Ce0.45Zr0.45Y0.07La0.03O1.95/La-Ba-Al2O3质量比对整体式铁基催化剂上稀薄甲烷燃烧的影响
2012-12-21陈永东唐水花曹红岩龚茂初陈耀强
陈永东 王 磊 唐水花,* 曹红岩 龚茂初 陈耀强,*
(1西南石油大学,油气藏地质及开发工程国家重点实验室,成都610500;2四川中自尾气净化有限公司,成都610225; 3四川大学化学学院,绿色化学与技术教育部重点实验室,成都610064)
Ce0.45Zr0.45Y0.07La0.03O1.95/La-Ba-Al2O3质量比对整体式铁基催化剂上稀薄甲烷燃烧的影响
陈永东1王 磊2唐水花1,*曹红岩3龚茂初3陈耀强3,*
(1西南石油大学,油气藏地质及开发工程国家重点实验室,成都610500;2四川中自尾气净化有限公司,成都610225;3四川大学化学学院,绿色化学与技术教育部重点实验室,成都610064)
采用共沉淀法和胶溶法分别制备了高性能的储氧材料Ce0.45Zr0.45Y0.07La0.03O1.95(OSM)和耐高温高比表面的La-Ba-Al2O3,并以它们为载体,制备了一系列整体式铁基催化剂.考察了该系列催化剂对甲烷稀薄燃烧的催化性能.并用低温N2吸附-脱附,储氧量(OSC)测试,X射线衍射(XRD)和H2程序升温还原(H2-TPR)等测试手段考察了不同Ce0.45Zr0.45Y0.07La0.03O1.95/La-Ba-Al2O3质量比对催化剂特性的影响.活性测试结果表明,当Ce0.45Zr0.45Y0.07La0.03O1.95/La-Ba-Al2O3质量比为1:1时新鲜和老化催化剂的活性均最好,新鲜催化剂可在50000 h-1的高空速条件下使含量为1%(体积分数)的甲烷在446°C起燃,553°C完全转化;低温氮气吸附-脱附测试结果和H2-TPR表明,不同的Ce0.45Zr0.45Y0.07La0.03O1.95/La-Ba-Al2O3质量比使催化剂表现出不同的织构性能和还原性能;XRD测试结果表明,OSM以均一固溶体存在,Fe高度分散在载体上.综合以上表征手段得出:合适的Ce0.45Zr0.45Y0.07La0.03O1.95/La-Ba-Al2O3质量比导致催化剂具有优异的稀薄甲烷催化燃烧活性和热稳定性.
Fe2O3基整体式催化剂;OSM/La-Ba-Al2O3质量比;稀薄甲烷催化燃烧
1 Introduction
The catalytic combustion of methane has attracted considerable attention in recent years.Good catalysts must be able to totally convert methane in low concentration and at high gas velocity,and should also have high thermal stability because of the high temperatures under reaction conditions.Initially,the noble metal catalysts that were studied showed high catalytic activities but had poor thermal stability and were expensive due to the sintering of the active sites of the catalyst.1,2As an alternative,transition metal oxide catalysts have been studied.3-5At present,the research on Fe2O3-based catalysts for the catalytic combustion of methane has mainly focused on perovskite6,7and hexaaluminate.8,9In addition,Fe2O3is widely applied as a catalyst promoter.10,11However,only a few studies on the catalytic combustion of thin methane on supported Fe2O3-based monolithic catalysts have been reported.12,13
One of the Fe2O3catalyst supports is γ-Al2O3,which is the most common support for monolithic catalysts.The specific surface area and pore volume of γ-Al2O3decrease in water-thermal conditions and at high temperatures due to the formation of α-Al2O3.However,doping γ-Al2O3with alkali,alkalineearth,and rare-earth metals can increase the thermal stability and suppress the decrease in specific surface area of the catalysts.14-16
Another common Fe2O3catalyst support is CeO2-ZrO2oxygen storage material,which is widely used in the three-way catalysts of gasoline vehicles.CeO2-ZrO2solid solutions not only disperse the active components but also improve the catalytic activity,owing to their oxygen storage capability.However, when methane combusts the local temperature of the catalyst surface can become very high,resulting in sintering of the CeO2-ZrO2solid solution which has poor thermal stability. Turko,17Zhang,18Lv19et al.found that CeO2-ZrO2solid solutions doped with La3+,Y3+,Ba2+,Nd3+,Pr3+,or Sm3+had large oxygen storage capacities(OSCs),large specific surface areas, and high thermal stabilities.
Liu et al.20studied Fe2O3/Ce0.67Zr0.33O2-Al2O3catalysts with a mass ratio of Ce0.67Zr0.33O2to Al2O3of 1:2 for methane catalytic combustion at GHSVs around 15000 h-1.When the loading amount of Fe2O3was 8%(w,mass fraction),the catalyst showed the highest activity,the best temperature specialty,and the highest thermal stability.However,the catalyst was not studied at high gas velocities.
In our early work,Fe/CexZr0.9-xLa0.1O1.95-Al2O3monolithic catalysts were preparedfor the catalytic combustion of thin methane at a GHSV of 50000 h-1,21and the catalyst with x=0.7 showed the highest activity.Using this catalyst,methane started to convert at 465°C and completely converted at 615°C. We have also studied Fe-based catalystssupported on Ce0.35Zr0.55Y0.07La0.03O1.95(OSM)and La-Al2O3,22and found that methane started to convert at 474°C and completely converted at 565°C on the monolithic catalyst of Fe/OSM+La-Al2O3with a GHSV of 50000 h-1.However,the catalytic activity and thermal stability of the catalysts need to be improved.
In this work,to improve the thermal stability,Ba has been introduced into La-Al2O3to form La-Ba-Al2O3,and the Ce ratio in the compound oxide CeZrYLaO has been increased to improve its oxygen storage capacity.Fe2O3monolithic catalysts coated by mixed catalysts of Fe/Ce0.45Zr0.45Y0.07La0.03O1.95and Fe/ La-Ba-Al2O3were prepared for the catalytic combustion of methane with 1%(volume fraction)CH4at a GHSV of 50000 h-1.The effect of the OSM/La-Ba-Al2O3mass ratio on the physicochemical properties of the catalysts was investigated by nitrogen adsorption-desorption,OSC measurements,XRD,and H2-TPR.
2 Experimental
2.1 Catalyst preparation
Ce0.45Zr0.45Y0.07La0.03O1.95was prepared by co-precipitation and was then calcination at 600°C for 3 h.Fresh La-Ba-Al2O3(5% (w)La2O3,5%(w)BaO)was prepared by peptization and then calcination at 1000°C for 3 h.All aged samples were calcined at 1050°C for 5 h in an air atmosphere.
The catalysts were prepared by wet impregnation.The loading of Fe2O3was 8%(w).In a typical preparation,the support was impregnated with the desired amount of Fe(NO3)3solution.The resulting powder was dried at 120°C for 2 h and calcined in air at 600°C for 2 h.The dried powder was then ball-milled with distilled water to form a slurry.The slurry was coated on cordierite(62 cell·cm-2,Φ=9 mm,L=24 mm)and the excess slurry was blown off.Finally,the catalyst was dried and calcined in air at 600°C for 2 h to give the fresh catalyst. Catalysts were prepared with Fe/(OSM+La-Ba-Al2O3(m1:m2)) ratios of m1:m2=0:1,1:1,2:1,1:2,1:0(mass ratio of OSM to La-Ba-Al2O3).The aged samples were obtained by calcining the fresh catalysts in air at 1050°C for 5 h.The catalyst load-ing amount of all the samples on cordierite was 140 mg·mL-1.
2.2 Activity measurements
Catalytic activity tests were carried out on the monolithic samples in a multiple fixed-bed continuous flow microreactor with a gas mixture composed of 1%CH4,4%O2,and 95%N2. The GHSV was 50000 h-1.Before the reactions,all of the catalysts were treated at 600°C for 1 h in a N2atmosphere regulated by a mass-flow controller.The reaction temperature range was 450-800°C.The reactants and products were separated by a carbon molecular sieve 601 column(stainless steel,120°C) through pulse injection with an entrance temperature of 100°C and detected by an on-line GC-2000 II gas chromatography equipped with a thermal conductivity detector(TCD),using H2as the load gas with a flow speed of 50 mL·min-1at 120°C.
2.3 Characterization of the catalysts
The specific surface area and pore size of the catalysts and supports were determined by N2adsorption-desorption at-196°C on a ZXF-06 automatic surface analyzer(Xibei Chemical Institute,China).Before the measurements,the samples were degassed in vacuum at 350°C for 1 h.
The crystal structures of the samples were determined by XRD on a Netherland Phillip XʹPert Por MPD diffractometer with Cu Kαradiation(λ=0.15406 nm)at 40 kV and 40 mA. The XRD data were recorded for 2θ values from 25°to 80°at an interval of 0.03°and the results were processed using Jade-6.
H2-TPR experiments were performed on a quartz tubular microreactor,equipped with a TCD.A 100 mg sample was pretreated in the flow of a 10%O2and 90%N2gas mixture(20 mL·min-1)at 600°C for 45 min,and then cooled to room temperature in N2(30 mL·min-1).The reduction was carried out in a flow of a 5%H2and 95%N2gas mixture(20 mL·min-1) from room temperature to 1000°C with a heating rate of 8°C· min-1,and detected by a TCD at 120°C.
Oxygen pulse-adsorption was used to measure the OSC of the samples by gas chromatography in pure N2flow(20 mL· min-1)for a 200 mg sample at 200°C.Prior to the measurements,the samples were reduced in pure H2at 550°C for 45 min,and then cooled to 200°C in pure N2flow.Finally,pulses of oxygen(20 mL·min-1)were injected into the sample in intervals of 5 min until no further oxygen consumption could be detected by the TCD at 120°C.
3 Results and discussion
3.1 Catalytic activity of the catalysts for methane
combustion
The effects of the OSM/La-Ba-Al2O3mass ratio of the Fe-based monolithic catalysts for the combustion of thin methane are shown in Fig.1.
As shown in Fig.1,the activity of the catalysts depends on the mass ratio of OSM to La-Ba-Al2O3.The activity of the fresh catalysts is in the order of 1:1>2:1>1:2>1:0>0:1,and the activity of the aged catalysts is in the order of 1:1>2:1>1:2>0: 1>1:0.This indicates that OSM increases the activity of the Fe-based monolithic catalyst.Furthermore,it is possible that OSM releases oxygen,which can decrease the conversion temperature.When OSM is used,the optimum mass ratio of OSM to La-Ba-A12O3is 1:1.At this ratio,the catalyst has the highest activity for both aged and fresh catalysts.The temperatures (T10,T50,and T90)at which 10%(T10),50%(T50),and 90%(T90) of methane are converted are listed in Table 1.
From Table 1,it can be seen that on the fresh Fe/(OSM+ La-Ba-Al2O3(1:1))catalyst methane starts to convert at 446°C and is completely converted at 553°C,with 1%CH4and GHSV of 50000 h−1.The difference(ΔT)between the light-off temperature(T10)and the complete-conversion temperature (T90)is 107°C.The activity of the fresh catalyst Fe/(OSM+ La-Ba-Al2O3(2:1))is slightly higher than that of Fe/(OSM+ La-Ba-Al2O3(1:2)),indicating that OSM slightly improves the catalytic activity.However,aged Fe/(OSM+La-Ba-Al2O3(1:2))
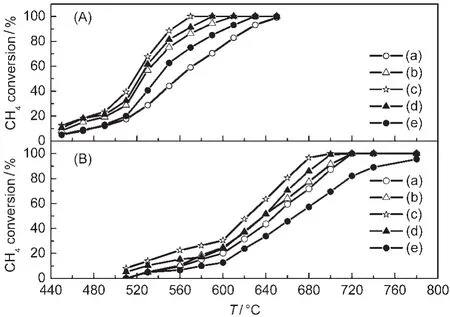
Fig.1 Catalytic activity of fresh(A)and aged(B)catalyst samples for thin methane combustion(a)Fe/La-Ba-Al2O3,(b)Fe/(OSM+La-Ba-Al2O3(1:2)),(c)Fe/(OSM+La-Ba-Al2O3 (1:1)),(d)Fe/(OSM+La-Ba-Al2O3(2:1)),(e)Fe/OSM.reaction conditions:feed1%CH4-4%O2-95%N2;catalyst concentration:140 g·L-1;GHSV:50000 h-1

Table 1 Catalytic activity of fresh and aged samples
-T10shows higher catalytic activity than aged Fe/(OSM+La-Ba-Al2O3(2:1))in the reaction temperature range from 600 to 680°C, indicating that La-Ba-Al2O3is the better anti-aging component in the catalysts.The catalyst Fe/(OSM+La-Ba-Al2O3(1:1))shows the lowest light-off temperature and the smallest ΔT value for both aged and fresh catalysts,which can be attributed to the coordinating effect of OSM and La-Ba-Al2O3on the catalyst.
3.2 Textural properties and OSC
Specific surface area and pore volume play an important role in the performance of catalytic supports,especially in high space velocity catalytic reactions.Thus,the supports must have suitable specific surface area and pore volume.The textural properties and OSC of the catalytic supports and catalysts are shown in Table 2.
As seen in Table 2,the OSM calcined at 1050°C for 5 h retains a high specific surface area(37 m2·g-1),large pore volume(0.20 mL·g-1),and large oxygen storage capacity(342.5 μmol·g-1).The BET specific surface area and BJH pore volume for La-Ba-Al2O3are 137 m2·g-1and 0.48 mL·g-1,respectively.The textural properties indicate that the good thermal stability of La-Ba-Al2O3can improve the dispersion of active sites and enhance anti-sintering of the catalysts.15Therefore, these materials are good catalytic supports.As expected,the specific surface areas of fresh and aged catalysts decrease with increasing OSM/La-Ba-Al2O3ratio,while the OSC of the catalysts increases.From Table 2,the OSC of Fe/OSM is larger than that of OSM,because adding iron increases the oxygen vacancies in the OSM structure after impregnation and improves the oxidizing capability and oxygen storage capacity of the OSM.In the OSC testing procedure,the samples were pre-reduced with hydrogen at 550°C for 45 min,with the iron oxides reduced to elementary substances by hydrogen and then oxidized to oxides during the testing.Therefore,the OSC of Fe/ OSM tends to be larger than that of OSM.Fe/La-Ba-Al2O3has the largest specific surface area(SBET)and pore volume,but no OSC.While Fe/OSM has the largest OSC,but its textural properties are poor.From Fig.1,the catalytic activities of Fe/ La-Ba-Al2O3and Fe/OSM are lower than the other catalysts,indicating that for a catalyst to have good activity it should have both good textural properties and large OSC.
3.3 XRD characterization
X-ray diffraction(XRD)was used to determine the bulk crystalline phases of the catalysts.The diffraction patterns of the fresh and aged catalysts are shown in Fig.2.

Fig.2 XRD patterns of fresh(A)and aged(B)samples(a)Fe/Al2O3,(b)Fe/La-Ba-Al2O3,(c)Fe/(OSM+La-Ba-Al2O3(1:2)), (d)Fe/(OSM+La-Ba-Al2O3(1:1)),(e)Fe/(OSM+La-Ba-Al2O3(2:1)),(f)Fe/OSM
As shown in Fig.2,OSM exists in uniform CeO2-ZrO2solid solution.17The crystalline structure of La-Ba-Al2O3calcined at 1000°C retains the γ-Al2O3phase,which indicates that La2O3and BaO stabilize γ-Al2O3.The crystalline structure of Fe2O3was not observed in any of the catalysts,indicating that Fe2O3is well dispersed on the support matrix.
As shown in Fig.2(A),the catalysts with OSM:La-Ba-Al2O3mass ratios of 1:2(c),1:1(d),and 2:1(e)have well-defined peaks,corresponding to the CeO2-ZrO2solid solution structure and the γ-Al2O3phase.From Fig.2(B),the crystalline structures of all the fresh catalysts change after being calcined at 1050°C. The XRD peaks become sharper and more intense,indicating that the structures are more crystalline.The α-Al2O3phase is also observed.Mixed OSM and La-Ba-Al2O3improves the dispersion and thermal stability of the supports towards Fe2O3, which maintains a high catalytic activity for the conversion of methane.
3.4 H2-TPR analysis
TPR curves for Fe-based monolithic catalysts with various OSM/La-Ba-Al2O3ratios are shown in Fig.3.From Fig.3(A), the fresh catalysts have a large peak(γ)in the range of 400-480°C,which corresponds to Fe2O3→Fe3O4.23The shoulder peak(α)of Fe/(OSM+La-Ba-Al2O3)and Fe/OSM catalysts in the range of 470-500°C can be attributed to the reduction of Ce4+on the surface.The peak at 800°C is due to the reduction of the lattice Ce4+,24,25which is the same as the reduction of Ce0.33Zr0.53Y0.10La0.04O1.93.17These peaks become more intense andthe area of the peaks increase as the OSM/La-Ba-Al2O3mass ratio increases.It is known that the peak area is related to the amount of reducible species on the surface of the catalyst.The larger the peak area,the greater the amount of reducible species and the stronger the reducing capability.This is consistent with the activity of the catalysts increasing in the order of 0:1<1:2<1:1 for the OSM/La-Ba-Al2O3mass ratio.When the mass ratio of OSM/La-Ba-Al2O3is 1:1,there is overlap between the Fe2O3→Fe3O4and Ce4+→Ce3+reduction peaks and its intensity significantly increases,which indicates that there is interaction between the two components and that the interaction increases the reduction of Fe3+and Ce4+on the surface.
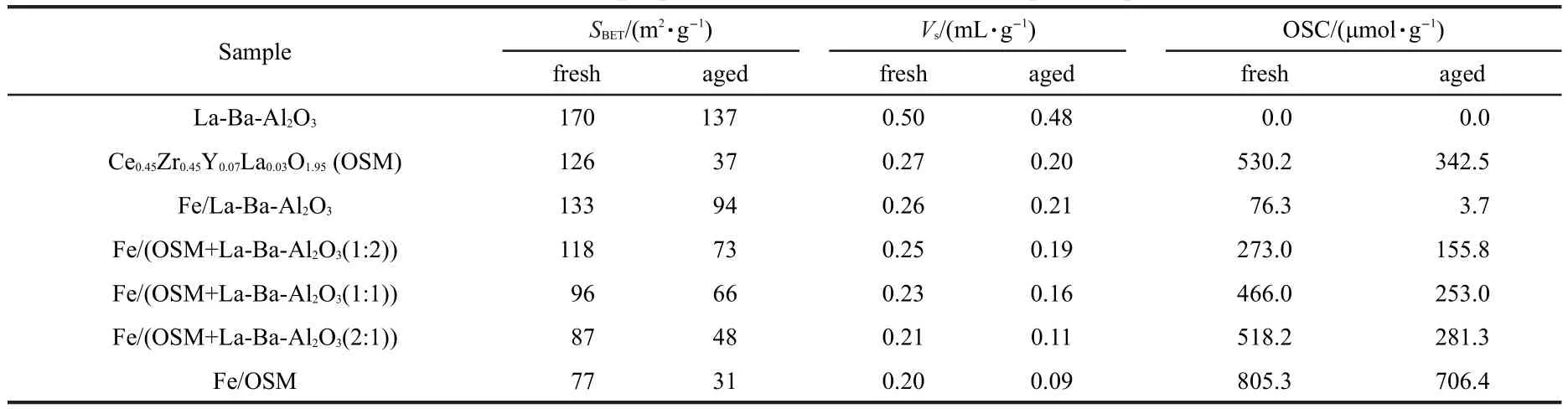
Table 2 Textural properties and OSC of fresh and aged samples

Fig.3 H2-TPR profiles of fresh(A)and aged(B)catalysts(a)Fe/La-Ba-Al2O3,(b)Fe/(OSM+La-Ba-Al2O3(1:2)),(c)Fe/(OSM+La-Ba-Al2O3 (1:1)),(d)Fe/(OSM+La-Ba-Al2O3(2:1)),(e)Fe/OSM.The main peaks are signed as α,β,and γ.
The overlap of the reduction peaks Fe2O3→Fe3O4and Ce4+→Ce3+on the surface improves the catalytic activity and oxygen storage capacity of Ce0.45Zr0.45Y0.07La0.03O1.95,and thus improves the catalytic activity of Fe/(OSM+La-Ba-Al2O3)for methane combustion.The temperature of the Fe2O3→Fe3O4peak reflects the activities of the samples.26Among the catalysts,Fe/(OSM+ La-Ba-Al2O3(1:1))has the lowest peak temperature of 406°C, which is in agreement with it having the lowest light-off and total conversion temperatures(Fig.1).
For the aged samples in Fig.3(B),the reduction peaks are at higher temperatures than the corresponding peaks for the fresh samples.The specific surface area of the samples decreased, the pore canal collapsed,and active components were sintered, resulting in the TPR peak of the samples moving to a higher temperature.Compared with Fig.3(A),the change of the reduction temperature for aged Fe/La-Ba-Al2O3is less than that for aged Fe/OSM,indicating that the thermal stability of Fe/ La-Ba-Al2O3is greater than that of Fe/OSM.With increasing La-Ba-Al2O3content(from(e)to(a)in Fig.3),the γ peak temperature decreases,which indicates that La-Ba-Al2O3resists aging.The ΔT for the γ peak temperature between fresh and aged Fe/(OSM+La-Ba-Al2O3(1:1))of 134°C is the smallest among all the Fe/(OSM+La-Ba-Al2O3)samples,which indicates that it has the best thermal stability due to the balance of OSM and La-Ba-Al2O3.
4 Conclusions
Ce0.45Zr0.45Y0.07La0.03O1.95prepared by co-precipitation has a high oxygen storage capacity,while La-Ba-Al2O3made by peptization has excellent texture and high thermal stability. Ce0.45Zr0.45Y0.07La0.03O1.95can improve the OSC and redox ability of the catalyst,both of which are important factors of fresh catalysts for methane combustion.La-Ba-Al2O3increases the dispersion of the active species of the catalysts and their thermal stability.Fe/(OSM+La-Ba-Al2O3)monolithic catalysts supported by a mixture of Ce0.45Zr0.45Y0.07La0.03O1.95and La-Ba-Al2O3have good catalytic activities and high temperature stabilities. When the mass ratio of Ce0.45Zr0.45Y0.07La0.03O1.95to La-Ba-Al2O3is 1:1,methane starts converting at 446°C and is completely converted at 553°C,which shows the highest activity for the combustion of thin methane of the catalysts studied.These catalysts show potential for the use in practical applications.
(1) Gélin,P.;Primet,M.Appl.Catal.B 2002,39(1),1.
(2) Choudhary,T.V.;Banerjee,S.;Choudhary,V.R.Appl.Catal.A 2002,234(1-2),1.
(3) Hasan,M.A.;Zaki,M.I.;Pasupulety,L.;Kumari,K.Appl. Catal.A 1999,181(1),171.
(4) Xu,J.;Li,P.;Song,X.F.;He,C.H.;Yu,J.G.;Han,Y.F. J.Phys.Chem.Lett.2010,1,1648.
(5) Ren,X.G.;Zheng,J.D.;Song,Y.J.;Liu,P.Catal.Commun. 2008,9(5),807.
(6) Petrović,S.;Terlecki-Baričević,A.;Karanović,L.;Kirilov-Stefanov,P.;Zdujić,M.;Dondur,V.;Paneva,D.;Mitov,I.; Rakić,V.Appl.Catal.B 2008,79(2),186.
(7)Tian,T.F.;Zhan,M.C.;Wang,W.D.,Chen,C.S.Catal. Commun.2009,10(5),513.
(8)Artizzu-Duart,P.;Millet,J.M.;Guilhaumea,N.;Garbowski,E.; Primet,M.Catal.Today 2000,59(1-2),163.
(9) Jiang,Z.;Hou,H.X.;Hao,Z.P.;Kang,S.F.;Li,J.J.;Hu,C. Acta Phys.-Chim.Sin.2004,20(11),1313. [蒋 政,侯红霞,郝郑平,康守方,李进军,胡 春.物理化学学报,2004,20 (11),1313.]
(10)Yue,B.H.;Zhou,R.X.;Wang,Y.J.;Zheng,X.M.Appl.Surf. Sci.2006,252(16),5820.
(11) Kucharczyk,B.;Tylus,W.Catal.Today 2008,137(2-4),324.
(12) Li,L.N.;Chen,Y.Q.;Gong,M.C.;Xiang,Y.Chem.J.Chin. Univ.2003,24(12),2235.[李丽娜,陈耀强,龚茂初,向 云.高等学校化学学报,2003,24(12),2235.]
(13) Choudhary,V.R.;Deshmukh,G.M.;Pataskar,S.G.Environ. Sci.Technol.2005,39(7),2364.
(14) Zhang,L.J.;Dong,W.P.;Guo,J.X.;Yuan,S.H.;Zhang,L.; Gong,M.C.;Chen,Y.Q.Acta Phys.-Chim.Sin.2007,23(11), 1738.[张丽娟,董文萍,郭家秀,袁书华,张 磊,龚茂初,陈耀强.物理化学学报,2007,23(11),1738.]
(15) Reddy,B.M.;Reddy,G.K.;Rao,K.N.J.Mol.Catal.A:Chem. 2009,306(1-2),62.
(16) Wilcox,L.;Burnside,G.;Kiranga,B.;Shekhawat,R.; Mazumder,M.K.;Hawk,R.M.;Lindquist,D.A.Chem.Mater. 2003,15(1),51.
(17) Turko,G.A.;Ivanova,A.S.;Plyasova,L.M.;Litvak,G.S.; Rogov,V.A.Kinet.Catal.2005,46(6),884.
(18) Zhang,L.;Zheng,L.M.;Guo,J.X.;Wu,D.D.;Gong,M.C.; Wang,J.L.;Chen,Y.Q.Acta Phys.-Chim.Sin.2008,24(8), 1342.[张 磊,郑灵敏,郭家秀,吴冬冬,龚茂初,王健礼,陈耀强.物理化学学报,2008,24(8),1342.]
(19) Lv,J.D.;Wang,L.;Lei,D.;Guo,H.X.;Kumar,R.V.J.Alloy. Compd.2009,467,376.
(20) Liu,Z.M.;Chen,Y.Q.;Zhong,J.B.;Wang,J.L.;Yan,S.H.; Gong,M.C.J.Rare Earths 2007,25(5),585.
(21) Chen,Y.D.;Liao,C.W.;Cao,H.Y.;Liu,Z.M.;Wang,J.L.; Gong,M.C.;Chen,Y.Q.Chin.J.Catal.2010,31(5),562. [陈永东,廖传文,曹红岩,刘志敏,王健礼,龚茂初,陈耀强.催化学报,2010,31(5),562.]
(22)Chen,Y.D.;Zhu,Y.;Wang,J.L.;Chen,Y.Q.;Gong,M.C. Chin.J.Inorg.Chem.2009,25(10),1771. [陈永东,朱 艺,王健礼,陈耀强,龚茂初.无机化学学报,2009,25(10),1771.]
(23) Scirè,S.;Minicò,S.;Crisafulli,C.;Galvagno,S.Catal. Commun.2001,2(6-7),229.
(24)Jen,H.W.;Graham,G.W.;Chun,W.;McCabe,R.W.;Cuif,J. P.;Deutsch,S.E.;Touret,O.Catal.Today 1999,50(2),309.
(25) Zeng,S.H.;Wang,L.;Gong,M.C.;Chen,Y.Q.J.Nat.Gas Chem.2010,19(5),499.
(26)Li,K.Z.;Wang,H.;Wei,Y.G.;Yan,D.X.Chem.Eng.J.2010, 156(3),512.
November 25,2011;Revised:March 7,2012;Published on Web:March 14,2012.
Effect of Ce0.45Zr0.45Y0.07La0.03O1.95/La-Ba-Al2O3Mass Ratio on the Activity of Fe-Based Monolithic Catalysts for the Combustion of Thin Methane
CHEN Yong-Dong1WANG Lei2TANG Shui-Hua1,*CAO Hong-Yan3GONG Mao-Chu3CHEN Yao-Qiang3,*
(1State Key Laboratory of Oil and Gas Reservoir Geology and Exploitation,Southwest Petroleum University,Chengdu 610500, P.R.China;2Sichuan Zhongzi Exhaust Purge Co.,Ltd.,Chengdu 610225,P.R.China;3Key Laboratory of Green Chemistry& Technology of Ministry of Education,College of Chemistry,Sichuan University,Chengdu 610064,P.R.China)
Ce0.45Zr0.45Y0.07La0.03O1.95,an oxygen storage material(OSM),and La-Ba-Al2O3have been prepared by co-precipitation and peptization,respectively,to be used as supports for Fe2O3catalysts.The Fe2O3catalysts were obtained by impregnation methods and then coated on the monolith.The catalytic combustion of low concentration(thin)methane was investigated over the prepared catalysts.The effect of the OSM/La-Ba-Al2O3mass ratio on the physicochemical properties of the catalysts was investigated using nitrogen adsorption-desorption,oxygen storage capacity(OSC)measurements,X-ray diffraction(XRD), and H2-temperature-programmed reduction(H2-TPR).The highest catalytic activity of both fresh and aged Fe2O3catalysts for thin methane combustion was observed when the mass ratio of OSM/La-Ba-AL2O3was 1:1.At this OSM/La-Ba-AL2O3mass ratio,methane started to convert at 446°C and completely converted at 553°C,with 1%(volume fraction)CH4and a gas hourly space velocity(GHSV)of 50000 h-1.The Fe-based monolithic catalysts with different mass ratios of OSM to La-Ba-Al2O3had different specific surface areas and reducibilities.XRD results showed that OSM existed in uniform solid solution and Fe2O3was well dispersed on the binary supports.Based on the characterizations,the high catalytic activity and thermal stability of the catalysts can be attributed to the proper coordination of OSM and La-Ba-AL2O3for thin methane combustion.
Fe2O3based monolithic catalyst;OSM/La-Ba-Al2O3mass ratio;Catalytic combustion of thin methane
10.3866/PKU.WHXB201203141
∗Corresponding authors.TANG Shui-Hua,Email:shuihuatang@swpu.edu.cn;Tel:+86-28-83032879.
CHEN Yao-Qiang,Email:chenyaoqiang@scu.edu.cn;Tel:+86-28-85418451.
The project was supported by the National Natural Science Foundation of China(21173153)and Opening Project of Key Laboratory of Green Catalysis of Sichuan Institutes of High Education,China(LYY1101).
国家自然科学基金(21173153)和绿色催化四川省高校重点实验室开放课题基金(LYY1101)资助项目
O643