C-MYC基因表达与胃癌病理进展无关
2013-05-11王克强韩国新刘振中东野圣伊
王克强 朱 蓓 韩国新 刘振中 东野圣伊 李 雁
(1.泰山医学院附属医院,山东 泰安 271000;2.武汉大学医学院肿瘤研究所,武汉大学中南医院肿瘤科,湖北武汉 430071)
The c-myc gene,mapped on human chromosome 8q24,encodes the transcription factor c-myc protein,which heterodimerizes with a partner protein,Max,to regulate gene expression.c- myc positively affects cell cycle regulation,apoptosis and metabolism,and negatively affects cellular differentiation and cell adhesion.Ectopic expression of the c-myc oncoprotein prevents cell cycle arrest in response to growth-inhibitory signals,leading to uncontrolled cell proliferation.Moreover,c- myc activation in quiescent cells is sufficient to induce cell cycle entry in the absence of growth factors.[1]Deregulated c - myc expression and signal pathway contribute to the neoplastic phenotype of deregulated growth,anchorage-independent growth,and glycolytic metabolism.c-myc overexpression is observed in many different malignancies such as breast cancer,ovarian cancer,gastric cancer,lung cancer,colorectal cancer and other cancers[2-9].In this paper,we studied c-myc expression profile in GC tissues using in situ hybridization,and explored the significance of this gene in GC pathological progression.
1 Materials and Methods
1.1 Clinical specimens Eight- one pathology- confirmed gastric carcinoma specimens were collected from Jan 2012 to Jun 2013 from the Affiliated Hospital of Taishan Medical College and the Institute of Oncology,Medical College of Wuhan University.There were 53 males and 28 females aged between 37 to 65 years old(mean age 58.6 years).Among these specimens were 35 cases with carcinomas at gastric cardia,17 cases at gastric body and 29 cases at antrum.According to Lauren classification,there were 33 cases with intestinal type carcinoma and 48 cases with diffused type carcinoma.Thirty-five cases were with well-differentiated carcinoma and 46 were poorly differentiated.In 11 cases cancer invaded superficial muscle layer and in the rest cancer invaded beyond deep muscle.Fiftyseven of these cases had lymph node metastases and 24 had no lymph node metastases.After surgical removal,the specimens were snap-frozen in liquid nitrogen and cryo-sectioned at-260C,with thickness between 6 to 10?m,which were mounted on aminopropyltriethoxysilane(APES,Sigma,St Louis,Ohio,USA)treated slides.
1.2 In situ hybridization H -C-myc probe was purchased from Sina-American Biotechnology Company(No.007,Sanshan Road,National Hi-Tech Industry Development Zone,Luoyang,Henan Province,China),with the length of 1.4 kb(Exon 3).Non -radioactive digoxigenin-11-UTP labeling was performed using random priming method,with reagent kit from Boehringer mannheims(purchased from Sino-A-merican Biotechnology Company,No.007,Sanshan Road,National Hi-Tech Industry Development Zone,Luoyang,Henan Province,China).The probe concentration was 15?g/ml and sensitivity was 0.1 pg DNA.After treatment with 0.1N HCl,RNase A,protease K and 0.1%Glycin,the slides were pre-hybridized for 1 h at room temperature in pre-hybridization solution(4×SSC,50%formamide,1×Denhardt solution,0.5%PEG and 0.5 mg/ml ssDNA).After washing the hybridization solution(probe concentration 0.2?g/ml)was added,the slides were sealed with cover glass and treated in formamide chamber at 95℃for 10-15 min.Then the slides were hybridized overnight at 42℃ in a humid chamber,after which the cover glass was removed in 2×SSCsolution,and alkaline phosphatase labeled anti-DIG antibody(1:500 dilution)was added and incubated for 2 h.After washing,5-bromo-4-chloro-3-indoxyl phosphate disodium(BCIP)/nitroblu-tetrazolium(NBT)color development substrate was added and kept in the dark for color reaction,which was terminated by TE buffer washing when color reaction was sufficient.The slides were counter-stained with eosin,and sealed.Normal gastric tissues from the same specimens were treated in the same fashion as negative controls,and positive control slides were provided by the probe supplier.
1.3 Slides interpretation When the slides were viewed under microscope(400×),dark brown-blue granules and particles could be found in positively stained cells,and the background was stained red by eosin.Positive and negative stained specimens were recorded respectively.Data on conventional pathology of the specimens were collected from routine pathological report based on gross pathology and hematoxylin and eosin(HE)stained tissue slides.
1.4 Statistical analysisComparisons between c - myc positive rates in different pathological subgroups were analyzed using Chi- square test,with P <0.05 as statistical significance.
2 RESULTS
2.1 c - myc expression in GC tissues Of the 81 GC specimens,55(67.9%)were found to have c - myc overexpression,which showed dark-brown granules in cancer tissue.The relationship of c-myc overexpression and the pathologic characteristics of tumors were summarized in Table 1.
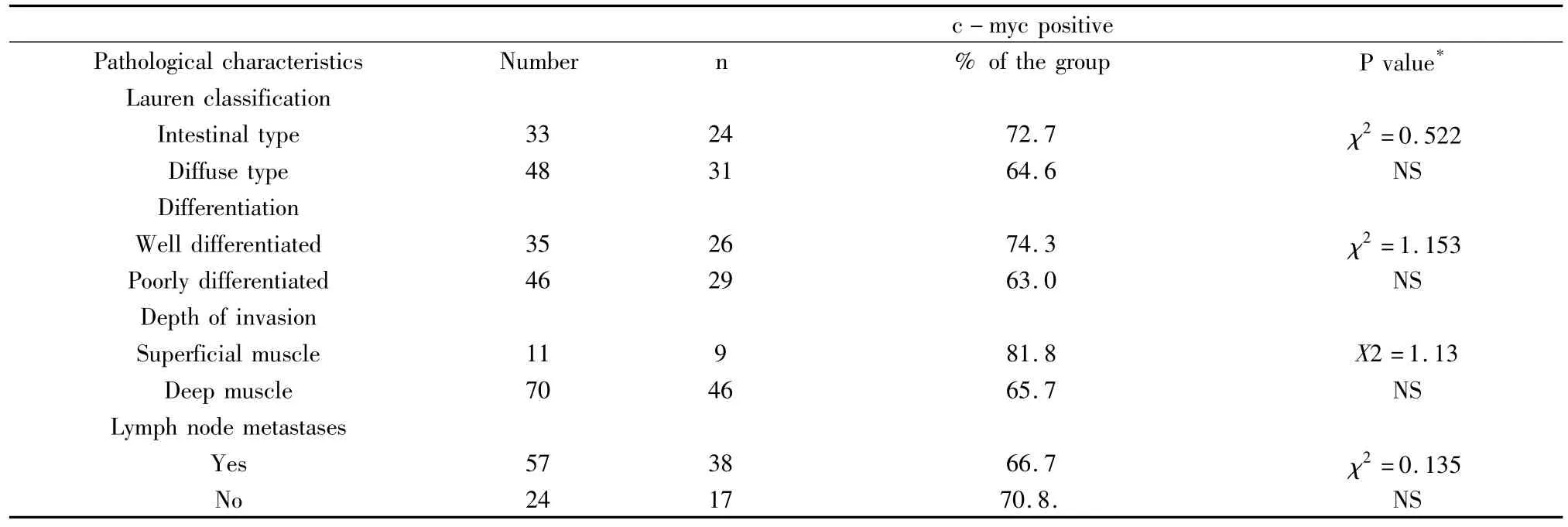
Table 1 c-myc expression and the pathological characteristics of the GC studied
2.2 Correlation between c - myc overexpression and major pathological characteristics Three major pathological parameters,tumor differentiation,depth of tissue invasion and lymph node metastasis-were particularly analyzed in this study to explore their correlation with c-myc expression.
2.2.1 In terms of tumor differentiation There were 35(43.2%,35/81)cases with well differentiated tumors and 46(56.8%.46/81)poorly differentiated tumors.Among the 55 c-myc positive cases,26 were well differentiated tumor,accounting for 74.3%(26/35)the well differentiated group,and 29 were poorly differentiated,accounting for 63.0%(29/46)of the poorly differentiated group(χ2=1.153,P >0.05)(Fig 1).
2.2.2 In terms of tumor invasion There were 11 cases(13.6%,11/81)with tumors invasion limited to the superficial muscle layer and 70 cases(86.4%,70/81)with tumors invading beyond the deep muscle layer.In cases with superficial muscle layer invasion,c -myc overexpression was found in 9 cases,accounting for 81.8%(9/11)of the group.In cases with deep muscle invasion,c-myc overexpression was observed in 46 cases,accounting for 65.7%(46/70)of the group.The difference in c-myc expression between these two groups were statistically very significant(χ2=1.13,P >0.05)(Fig 1).
2.2.3 In terms of lymph node metastases There were 57 cases(70.4%,57/81)with lymph node metastases and 24 cases(29.6%,24/81)without lymph node metastases.Of the lymph node positive group,c-myc overexpression was found in 38 cases,which was 66.7%(38/57)of the group.In contrast,in the lymph node negative group,c-myc overexpression was found in 17 cases,accounting for 70.8%(17/24)of the group.The differences between these two groups did not reached statistically significance(χ2=0.135,P >0.05)(Fig 1).
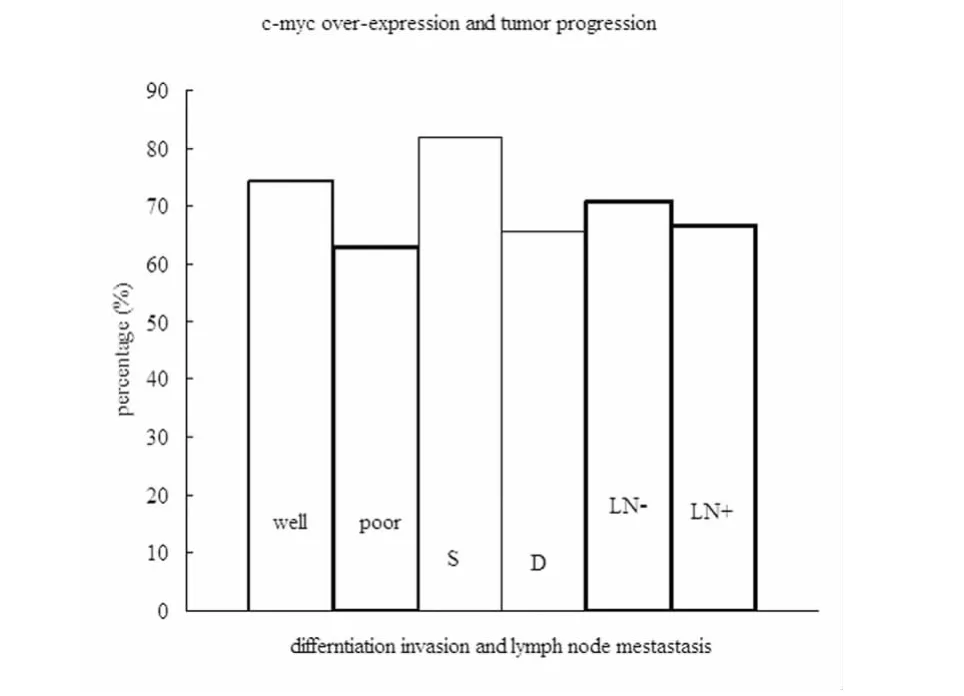
Figure 1 Relationship between c-myc overexpression and pathological characteristics in gastric cancer.Well=well differentiated,poor=poorly differentiated;S=tumor invading superficial muscle layer,D=tumor invading deep muscle layer;LN- =lymph node metastasis negative,LN+ =lymph node metastasis positive.The percentage of c-myc overexpression was not statistically significant between poorly differentiated tumors(63.0%)and well differentiated tumors(74.3%)(P >0.05),between tumors invading deep muscle(65.7%)than those invading superficial muscle(81.8%)(P > 0.05),and between tumors with lymph node metastases(66.7%)and those without lymph node metastases(70.8%)(P > 0.05)(Chisquare test).
3 DISCUSSION
GC is one of the most common malignant tumors worldwide and continues to be a highly aggressive malignancy with poor prognosis and low long-term survival rates.The survival of patients with GC depends mainly on the stage of the disease,with early GC having a 5 year survival of 90-100% and advanced tumors a 5 year survival of 15-25%.The role of other prognostic factors in these tumors is still under investigation.[3]Conventional pathology demonstrated that certain types of GC usually showed much more aggressive biological behavior than other types,and such pathological features include tumor differentiation,depth of tumor invasion and lymph node metastasis.[10]With the development of molecular biology,it has been found that there are many genes involved in GC development and pathological progression,including the plasminogen activator(uPA)and its inhibitor PAI-1(plasminogen activator inhibitor type 1),the cell-cycle regulator cyclin E,epidermal growth factor(EGF),cell proliferation related c-myc,the apoptosis inhibitor bcl-2,the cell adhesion molecule E -cadherin,and the multifunctional protein beta - catenin.[11]The relationship between these molecular markers and the conventional pathological parameters is currently under intensive investigation,as elucidation of these molecular profiles may provide more information on biological behavior of GC.
Using highly sensitive in situ hybridization method,we found 67.9%(55/81)of the gastric tumors had c-myc overexpression in the present work,which is similar to the results by Onoda et al,who found that 68.6%of primary gastric carcinoma had c - myc mRNA overexpression.[12]c - myc expression is known to be continuously high in proliferating cells but not in most quiescent or terminally differentiated cells.[13,14]Although the incidence of cells with c-myc DNA amplification is low(less than 12%)in carcinogenesis of the stomach,Onoda et al reported that c-myc mRNA and c-myc protein were overexpressed in the early stage of stomach cancer:mRNA overexpression in 54.8%of advanced cases and in 90.0% of early cases.[12]c - myc plays a central role in normal growth and development,as well as in cellular transformation and carcinogenesis in vitro.[15]Expression of c - myc protein correlated with proliferative activity of gastric cancer cells.However,we could not demonstrate a direct relationship between expression of c-myc mRNA and progression or extent of diseases.
Our results suggested that c-myc overexpression was very common in GC and such expression was not related to GC pathological progression.The percentage of c-myc overexpression was not significantly different between poorly differentiated GC and well differentiated GC,between tumors invading beyond deep muscle and those limited to superficial muscle,and between tumors with lymph node metastases than those without lymph node metastases.These results are in line with previous findings suggesting that c-myc overexpression had little influence on the histological features.[12,16]As c -myc may play an important role to predict more rapid growth characteristics and fast tumor progression of gastric cancer,this molecule could be used as a valuable marker for GC proliferation.
[1] Amati B,Alevizopoulos K,Vlach J.Myc and the cell cycle[J].Front Biosci,1998,3:D250 -268.
[2] Liao DJ,Dickson RB.C - myc in breast cancer[J].Endocr relat cancer,2000,7:143 -164.
[3] Sanz- Ortega J,Steinberg SM,Moro E,et al.Comparative study of tumor angiogenesis and immunohistochemistry for p53,c-ErbB2,c-myc and EGFr as prognostic factors in gastric cancer[J].Histol Histopathol,2000,15:455 - 462.
[4] Ishii HH,Gobe GC,Pan W,et al.Apoptosis and cell proliferation in the development of gastric carcinomas:associations with c-myc and p53 protein expression[J].JGastroenterol Hepatol,2002,17:966-972.
[5] Zajac-Kaye M.Myc oncogene:a key component in cell cycle regulation and its implication for lung cancer[J].Lung Cancer,2001,34(2):S43 -46.
[6] Xie D,Sham JS,Zeng WF,et al.Heterogeneous expression and association of beta-catenin,p16 and c-myc in multistage colorectal tumorigenesis and progression detected by tissue microarray[J].Int JCancer,2003,107:896 -902.
[7] Li Fu - iun,Deng Hui,Wang Hong - wei,et al.Effects of external application of chinese mcdicine on diabetic ulcers and the expressions ofβ - catenin,c - myc and K6[J].Chin J Integr,2011:17(4):261-266.
[8] A lbihn A ,Johnsen JI,Henriksson MA .MYCin oncogenesis and as a target for cancer therapies[J].Adv Cancer Res,2010,107:163-224.
[9] Skvortsova I,Skvortsov S,Raju U ,et al.Epithelial-to-mesenchymal transition and c-myc edpression are the detemninants of cetuximab-induced enhancement of squamous cell carcinoma radioresponse[J].Radiother Oncol,2010,96(1):108 -115.
[10] Kim HJ,Karpeh MS.Surgical approaches and outcomes in the treatment of gastric cancer[J].Semin Radiat Oncol,2002,12:162-169.
[11] Hofler H,Becker KF.Molecular mechanisms of carcinogenesis in gastric cancer[J].Recent Results Cancer Res,2003,162:65 -72.
[12] Onoda N,Maeda K,Chung YS,et al.Overexpression of c-myc messenger RNA in primary and metastatic lesions of carcinoma of the stomach[J].JAm Coll Surg 1996;182:55 - 59.
[13] Hurlin PJ,Poley KP,Ayer DE,et al.Regulation of c- myc and Mad during epidermal differentiation and HPV-associated tumorigenesis[J].Oncogene,1995,11:2487 -2501.
[14] Chin L,Schreiber- Agus N,Pellicer I,et al.Contrasting roles for Myc and Mad protein in cellular growth and differentiation[J].Proc Natl Acad Sci USA,1995,92:8488-8492.
[15] Lee LA,Dolde C,Barrett J,et al.A link between c-myc-mediated transcriptional repression and neoplastic transformation[J].J Clin Invest,1996,97:1687 -1695.
[16] Kim YJ,Ghu HD,Kim DY,et al.Expression of cellular oncogenes in human gastric carcinoma:c-myc,c-erb B2,and c-Ha - ras[J].J Surg Oncol,1993,54:167 -170.
