Study on Deactivation by Sulfur and Regeneration of Pd/C Catalyst in Hydrogenation of N-(3-nitro-4-methoxyphenyl) Acetamide*
2013-06-07张群峰吕井辉马磊卢春山刘维李小年
(张群峰)(吕井辉)(马磊)(卢春山)(刘维)(李小年)**
Industrial Catalysis Institute, State Key Laboratory Breeding Base of Green Chemistry Synthesis Technology, Zhejiang University of Technology, Hangzhou 310032, China
Study on Deactivation by Sulfur and Regeneration of Pd/C Catalyst in Hydrogenation of N-(3-nitro-4-methoxyphenyl) Acetamide*
ZHANG Qunfeng(张群峰), LÜ Jinghui(吕井辉), MA Lei(马磊), LU Chunshan(卢春山), LIU Wei(刘维)and LI Xiaonian(李小年)**
Industrial Catalysis Institute, State Key Laboratory Breeding Base of Green Chemistry Synthesis Technology, Zhejiang University of Technology, Hangzhou 310032, China
Deactivation of Pd/C catalyst often occurs in liquid hydrogenation using industrial materials. For instance, the Pd/C catalyst is deactivated severely in the hydrogenation of N-(3-nitro-4-methoxyphenyl) acetamide. In this study, the chemisorption of sulfur on the surface of deactivated Pd/C was detected by energy dispersive spectrometer and X-ray photoelectron spectroscopy. Sulfur compounds poison the Pd/C catalyst and increase the formation of azo deposit, reducing the activity of catalyst. We report a mild method to regenerate the Pd/C catalyst: wash the deposit by N,N-dimethylformamide and oxidize the chemisorbed sulfur by hot air. The regenerated Pd/C catalyst can be reused at least ten runs with stable activity.
Pd/C catalyst, hydrogenation, deactivation, regeneration, sulfur
1 INTRODUCTION

The deactivation of Pd/C catalyst often occurs in liquid hydrogenation using industrial materials. For example, since the reduction by sodium sulfide is applied in industry, the industrial material may contain sulfide, which is a poison to Pd/C catalyst. N-(3-amino-4-methoxyphenyl) acetamide (AMA) is an important intermediate of dyes and is produced by hydrogenation of N-(3-nitro-4-methoxyphenyl) acetamide (NMA) over Pd/C catalyst [19], which may be deactivated in the synthesis of AMA. In this work, sulfur compounds in industrial NMA poisons Pd/C catalyst and produces the deposit, azo, to block pores of the Pd/C catalyst. It is difficult to regenerate the catalyst using some methods available. We report a mild method to regenerate the Pd/C catalyst: wash the deposit using N,N-dimethylformamide (DMF) and oxidize chemisorbed sulfur using hot air.
2 EX PERIMENTAL
2.1 Catalyst preparation
150 ml aqueous suspension with 10 g activated carbon in a 500 ml flask was heated to 80 °C, and then 10 ml of 0.05 g·ml−1H2PdCl4aqueous solution was added with stirring for 2 h. The suspension pH of 7-9 was obtained by addition of 10% (by mass) NaOH aqueous solution. The precipitated sample was reduced by an excess of 85% (by mass) hydrazine hydrate aqueous solution at 30 °C. It was dried in the vacuum at 110 °C for 10 h.
2.2 Catalyst characterization
X-ray photoelectron spectroscopy (XPS) analysis was performed with a Kratos AXIS Ultra DLD system. The binding energy values were referenced to the C1s level (284.6 eV) resulted from surface contaminants. The surface area was determined by N2adsorption at 77K using NOVA 1000e (Quantachrome Instruments Corp) automatic surface area and pore radius distributionapparatus. The elemental composition of catalysts was determined by energy dispersive spectrometer (EDS, Thermo Vantage ESI).
2.3 Catalytic hydrogenation of NMA
Hydrogenation of NMA over Pd/C catalyst was carried out in a 500 ml stainless steel autoclave (Weihai Zikong Autoclave Co. Ltd) at2HP of 1 MPa and 90 °C, which contained NMA (50 g, 0.02 mol), 150 ml water and 0.2 g Pd/C catalyst. The products were analyzed by a liquid chromatography (Agilent 1100) equipped with a C18 column and a UV detector.
3 RESUL TS AND DISCUSSION
3.1 Catalytic performance of Pd/C catalyst
Chemically pure NMA (99.5%, Jihua Chemical Co., Ltd.) was used to assess the catalytic performance of Pd/C catalyst in the hydrogenation of NMA. Table 1 shows that 100% conversion of NMA and the selectivity of AMA of 99.37% can be reached in 55 min, and the deactivation of Pd/C is insignificant.
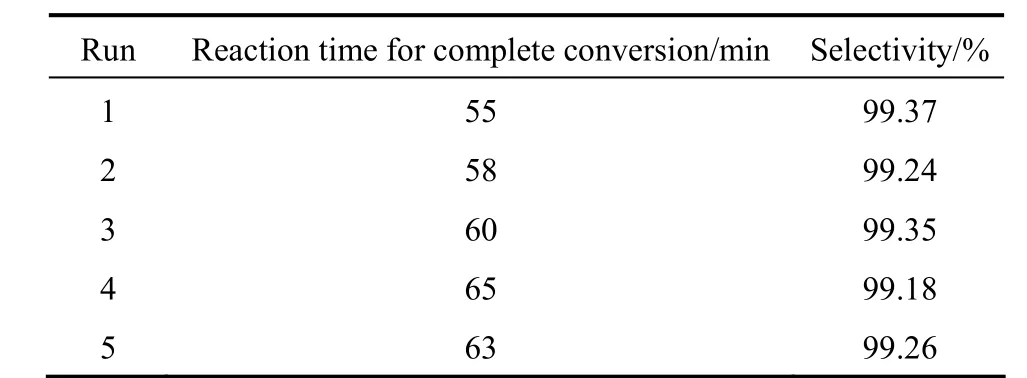
Table 1 Reusability test of Pd/C in hydrogenation of NMA

Figure 1 Conversion of NMA as function of reaction time▲ chemically pure raw; ▼ industrial raw
The relationship between the conversion of NMA and reaction time is shown in Fig. 1. The hydrogenation rate of chemically pure NMA is independent of NMA concentration, which is consistent with the classical kinetic characteristics of liquid hydrogenation of aromatic nitro compounds [20]. For industrial NMA (98.6 %, Mingsheng Chemical Co., Ltd.), the reaction rate decreases with time in the hydrogenation and depends on the concentration of NMA, suggesting that Pd/C catalyst is deactivated in the process. Table 2 show that 100% conversion of NMA needs 140 min with the same Pd/C catalyst, much longer than that using chemically pure NMA. In the second or third run, the time for complete conversion of NMA is even longer.

Table 2 Reusability test of Pd/C in NMA hydrogenation
3.2 Catalyst deactivation by sulfur
In order to examine the deactivation of Pd/C catalyst in the hydrogenation of industrial NMA, the surface areas of fresh and used catalysts were measured (Table 3). The surface area of used Pd/C is decreased considerably with industrial NMA, while it can maintain much higher value with chemically pure NMA. The decline of surface area should be attributed to the deposition of organic compounds, which may be the major reason for the deactivation of Pd/C catalyst.

Table 3 Surface area of fresh and used Pd/C catalysts
An EDS examination was made to determine the elemental compositions for the fresh and used catalysts with industrial NMA, as shown in Table 4. Pd mass content in the fresh Pd/C catalyst is 9.68%, and that in used catalyst with industrial raw is 7.64%. The decrease in Pd content may be attributed to the deposition of organic compounds on the Pd/C catalyst. In addition, more elements are present in the used catalyst with industrial NMA, such as K, Ca and S. The XPS spectra for used Pd/C catalyst are illustrated in Fig. 2 (a). The peaks of S2pspectra at 163.33, 164.87 and 167.85 eV show three states of S element, S(−2), S(0) and S(+6). S(−2) and S(0) are poisons to the Pd/C catalyst.
The deposits of used Pd/C catalysts were washed by alcohol to illustrate the relationship between the sulfur poisoning and the deposition of organic compounds. With liquid chromatography-mass spectrometry, azo was detected in the alcohol solution with industrial NMA, while azo was not found with chemically pureNMA. Azo is an intermediate in the hydrogenation and is generally converted to aromatic amine compound immediately, which is rarely accumulated and remains in the reaction system. However, azo produced in the reaction will be precipitated from the solution and block the pore of the Pd/C catalyst, due to its difficult dissolution in water for its puny polar and high molecular weight. Since the industrial NMA contains sulfur compounds, azo may form and deposit in the Pd/C catalyst in the hydrogenation, reducing the activity of catalyst. Similar result has been reported, e.g., dimethyl sulfoxide works as an additive and increases the selectivity of azo and N-phenylhydroxylamine effectively in the hydrogenation of nitrobenzene [21].

Table 4 Elemental compositions of fresh and used Pd/C catalysts

Figure 2 XPS spectra of S2pfor used (a) and regenerated (b) Pd/C catalysts
3.3 Regeneration of the deactivated Pd/C catalyst
The removal of azo deposit from the catalyst surface and exposure of Pd active sites are necessary for the regeneration of deactivated Pd/C catalyst. Washing organic foulants from catalysts is a general way, especially for carbon supported metal catalysts. 2.0 g deactivated Pd/C catalyst was washed at 1 MPa of hydrogen and 80 °C for 3 h in a 500 ml stainless steel autoclave using 100 ml NaOH aqueous solution [10% (by mass)], methanol, acetic acid and DMF separately. Table 5 presents the surface area and catalytic activity of the regenerated catalyst, indicating that DMF is more effective since the surface area increases to 1047 m2·g−1and the reaction time for complete conversion reduces to 200 min. DMF can be recovered by distillation and reused. However, the activity of the regenerated Pd/C catalyst is still much lower than that of fresh catalyst. The solvent dissolves the deposit of organic compounds but can not remove the sulfur compounds chemisorbed on catalyst surface. Complete regeneration of deactivated Pd/C catalyst could not be achieved with washing process only.
It is difficult to remove the chemisorbed sulfur from metal catalyst by physical treatments. It has been found that the treatment with sodium hypochlorite as oxidant regenerates Pd/Al2O3catalyst quickly and completely, which was applied in reductive treatment of waters contaminated with halogenated hydrocarbons and deactivated by sulfur [5, 6]. However, the treatment with sodium hypochlorite is not a green process. In the present work, we use a simple and sustainable way to regenerate the deactivated Pd/C catalyst: oxidation by hot air. The oxidation of the catalyst after being washed by DMF was carried out at 110 °C under air in the oven for 2 h and 4 h separately. The catalyst with oxidation for 4 h was analyzed by XPS to determine the state of S element [Fig. 2(b)]. The intensity of S2psignal is much weaker than that in Fig. 2(a), which suggests that the content of S is decreased after regeneration by washing and oxidation. The mass content of S is also proved by EDS, about 0.14% in the catalyst after oxidation. The peaks of S2pspectra at 163.59 and 167.90 eV show two states of S, S(−2) and S(+6), at the regenerated Pd/C catalyst. The intensity of S(−2) in Fig. 2 (b) is much weaker than that in Fig. 2 (a), indicating that most of S(−2) in the deactivated catalyst is oxidized and removed. The interaction between Pd and S(−2) is much stronger than that between Pd and S(+6), so the removal of sulfur by oxidation is appropriate. The catalytic performance of regenerated Pd/C catalyst is given in Table 6. The reaction time for complete conversion is greatly reduced.

Table 5 The surface area and activity of regenerated Pd/C catalyst by washing process

Table 6 Regeneration of Pd/C catalyst by DMF washing and oxidation process
3.4 Stability of the regenerated Pd/C catalyst
The activity for the regenerated Pd/C catalyst was tested to assess the efficiency of the regeneration process with washing by DMF and oxidation by hot air. After each reaction, the Pd/C catalyst was washed by DMF at 1 MPa of hydrogen and 80 °C for 3 h and oxidized at 110 °C under air for 4 h. 0.05 g Pd/C catalyst was added to the reaction system in each run. The result is listed in Table 7. The regenerated Pd/C catalyst can be reused at least ten runs with stable activity.

Table 7 Reusability of regenerated Pd/C catalyst
4 CONCLU SIONS
Sulfur compounds in the industrial NMA poison Pd/C catalyst in its hydrogenation and changes the progress of hydrogenation reaction due to the formation of azo deposit, which decreases the activity of Pd/C catalyst. The surface area of Pd/C catalyst is partly recovered by washing the deposit on the surface using organic solvent, but the sulfur compounds chemisorbed on the catalyst are not removed. Most of S(−2) in the deactivated catalyst can be oxidized and removed from the catalyst. It is an effective and sustainable way to regenerate the Pd/C catalyst deactivated by sulfur during industrial hydrogenation of aromatic nitro compounds.
REFERENCES
1 Huu, T.T., Chizari, K., Janowska, I., Moldovan, M.S., Ersen, O., Nguyen, L.D., Ledoux, M.J., Huu, C.P., Begin, D., “Few-layer graphene supporting palladium nanoparticles with a fully accessible effective surface for liquid-phase hydrogenation reaction”, Catalysis Today,189, 77-82 (2012).
2 Guo, S.Z., Xu, Z.H., Xia, R.H., Zhou, F., Fang, D.Y., “Kinetics on hydrogenation of cyclopentadiene over Pd/γ-Al2O3catalyst”, Chin. J. Chem. Eng.,13, 623-627 (2005).
3 Albers, P., Pietsch, J., Parker, S.F., “Poisoning and deactivation of palladium catalysts”, Journal of Molecular Catalysis A: Chemical,173, 275-286 (2001).
4 Trimm, D.L., “The regeneration or disposal of deactivated heterogeneous catalysts”, Applied Catalysis A: General,212, 153-160 (2001).
5 Lowry, G.V., Reinhard, M., “Pd-Catalyzed TCE dechlorination in groundwater: Solute effects, biological control, and oxidative catalyst regeneration”, Environmental Science & Technology,34, 3217-3223 (2000).
6 Munakata, N., Reinhard, M., “Palladium-catalyzed aqueous hydrodehalogenation in column reactors: Modeling of deactivation kinetics with sulphide and comparison of regenerants”, Applied Catalysis B: Environmental,75, 1-10 (2007).
7 Xiao, T.C., An, L.D., “Mechanism of sulfur poisoning on supported noble metal catalyst—the adsorption and transformation of sulfur on palladium catalysts with different supports”, Catalysis Letters,12, 287-296 (1992).
8 Yurkina, O.V., De Vekki, A.V., Kraev, Y.L., “Mechanism of deactivation of palladium-containing hydrogenation catalysts in the presence of sulfur compounds”, Petroleum Chemistry,44(3), 160-165 (2004).
9 Mikołajczuk, A., Borodzinski, A., Kedzierzawski, P., Stobinski, L., Mierzwa, B., Dziura, R., “Deactivation of carbon supported palladium catalyst in direct formic acid fuel cell”, Applied Surface Science,257, 8211-8214 (2011).
10 Zhang, X.X., Zong, B.N., Min, E.Z., Lu, L.J., “Method for regenerating a palladium catalyst”, China Pat., 03122844.5 (2003).
11 Li, W.X., Bo, Z.F., Qian, Z.M., “Palladium-carbon catalyst on-line regeneration method for terephthalic acid production apparatus”, China Pat., 00112558.3 (2000).
12 Zhang, X.P., Sui, Z.J., Zhou, X.G., Yuan, W.K., “Modeling and simulation of coke combustion regeneration for coked Cr2O3/Al2O3propane dehydrogenation catalyst”, Chin. J. Chem. Eng.,18, 618-625 (2010).
13 Schwartz, W.R., Ciuparu, D., Pfefferle, L.D., “Combustion of methane over palladium-based catalysts: Catalytic deactivation and roleof the support”, Journal of Physical Chemistry C, 116 (15), 8587-8593 (2012).
14 Ping, E.W., Pierson, J., Wallace, R., Miller, J.T., Fuller, T.F., Jones, C.W., “On the nature of the deactivation of supported palladium nanoparticle catalysts in the decarboxylation of fatty acids”, Applied Catalysis A: General, 396 (1-2), 85-90 (2011).
15 Gross, M.S., Pisarello, M.L., Pierpauli, K.A., Querini, C.A., “Catalytic deoxygenation of water: Preparation, deactivation, and regeneration of palladium on a resin catalyst”, Industrial & Engineering Chemistry Research, 49 (1), 81-88 (2010).
16 Ordonez, S., Diez, F.V., Sastre, H., “Characterisation of the deactivation of platinum and palladium supported on activated carbon used as hydrodechlorination catalysts”, Applied Catalysis B: Environmental, 31 (2), 113-122 (2001).
17 Salame, I.I., Bandosz, T.J., “Surface chemistry of activated carbons: Combining the results of temperature-programmed desorption, Boehm, and potentiometric titrations”, Journal of Colloid and Interface Science, 240, 252-258 (2001).
18 Bandosz, T.J., Jagiello, J., Schwarz, J.A., “Comparison of methods to assess surface acidic groups on activated carbons”, Analytical Chemistry, 64, 891-895 (1992).
19 Zhang, Q.F., Lü, J.H., Ma, L., Lu, C.S., Xu, X.L., Liang, Q.X., Li, X.N., “Catalytic hydrogenation for the green synthesis of N-(3-Amino-4-methoxyphenyl) acetamide”, Fine Chemical Intermediate, 38, 41-43 (2008).
20 Nishimura, S., Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis, John Wiley & Sons Inc., New York, 325 (2001).
21 Takenaka, Y., Kiyosu, T., Choi, J.C., Sakakura, T., Yasuda, H., “Selective synthesis of N-aryl hydroxylamines by the hydrogenation of nitroaromatics using supported platinum catalysts”, Green Chemistry, 11, 1385-1390 (2009).
2012-12-13, accepted 2013-03-09.
* Supported by the Natural Science Foundation of Zhejiang Provincial (LY12B03009) and Program for Zhejiang Leading Team of Science and Technology Innovation (2011R09020-03).
** To whom correspondence should be addressed. E-mail: xnli@zjut.edu.cn
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Preparation of Mesoporous Carbons from Acrylonitrile-methyl Methacrylate Copolymer/Silica Nanocomposites Synthesized by in-situ Emulsion Polymerization*
- Immobilization of Papain in Biosilica Matrix and Its Catalytic Property*
- Comparison on Thermal Conductivity and Permeability of Granular and Consolidated Activated Carbon for Refrigeration*
- Effect of Hydrogen Reduction of Silver Ions on the Performance and Structure of New Solid Polymer Electrolyte PEI/Pebax2533/AgBF4Composite Membranes*
- Synthesis of 2-Methyl-4-methoxyaniline from o-Nitrotoluene Using Pt/C and Acidic Ionic Liquid as Catalyst System*
- Adsorption and Desorption Behavior of Tannic Acid in Aqueous Solution on Polyaniline Adsorbent*
