tend to react with some poisoning compounds, causing carrier inactivation except for complexing with olefins. Silver ions can be reduced to silver metal particles under the exposure of light or reducing gases (such as hydrogen). Further more, silver ions can react with hydrogen sulfide or acetylenic compounds to form silver compounds (silver acetylide or silver sulfide) [18]. These side reactions can inactivate the silver carrier to complex olefin molecules. Consequently, facilitated transport membranes lost their olefin/paraffin selectivity with exposure time. Fortunately, poisoned carriers in the facilitated transport membrane process can be refreshed by hydrogen peroxide (except silver sulfide due to its irreversible effect on the silver carrier) [10, 19]. Nowadays, H2O2/HBF4is used as the stabilizer in facilitated transport membranes [20].
In order to study the influence of carrier reduction on the permeation properties of propylene and propane, some new PEI/Pebax2533/AgBF4composite membranes are prepared with 5.0 mol·L−1and 8.0 mol·L−1AgBF4solutions. Figs. 7 and 8 show the permeances of propylene, propane and hydrogen for PEI/Pebax2533/AgBF4composite membranes reduced by hydrogen. In this test, hydrogen transports across the membranes continuously at 0.6 MPa feed pressure and 0.1 MPa permeate pressure except for periodic interruptions where the permeation fluxes of purepropylene and propane are measured. From Fig. 7, it is clearly that the permeance of propylene decreases sharply in the first 10 h by hydrogen reduction, but the propane permeance almost does not change. After the first 10 h, the propane permeance increases continually, but the propylene permeance is relatively constant. From Fig. 8, the propylene permeance of PEI/Pebax2533/AgBF4composite membrane prepared with 8.0 mol·L−1AgBF4solution also decreases with the increase of hydrogen reduction time and then almost does not change, but the propane permeance increases simultaneously. From Figs. 7 and 8, the permeance of hydrogen is independent of the hydrogen reduction time.
Figure 9 shows that ideal selectivity of propylene/ propane decreases rather rapidly after the membranes are reduced continuously. Figs. 7 and 8 indicate that this loss in selectivity is primarily due to the decreasing propylene permeance and increasing propane permeance. Fig. 10 illustrates the effect of hydrogen reduction on the surface color of a PEI/Pebax2533/AgBF4composite membrane prepared with 8.0 mol·L−1AgBF4solution. Hydrogen-reduced membrane shows a clearly color change from white to brown. With the increase of hydrogen reduction time, more silver ions are reduced to metal particles, which appear deep dark in color.
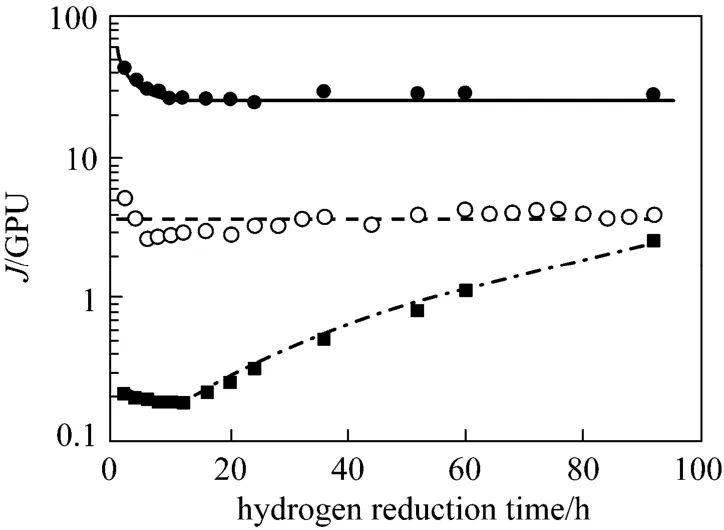
Figure 7 Th e p ermeances o f p ropylene, p ropane an d hydrogen as a function of h ydrogen reduction time for a PEI/Pebax2533/AgBF4composite membrane prepared with 5.0 mol·L−1AgBF4solution(testing pressure difference: 0.5 MPa; testing temperature:25 °C)● C3H6; ■ C3H8; ○ H2

Figure 8 Th e p ermeances o f p ropylene, p ropane an d hydrogen as a function of h ydrogen reduction time for a PEI/Pebax2533/AgBF4composite membrane prepared with 8.0 mol·L−1AgBF4solution(testing pressure difference: 0.5 MPa; testing temperature: 25 °C).● C3H6; ■ C3H8; ○ H2
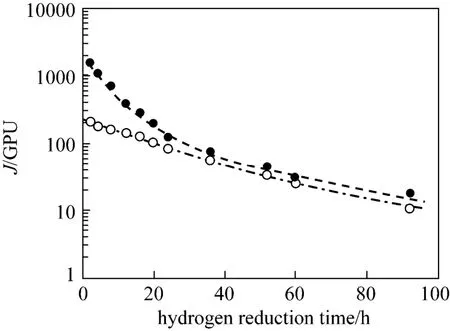
Figure 9 Ideal selectivity of propylene/propane as a function of hy drogen reduction time for PEI/Pebax2533/AgBF4composite membranes prepared with 5.0 and 8.0 mol·L−1AgBF4solution(testing pressure difference: 0.5 MPa; testing temperature: 25 °C)○ 5.0 mol·L−1AgBF4; ● 8.0 mol·L−1AgBF4
The structure of PEI/Pebax2533/AgBF4composite membranes after hydrogen reduction studied by X-ray diffraction using CuKαradiation with 2θ from 5° to 65°. From Fig. 11, the Bragg’s reflections exhibit 30° to 40° attribute to the existence of Ag0, which are found only in data of the hydrogen reduced membrane sample c. After hydrogen reduction, silver ions are reduced to Ag0particles and aggregate on the surface of membrane samples. The more the exposure time to hydrogen, the more silver ions are reduced to metal particles, which appear obvious diffraction peak in XRD spectrogram. The prominent non-crystal peaks at 2θ values of about 15° to 30° are attributable to thePebax2533, which weaken gradually with the increase of aggregated Ag0particles on the surface of PEI/Pebax2533/AgBF4composite membranes.

Figure 10 Photograph of a PEI/Pebax2533/AgBF4composite membrane prepared with 8.0 mol·L−1AgBF4solution with different hydrogen reduction time
For PEI/Pebax2533/AgBF4composite membranes, the interaction between ether oxygen and silver salt can also be reflected from the spectra of ether oxygen bond stretching mode. Fig. 12 shows FT-IR spectra of PEI/Pebax2533/AgBF4composite membranes with hydrogen reduction in the range 2100-700 cm−1. The peak appears at approximately 1100 cm−1corresponds to CO stretching frequency of ether linkages of Pebax2533 (sample a in Fig. 12). From the sample b in Fig. 12, the interaction of ether oxygen with silver ions makes ether oxygen bond stretching peak broaden and shift to lower wave numbers [10]. The interaction induces silver ions to keep disassociating and form complexes with olefins, resulting in the facilitated transport process of olefins. For PEI/Pebax2533/AgBF4composite membranes with hydrogen reduction (sample c), that the intensity of ether linkages diminishes due to the reduction of silver ions indicates the formation of silver particle layer. As the membrane sample is reduced by hydrogen, more and more silver particles aggregate on the surface of PEI/Pebax2533/AgBF4composite membrane.
The surface morphology of PEI/Pebax2533/AgBF4composite membranes with hydrogen reduction is also monitored by three-dimensional AFM images as shown in Fig. 13. The surface morphology of PEI/Pebax2533 composite membranes is relatively smooth and featureless (sample a in Fig. 13). Due to hydrogen reduction, silver particles gradually start to form and aggregate on the membrane surface, and the membrane surface becomes relatively tough, which is reflected from samples b, c and d in Fig. 13. This phenomenon is a good agreement with the results of FT-IR spectroscopy.
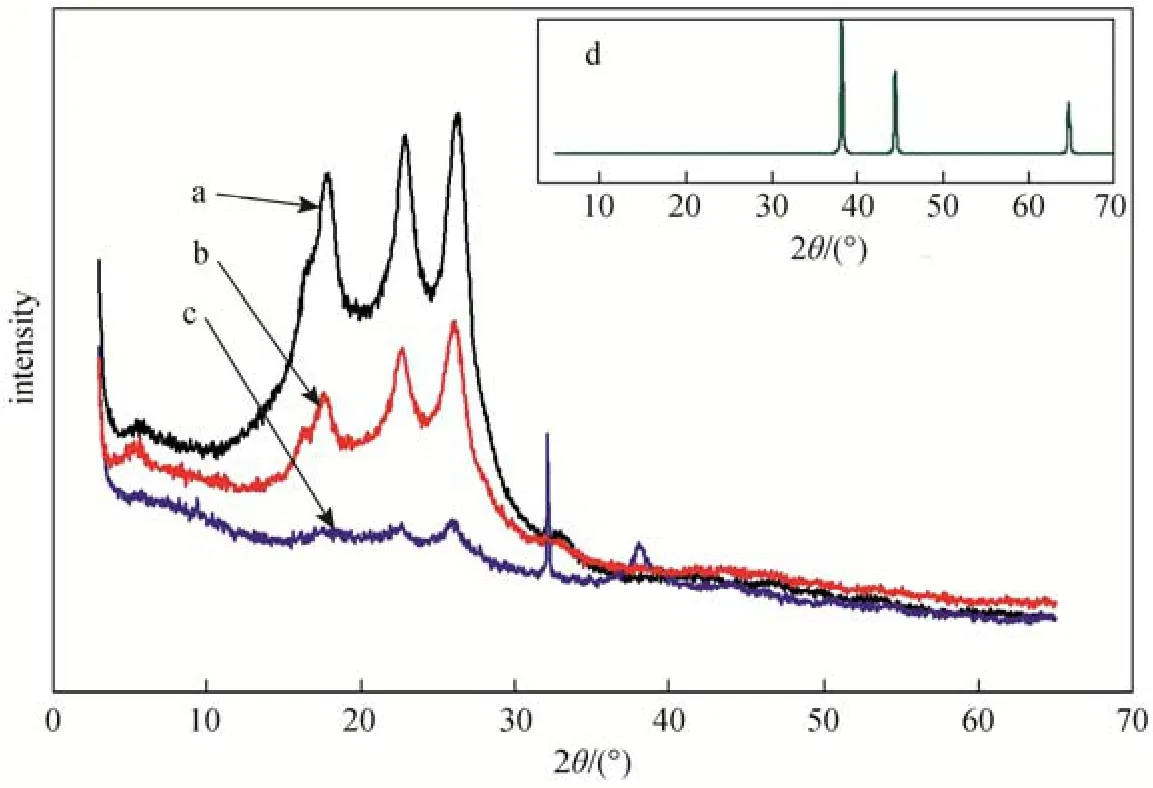
Figure 11 X-ray analysis of PEI/Pebax2533/AgBF4composite membranes with hydrogen reductiona—without AgBF4; b—prepared with 8.0 mol·L−1AgBF4; c—prepared with 8.0 mol·L−1AgBF4and reduced by hydrogen for 92 h; d—Ag

Figure 12 FT-IR spectra of PEI/Pebax2533/AgBF4composite membranes with hydrogen reductiona—without AgBF4; b—prepared with 8.0 mol·L−1AgBF4; c—prepared with 8.0 mol·L−1AgBF4and reduced by hydrogen for 92 h
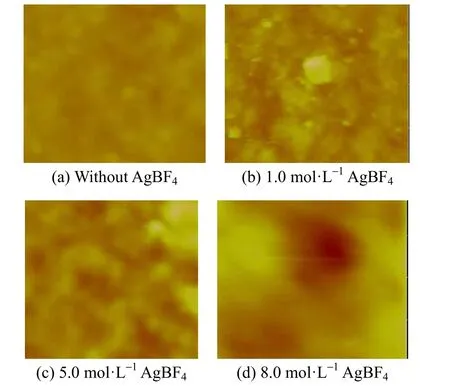
Figure 13 AFM images of PEI/Pebax2533/AgBF4composite membranes with hydrogen reduction for 92 h
The hydrogen reduction experiment is a relatively harsh test because pure hydrogen permeates the membrane continuously. In the actual application process, such at the bottom of a C2 splitter or on metathesis C4purge streams, the concentration of hydrogen is in the ppm range. Additionally, according to HSAB (hard and soft acid and base) theory, in a mixture with olefins, silver ions may be more resistant to hydrogen reduction because they are complexed with olefin molecules preferentially.
From above analysis, after hydrogen reduction, the selectivity of propylene/propane for PEI/Pebax2533/ AgBF4composite membranes decreases over time. This result may reflect that the silver ions become deactivated (reduced to silver metal particles) in the presence of hydrogen. In fact, peroxide/acid treatment is very effective for regenerating membranes deactivated by hydrogen exposure [10].
4 CONCLU SIONS
New PEI/Pebax2533/AgBF4composite membranes are prepared and the effect of hydrogen reduction of silver ions on the membrane performance and structure is investigated. Some conclusions can be made as follows:
(1) For PEI/Pebax2533/AgBF4composite membranes prepared with 5.0 to 8.0 mol·L−1AgBF4solutions, due to the carrier-facilitated transport, the permeances of propylene and ethylene increase with the increase of AgBF4concentration, and a high selectivity can be obtained.
(2) For propylene/propane mixture, the swelling of propylene in Pebax2533 matrix and co-permeance of propane and propylene induce the increase of propane permeance. And the competition of propane in the sorption sites decreases the propylene permeance. The mixed-gas selectivity is lower than its ideal selectivity.
(3) For hydrogen reduced membranes, the selectivity loss of propylene/propane is primarily due to the decreasing permeance of propylene and the increasing permeance of propane. With the increase of hydrogen reduction time, the membranes show a clearly color change from white to brown.
(4) The data of X-ray diffraction prove that silver ions are reduced to Ag0after hydrogen reduction and aggregated on the surface of PEI/Pebax2533/AgBF4composite membranes.
REFERENCES
1 Baker, R.W., “Future directions of membrane gas separation technology”, Ind. Eng. Chem. Res., 41, 1393-1411 (2002).
2 IIinitch, O.M., Semin, G.L., Chertova, M.V., Zamaraev, K.I., “Novel polymeric membranes for separation of hydrocarbons”, J. Membr. Sci., 66, 1-8 (1992).
3 Burns, R.L., Koros, W.J., “Defining the challenges for C3H6/C3H8 separation using polymeric membranes”, J. Membr. Sci., 211, 299-309 (2003).
4 Wind, J.D., Paul, D.R., Koros, W.J., “Natural gas permeation in polyimide membranes”, J. Membr. Sci., 228, 227-236 (2004).
5 Park, Y.S., Won, J., Kang, Y.S., “Facilitated transport of olefin through solid PAAm and PAAm-graft composite membranes with silver ions”, J. Membr. Sci., 183,163-170 (2001).
6 Pinnau, I., Toy, L.G., Casillas, C., “Olefin separation membrane and process”, U.S. Pat., 5670051 (1997).
7 LeBlanc Jr., O.H., Ward, W.J., Matson, S.L., Kimura, S.G., “Facilitated transport in ion-exchange membranes”, J. Membr. Sci., 6, 339-343 (1980).
8 Teramoto, M., Matsuyama, H., Yamashiro, T., Okamoto, S., “Separation of ethylene from ethane by a flowing liquid membrane using silver nitrate as a carrier”, J. Membr. Sci., 45, 115-136 (1989).
9 Pinnau, I., Toy, L.G., “Solid polymer electrolyte composite membranes for olefin/ paraffin separation”, J. Membr. Sci., 184, 39-48 (2001).
10 Merkel, T., Blanc, R., Zeid, J., Suwarlim, A., Firat, B., Wijmans, H., Asaro, M., Greene, M, “Separation of olefin/paraffin mixtures with carrier facilitated membrane”, Department of Energy Report No. DE-FC36-04GO14151 (2007).
11 Jose, B., Ryu, J.H., Lee, B.G., “Effect of phthalates on the stability and performance of AgBF4-PVP membrane for olefin/paraffin separation”, Chem. Commun., 20, 2046-2047 (2001).
12 Jose, B., Ryu, J.H., Kim, Y.J., Kim, H., Kang, Y.S., Lee, S.D., Kim, H.S., “Effect of plasticizers on the formation of silver nanoparticles in polymer electrolyte membranes for olefin/paraffin separation”, Chem. Mater., 14, 2134-2139 (2002).
13 Kim, J.H., Lee, D.H., Won, J., Jinnai, H., Kang, Y.S., “The structural transitions of π-complexes of poly(styrene-β-butadiene-β-styrene) block copolymers with silver salts and their relation to facilitated olefin transport”, J. Membr. Sci., 281, 369-376 (2006).
14 Müller, J., Peinemann, K.V., Müller, J., “Development of facilitated transport membranes for the separation of olefins from gas streams”, Desalination, 145, 339-345 (2002).
15 Wang, Y., Ren, J., Deng, M., “Ultrathin solid polymer electrolyte PEI/Pebax2533/AgBF4composite membrane for propylene/propane separation”, Sep. Purif. Technol., 77, 46-52 (2011).
16 Sunderrajan, S., Freeman, B.D., Hall, C.K., Pinnau, I., “Propane and propylene sorption in solid polymer electrolytes based on poly (ethylene oxide) and silver salts”, J. Membr. Sci., 182, 1-12 (2001).
17 Morisato, A., He, Z., Pinnau, I., Merkel, T.C., “Transport properties of PA12-PTMO/AgBF4solid polymer electrolyte membranes for olefin/paraffin separation”, Desalination, 145, 347-351 (2002).
18 Marcinkowsky, A.E., Keller II, G.E., Verma, S.K., “Olefin separation process”, U.S. Pat., 4174353 (1979).
19 Steigelmann, E.F., “Membrane process and product”, U.S. Pat., 4014665 (1977).
20 Kim, J.H., Min, B.R., Kim, H.S., Won, J., Kang, Y.S., “Facilitated transport of ethylene across polymer membranes containing silver salt: effect of HBF4on the photoreduction of silver ions”, J. Membr. Sci., 212, 283-288 (2003).
2012-02-08, accepted 2012-12-28.
* Supported by the National Natural Science Foundation of China (20776137) and the National High Technology Research and Development Program of China (2008AA06Z325).
** To whom correspondence should be addressed. E-mail: renjizhong@dicp.ac.cn
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Adsorption and Desorption Behavior of Tannic Acid in Aqueous Solution on Polyaniline Adsorbent*
- Synthesis of 2-Methyl-4-methoxyaniline from o-Nitrotoluene Using Pt/C and Acidic Ionic Liquid as Catalyst System*
- Supercritical Fluid Extraction of a Novel Template from Mesoporous Zirconia and the Effect on Porous Structure*
- Preparation of Mesoporous Carbons from Acrylonitrile-methyl Methacrylate Copolymer/Silica Nanocomposites Synthesized by in-situ Emulsion Polymerization*
- Comparison on Thermal Conductivity and Permeability of Granular and Consolidated Activated Carbon for Refrigeration*
- Immobilization of Papain in Biosilica Matrix and Its Catalytic Property*
