新型螺吡喃衍生物:离子传感和分子水平的信息处理
2012-12-11李颖若张洪涛齐传民郭雪峰
李颖若 张洪涛 齐传民,* 郭雪峰,3,*
(1北京师范大学化学学院,放射性药物教育部重点实验室,北京100875;2北京大学化学与分子工程学院,北京分子科学国家实验室,分子动态与稳态结构国家重点实验室,北京100871;3北京大学工学院先进材料与纳米技术系,北京100871)
新型螺吡喃衍生物:离子传感和分子水平的信息处理
李颖若1张洪涛2齐传民1,*郭雪峰2,3,*
(1北京师范大学化学学院,放射性药物教育部重点实验室,北京100875;2北京大学化学与分子工程学院,北京分子科学国家实验室,分子动态与稳态结构国家重点实验室,北京100871;3北京大学工学院先进材料与纳米技术系,北京100871)
为实现金属离子检测和分子水平的信息处理,合成了一类新型的含有功能配位基团的螺吡喃衍生物(SP1-SP4).研究发现:在没有UV光照的条件下,金属离子可以促进螺吡喃(SP2和SP4)开环并形成稳定可逆的络合物(MC-Mn+).紫外-可见吸收光谱研究表明,在UV光照前加入不同的金属离子会引起SP2和SP4的光学性质的特征变化,因此提供了一种简易的通过裸眼就能辨别金属离子的比色方法.荧光光谱研究表明,这类化合物能够高灵敏高选择性地检测锌离子.此外,基于吸收光谱和荧光光谱的变化,这类螺吡喃衍生物可以用于构建组合的逻辑门,执行分子水平的信息处理,从而展现了其在化学或环境传感和未来的分子计算机领域的潜在应用前景.
螺吡喃;化学传感;逻辑门;紫外-可见吸收光谱;荧光光谱
1 Introduction
Photochromic compounds have been extensively investigated in recent years for their high potential applications in optically rewritable storage,1optical switching,2chemical3and biological4sensings.In particular,considerable attention has been paid to spiropyran molecules,one of the most promising families of photochromic compounds,because of their unique optical and physical properties.5-10The stimulus-induced transformation of the ring-closed structure of spiropyrans(SPs)into their fully π-conjugated isomeric merocyanine forms(MCs)results not only in the variations of absorption spectra,but also in the profound alterations of other physical and chemical properties of the system,such as the dipole moments,nonlinear optic properties,emission spectra,and macroscopic properties (for example,conductance,rheological property,and surface wettability).By taking advantage of these remarkable characteristics of SPs,a number of spiropyran derivatives containing diverse functional groups have so far been designed and used as molecular sensors and molecular switches.11-17
Among the remarkable characteristics of SPs,one unique feature is that the photogenerated open merocyanine form processes the charge-separated zwitterionic state with a free negatively-charged oxygen atom,which can further interact with external stimuli through dipole-dipole interactions and coordination chemistry(Scheme 1).Recently,several groups have successfully utilized this for the purpose of optically detecting metal ions,18-20anions,21nucleobases,22amino acids,23and DNA,24etc.In this study,a new class of spirobenzopyrans SP2 and SP4 bearing electron-donating―OMe group and pyridine or quinoline moiety as binding sites were designed and synthesized(Scheme 1).We will explore the changes in their chemical and physical properties upon addition of different metal ions before and after UV irradiation and show the capability of selectively detecting metal ions with high sensitivity and constructing logic gates for information processing at the molecular level.25-31
2 Experimental
Functional spirobenzopyran derivatives SP1-SP4 were synthesized as shown in Scheme 2.Compounds 2-1 and 2-2 were prepared by modification of the procedure reported by Raymo and Giordani32bearing―OH as a functional group for the following reaction step.The pyridine or quinoline moiety was then linked to compounds 2-1 and 2-2 using EDCI/DMAP (EDCI:1-ethyl-3-(3-dimethylaminopropyl)carbodiimide,DMAP: 4-dimethylaminopyridine)esterification reaction to give SP1-SP4 as yellow crystals in high yield(~90%).
2.1 1-(2-hydroxyethyl)-2,3,3-trimethylindoliumbromide(1)
Under nitrogen atmosphere,a mixture of 2,3,3-trimethyl-3H-indole(4.77 g,30.0 mmol)and 2-bromoethanol(4.50 g, 36.0 mmol)in dry CH3CN(30 mL)was heated under reflux for 12 h.Removal of CH3CN and excess of 2-bromoethanol under the reduced pressure gave a dark purple residue.Repeated washing with anhydrous ether gave compound 1(7.85 g, 92.1%)as a white solid.All the reagents used areAR grade.
1H NMR(DMSO-d6,400 MHz):1.55(s,6H),3.38(s,3H), 3.87(t,2H,J=6.8 Hz),4.60(t,2H,J=6.8 Hz),7.60-7.64(m, 2H),7.84-7.87(m,1H),7.94-7.98(m,1H).Fourier transform mass spectroscopy(FTMS):m/z=204.1,[M-Br]+.
2.2 2-(3ʹ,3ʹ-dimethyl-6-nitrospiro[chromene-2,2ʹ-indolin]-1ʹ-yl)ethanol(2-1)
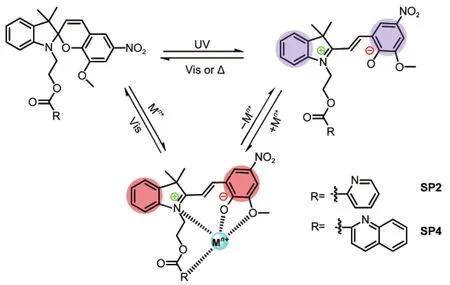
Scheme 1 Illustrations of the reversible structural transformations of SP2 and SP4 in responses to light,heat,and metal ions
Under nitrogen atmosphere,Compound 1(1.14 g,4.0 mmol) and 2-hydroxy-5-nitrob-enzaldehyde(0.80 g,4.8 mmol)were dissolved in dry tetrahydrofuran(THF)(25 mL).The solution was heated to reflux then triethylamine(0.49 g,4.8 mmol)in THF(5 mL)was added dropwise.The mixture was refluxed for 4 h.The solvent was removed by evaporation under reduced pressure.The crude residue was recrystallized from absolute ethanol giving compound 2-1(1.30 g,92.2%)as red purple crystals.
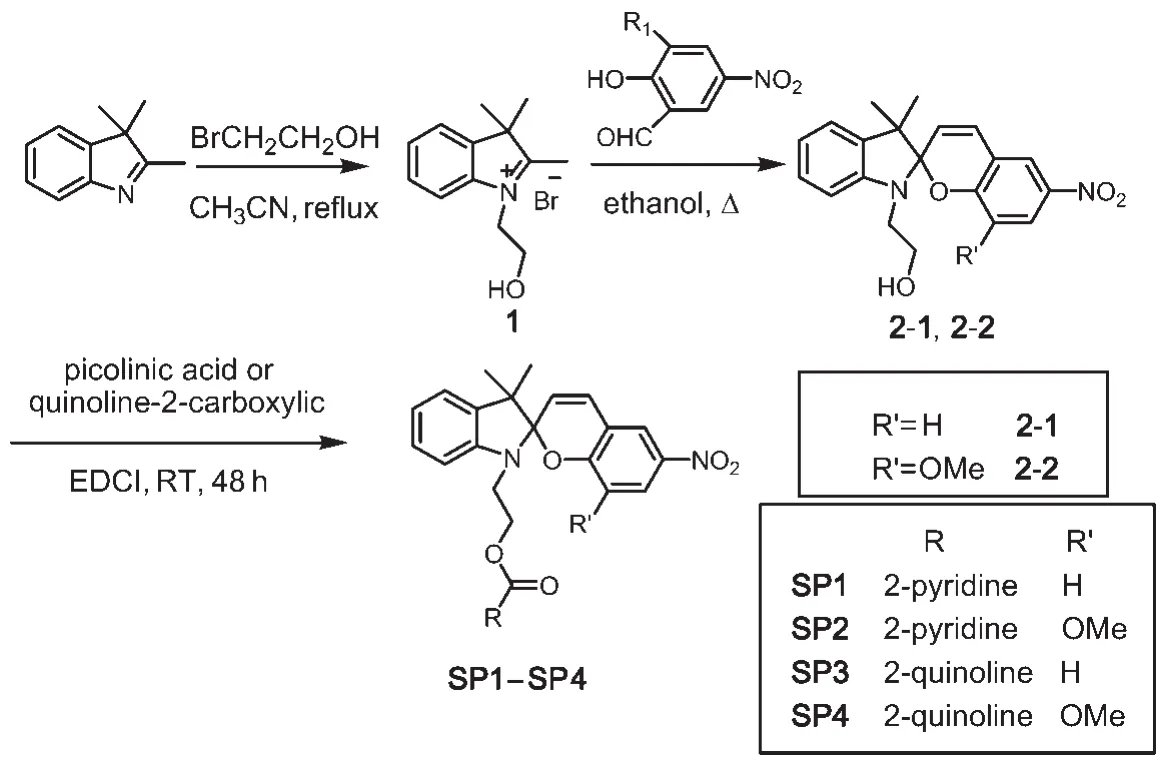
Scheme 2 Synthesis of spirobenzopyrans SP1-SP4
1H NMR(CDCl3,400 MHz):1.20(s,3H),1.29(s,3H), 3.33-3.50(m,2H),3.68-3.77(m,2H),5.89(d,1H,J=13.6 Hz),6.67(d,1H,J=10.4 Hz),6.76(d,1H,J=12.4 Hz), 6.87-6.93(m,2H),7.10(d,1H,J=9.0 Hz),7.20(t,1H,J=10.0 Hz),7.99-8.04(m,2H).FTMS:m/z=353.1,[M+H]+.
2.3 2-(8-methoxy-3ʹ,3ʹ-dimethyl-6-nitrospiro [chromene-2,2ʹ-indolin]-1ʹ-yl)ethanol(2-2)
Compound 2-2 was prepared according to a procedure similar to compound 2-1.After column chromatography on silica gel with ethyl acetate/petroleum(60-90°C)(1:1,V/V)as eluent,compound 2-2 was obtained as dark blue crystals(3.82 g, 91.6%).
1H NMR(CDCl3,400 MHz):1.18(s,3H),1.29(s,3H), 3.35-3.43(m,2H),3.51-3.63(m,2H),3.78(s,3H),5.82(d, 1H,J=14.0 Hz),6.65(d,1H,J=10.4 Hz),6.84-6.90(m,2H), 7.08(d,1H,J=10.0 Hz),7.15-7.21(m,1H),7.63(d,1H,J=3.6 Hz),7.69(d,1H,J=3.6 Hz).FTMS:m/z=383.2,[M+H]+.
2.4 2-(3ʹ,3ʹ-dimethyl-6-nitrospiro[chromene-2,2ʹ-indolin]-1ʹ-yl)ethylpicolinate(SP1)
Under nitrogen atmosphere,compound 2-1(0.35 g,1.0 mmol),picolinic acid(0.12 g,1.0 mmol),EDCI(0.38 g,2.0 mmol),DMAP(0.01 g,0.1 mmol)were dissolved into dry dichloromethane(15 mL).The reaction mixture was stirred at room temperature overnight.Evaporation of the solvent gave a brown tar.The obtained brown tar was dissolved into ethyl acetate,washed with H2O three times,and dried over anhydrous magnesium sulfate.Evaporation of the solvent gave a light brown residue.The crude residue was recrystallized from ethyl acetate/n-hexane giving SP1(0.82 g,90.5%)as a light yellow crystals.
1H NMR(CDCl3,400 MHz):1.15(s,3H),1.29(s,3H), 3.55-3.63(m,1H),3.68-3.74(m,1H),4.55-4.58(m,2H), 5.96(d,1H,J=10.4 Hz),6.73(d,1H,J=8.4 Hz),6.79(d,1H, J=8.0 Hz),6.87-6.92(m,2H),7.09(d,1H,J=6.4 Hz), 7.19-7.23(m,1H),7.46-7.49(m,1H),7.80-7.84(m,1H), 7.95-7.98(m,2H),8.06(d,1H,J=7.2 Hz),8.74(d,1H,J=4.8 Hz).13C NMR(CDCl3,100 MHz):165.05,159.32,149.87, 147.72,146.46,141.06,137.03,135.75,128.41,127.92, 127.05,125.92,125.19,122.76,121.86,121.83,119.97, 118.45,115.53,106.78,106.49,63.30,52.87,42.21,25.86, 19.85.FTMS:m/z=458.15,[M+H]+.
2.5 2-(8-methoxy-3ʹ,3ʹ-dimethyl-6-nitrospiro [chromene-2,2ʹ-indolin]-1ʹ-yl)ethyl picolinate (SP2)
SP2 was prepared according to a procedure similar to SP1. Compound 2-2 was used instead of compound 2-1.SP2 was obtained as yellow crystals(0.64 g,88.9%).
1H NMR(CDCl3,400 MHz):1.15(s,3H),1.27(s,3H), 3.58-3.66(m,1H),3.73(s,3H),3.74-3.80(m,1H),4.56(t, 2H,J=6.6 Hz),5.93(d,1H,J=10.4 Hz),6.78(d,1H,J=7.6 Hz),6.83-6.90(m,2H),7.08(d,1H,J=6.4 Hz),7.17-7.21 (m,1H),7.45-7.48(m,1H),7.56(d,1H,J=2.8 Hz),7.66(d, 1H,J=2.4 Hz),7.65-7.66(m,1H),8.02(d,1H,J=7.6 Hz), 8.73(d,1H,J=5.6 Hz).13C NMR(CDCl3,100 MHz):164.10, 149.84,149.03,147.73,147.33,146.42,140.45,136.99, 135.79,128.36,127.73,126.99,125.15,121.88,121.84, 119.68,118.18,115.30,107.72,106.70,106.33,63.27,56.12, 52.84,41.97,25.98,19.79.FTMS:m/z=488.19,[M+H]+.
2.6 2-(3ʹ,3ʹ-dimethyl-6-nitrospiro[chromene-2,2ʹ-indolin]-1ʹ-yl)ethylisoquinol-ine-3-carboxylate (SP3)
SP3 was prepared according to a procedure similar to SP1. Quinoline-2-carboxylic was used instead of acid picolinic acid. SP3 was obtained as light yellow crystals(0.69 g,90.6%).
1H NMR(CDCl3,400 MHz):1.20(s,3H),1.40(s,3H), 3.45-3.54(m,1H),3.81-3.91(m,1H),4.64-4.69(m,2H), 6.37(d,1H,J=14.4 Hz),6.77(d,1H,J=10.4 Hz),6.85-6.90 (m,2H),7.08(d,1H,J=10.0 Hz),7.15-7.19(m,1H),7.22(d, 1H,J=11.6 Hz),7.66-7.71(m,1H),7.81-7.87(m,1H), 7.89-7.93(m,2H),8.02(d,1H,J=4.0 Hz),8.16(d,1H,J= 11.6 Hz),8.28-8.33(m,2H).13C NMR(CDCl3,100 MHz): 165.37,159.41,147.67,147.57,146.46,141.00,137.29, 135.78,130.58,130.44,129.34,128.79,128.28,127.93, 127.64,125.87,122.72,122.27,121.88,120.95,119.94, 118.54,115.52,106.75,106.65,63.54,52.97,42.30,25.88, 19.88.FTMS:m/z=508.17,[M+H]+.
2.7 2-(8-methoxy-3ʹ,3ʹ-dimethyl-6-nitrospiro [chromene-2,2ʹ-indolin]-1ʹ-yl)ethyl isoquinoline-3-carboxylate(SP4)
SP4 was prepared according to a procedure similar to SP1. Compound 2-2 was used instead of compound 2-1 and quinoline-2-carboxylic was used instead of acid picolinic acid.SP4 was obtained as yellow crystals(0.72 g,90.1%).
1H NMR(CDCl3,400 MHz):1.17(s,3H),1.27(s,3H), 3.65-3.70(m,1H),3.72(s,3H),3.80-3.87(m,1H), 4.58-4.66(m,2H),6.10(d,1H,J=10.4 Hz),6.82(d,1H,J= 7.6 Hz),6.86(d,1H,J=10.4 Hz),6.88(t,1H,J=7.4 Hz),7.08 (d,1H,J=6.4 Hz),7.21(t,1H,J=7.6 Hz),7.53(d,1H,J=2.4 Hz),7.64(d,1H,J=2.4 Hz),7.67(t,1H,J=8.0 Hz),7.81(t, 1H,J=7.6 Hz),7.88(d,1H,J=9.2 Hz),8.09(d,1H,J=8.8 Hz), 8.27(d,2H,J=8.4 Hz).13C NMR(CDCl3,100 MHz):165.31, 149.12,147.70,147.54,147.32,146.42,140.40,137.22, 135.82,130.56,130.39,129.28,128.73,128.26,127.73, 127.60,122.33,121.86,120.92,119.64,118.28,115.28, 107.68,106.67,106.51,63.50,56.11,52.95,42.05,25.97, 19.79.FTMS:m/z=538.17,[M+H]+.
3 Results and discussion
3.1 Photochromic properties
Previous reports demonstrated that the introduction of an electron-withdrawing group(e.g.,―NO2,―CF3)into the benzene ring enhances the stability of the open form of SPs33,34whereas an electron-donating group(e.g.,―t-Bu,―OMe)produces a more stable photostationary closed form.35,36To gather the kinetic data of SP1-SP4 to evaluate the effect of―OMe on the properties of spiropyrans,we monitored the evolutions of the absorption spectra and the time dependence of absorbance at λmax(maximum absorption wavelength)of SP1-SP4 in ethanol solution upon UV irradiation,visible irradiation and in the dark(Figs.S1-S4(Supporting Information)).The kinetic of each process can be fit with a single exponential.Using the method from literature,32,11the rate constants and the percent conversions(χe)were calculated and summarized in Table 1. As expected,the rate constants for the conversions of MC2 to SP2 and MC4 to SP4 in the dark were determined to be~(1.4± 0.1)×10-2s-1,which is much larger than those for MC1 to SP1 (~(1.3±0.1)×10-3s-1)and MC3 to SP3(~(1.7±0.1)×10-3s-1). This indicates that MC2 and MC4 can thermally isomerize back to the corresponding SP2 and SP4 faster than the cases of SP1 and SP3.Visible irradiation can accelerate the conversion from MC to SP.Consistently,the rate constants for MC2 to SP2(~(1.1±0.1)×10-1s-1)and MC4 to SP4(~(8.9±0.1)×10-2s-1)under visible irradiation are still larger than those for MC1 to SP1(~(7.2±0.1)×10-3s-1)and MC3 to SP3(~(7.1±0.1)×10-3s-1),separately.On the basis of kinetic data listed in Table 1, the calculated conversions(χe)of SP2 and SP4 are 6.7%and 8.7%,respectively,which are much smaller than the cases for SP1 and SP3(56.4%and 50.3%,respectively),indicating that the introduction of―OMe apparently shifts the SP/MC equilibrium to favor the closed form of spiropyrans and thus decrease the stability of the open form most likely due to the increase of the electron density of the phenoxide ion unit affected by the electron-donating―OMe group.19
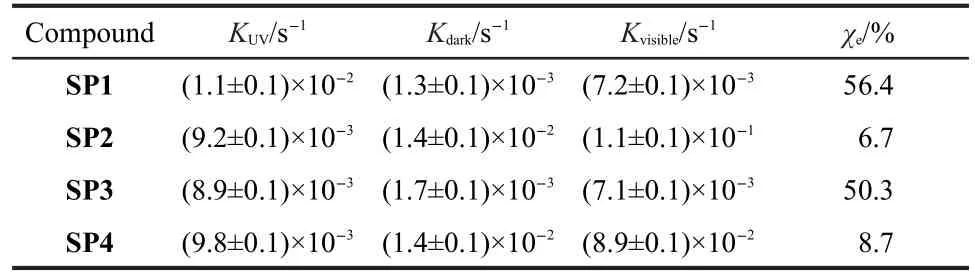
Table 1 Calculated rate constants and conversions of SP1-SP4 at 293 K
3.2 Sensing properties

Fig.1 Absorption spectra of SP1(a)and SP2(b)after addition of 1 equivalent of different metal ions in the darkconcentrations of SP1 and SP2:5.0×10-5mol·L-1,solvent:ethanol, temperature:293 K
Fig.1 and Fig.S5(Supporting Information)show the absorption spectra of SP1-SP4(5.0×10-5mol·L-1)in ethanol in the absence and the presence of 1 equivalent(equiv.)of different metal ions before and after UV irradiation.Interestingly,we found that the spectra for the solutions of SP2 and SP4 after addition of metal ions were significantly changed depending on the kind of metal ions(Figs.1b and S5a)whereas no obvious spectral changes were observed for control compounds SP1 and SP3(Figs.1a and S5b)before UV irradiation.In contrast, after further UV irradiation,SP1/SP3 showed the obvious absorption changes in the presences of different metal ions(Fig. S5(c,d))whereas SP2/SP4 showed the negligible spectral changes(data not shown).Tables 2 and S1 give a summary of the maximum absorption wavelength(λmax)of SP1-SP4 in the absence and the presence of different metal ions after UV irradiation and the corresponding changes in maximum absorption wavelengths(Δλmax).
On the basis of data in Tables 2 and S1,we found that the absorbance maxima of SP1 and SP3 in the presence of metal ions after UV irradiation showed the hyperchromatic shifts to different extents depending on different metal ions(Summaries of some important parameters for different metal ions can be found in Table S2 and Fig.S6).After separate additions of Fe3+, Cr3+,Cu2+,and Pb2+,the shoulder peaks at~400-450 nm with the large hypsochromic shifts of>100 nm appeared due to the formation of MC-Mn+complexes(Scheme 1),showing that the interactions between metal ions and MC are very strong.In the cases of Zn2+,Ni2+,Co2+,and Cd2+,only slight hypsochromic shifts of 9-14 nm were observed after addition of them,reflecting that the interactions between metal ions and MC are moderate.However,the maximum absorption wavelength(λmax)of MC did not change at all upon addition of Ca2+and Mg2+,showing that the interactions between Ca2+/Mg2+and MC are quite weak.Fig.S7(a,b)shows the corresponding photographs of SP1 upon addition of 1 equiv.of metal ions before and after UV irradiation,respectively,from which the observed color changes are consistent with UV-Vis absorption studies discussed above.Further irradiation of the UV-irradiated solutionsof SP1 and SP3 with visible light can turn all of them back to the original.In conjunction with UV-Vis studies before UV irradiation in Figs.1a and S5b,these results demonstrate that only the addition of metal ions can not lead to the conversion of SP1 and SP3 from the close form to the open form and that upon UV irradiation,metal ions can reversibly interact with the photoreleased negatively-charged phenolate oxygen with the different strengths and form MC-Mn+complexes.
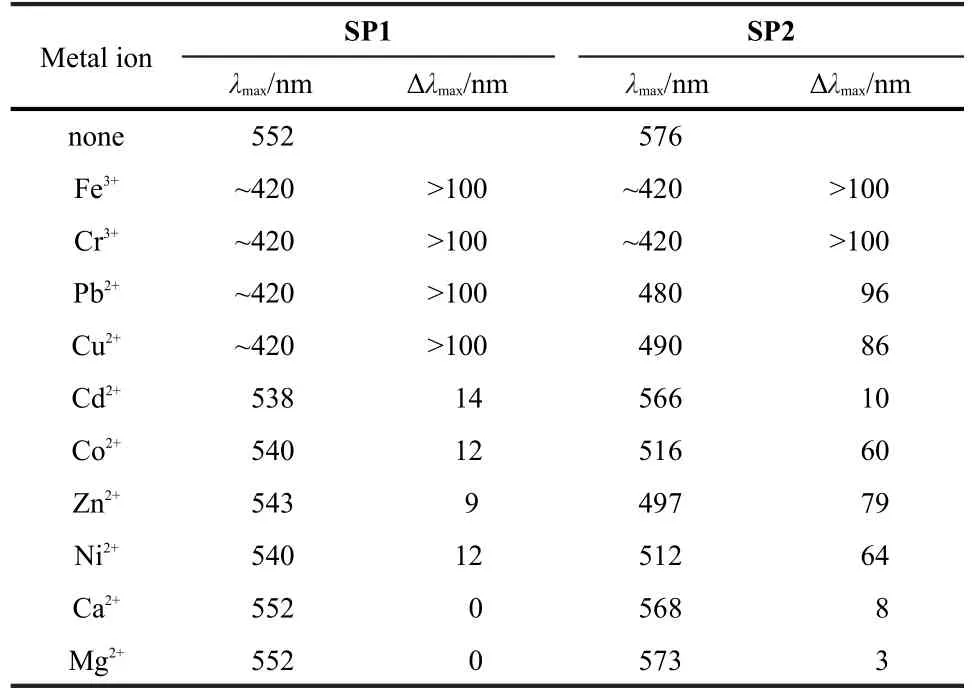
Table 2 Summaries of the maximum absorption wavelengths (λmax)of SP1 and SP2 in ethanol solution in the absence and presence of different metal ions and the corresponding changes in λmax(Δλmax)after UV irradiation
Remarkably,we found that only addition of metal ions led to the ring-opening isomerization of SP2 and SP4 with distinct color changes as shown in Figs.1b and S5a.For example,addition of Fe3+and Cr3+produced an immediate color changes from colorless to brilliant yellow.Correspondingly,a shoulder at about 420 nm,a significant hypsochromic shift in comparison with the open form,37was observed,which should be ascribed to MC-Mn+complexes as demonstrated above.Irradiating the colored solution with visible light did not liberate metal ions with regeneration of the original absorbance,showing the strong interactions between metal ions and MC.It is well known that metal ions with high charge density(Z2/r,where Z is the ion charge and r is the ionic radius)tend to form firm combinations with ligands.19,38-40Among metal ions studied here,both Cr3+and Fe3+possessing more charges and relatively smaller ionic radii afford a higher charge density,consistent with the experimental observation.In comparison with SP1 and SP3,we hypothesize that the metal-generated ring opening of SP should be ascribed to the synergistic effect of the strong affinity between metal ions and SP and the presence of the―OMe group that favors the formation of stable chelate complexes(MC-Mn+).When transition metals,such as Cu2+, Zn2+,Ni2+,Co2+and an IVA group metal ion Pb2+were used,the hypsochromic shifts in absorbance maxima in the range of 60-96 nm were detected.From Fig.S6,we can see that these metal ions have smaller but approximate charge density,thus resulting in the moderate binding between metal ions and MC that affords the reversible photochromism upon exposure to visible light.These also led to different hypsochromic shifts of λmaxof the resulted MC-Mn+complexes with the different colors that can be differentiated by a naked eye(Fig.S7(c,d)).For example,an orange color was observed for the Cu2+and Pb2+complexes with λmaxof 490 and 480 nm,respectively,while a pink color was observed for Ni2+and Co2+complexes with λmaxof 512 and 516 nm,respectively.The absorbance maxima of Zn2+complex(497 nm)was situated between them with a pink-orange color.In contrast,in the presence of Ca2+,Mg2+,and Cd2+, the absorption spectra change only slightly whenever spiropyran molecules were closed or open,reflecting that the interactions between metal ions and molecules are weak.For the alkaline-earth metal ions Mg2+and Ca2+,the missing of d electrons may decrease the coordination ability of metal ions with SP. Cd2+is another metal ion of group IIB similar to Zn2+,but the hypsochromic shift in absorbance maxima of Cd2+is smaller than that observed with Zn2+probably because of the larger size of Cd2+(ionic radius=97 pm)relative to Zn2+(ionic radius= 74 pm).The findings demonstrate that the interactions of metal ions with SP2 and SP4 are highly metal ion-dependent,thus potentially providing a useful colorimetric approach for detecting different metal ions with high sensitivity.
To further detect the capability of ion sensing,we investigated the emission properties of SP1-SP4.When excited at the maximum absorption wavelength of SP1-SP4/MC1-MC4,it was found that the closed forms SP1-SP4 had no emission while the zwitterionic opened forms MC1-MC4 fluoresced with the maximum absorption wavelengths of ca 626,650, 636,and 660 nm(Fig.2a),respectively,consistent with the previous report.41Importantly,addition of 1 equiv.of Zn2+to the SP2 solution led to a dramatic increase in emission intensity at 600 nm excited at λ=493 nm.Similar results were obtained with SP4(Fig.S8).The fluorescence increase is majorly due to the coordination of Zn2+with―OMe and fluorophores(pyridine or quinoline moiety)together with phenolate oxygen.The coordination can decrease the electron densities of the―OMe and the phenolate oxygen and thus increase the π-conjugation degree of the complex.On the other hand,the coordination of the ligands with the diamagnetic Zn2+containing a closed-shell d10electronic configuration would shut down the photoinduced electron-transfer pathway of the excited free ligand upon Zn2+coordination42-44and thus turn on the fluorescence.Another possibility is that the coordination would inhibit the photoinduced tautomerization of the phenolate moiety which leads to nonradiative deexcitation and thus improve the fluorescence by reducing the probability of radiationless relaxation.45,46
Addition of metal ions such as Pb2+,Mg2+,Cd2+to the solution of SP2 or SP4 also resulted in the fluorescence enhancement.However,the magnitude of the fluorescent enhancement of SP2 or SP4 in the presence of them is smaller than that observed with Zn2+,which could be attributed to the different binding affinities of pyridine and quinoline with them.Ca2+cannot turn on the fluorescence because of the larger ionic size (ionic radius=99 pm)and relative lower ionic electronegativity (1.01).Other metal ions studied,such as Fe3+,Cr3+,Cu2+,Co2+, and Ni2+,were unable to turn on the fluorescent signal of SP2 or SP4,which may be attributed to the proximity of the paramagnetic metal ions to the unpaired electrons of the ligands which lead to spin-orbit coupling and intersystem crossing.47To further explore the selectivity of Zn2+,competition experiments were also conducted in which solutions of SP2 or SP4 was first added with 1 equiv.of other metal ions separately and Zn2+was then added to the mixture.As shown in Figs.2d and S8b,the fluorescence of SP2 or SP4 after addition of Zn2+dramatically increased,demonstrating the excellent selectivity of Zn2+detection.It should be noted that the fluorescence increase was relatively small when Zn2+was added to the solution of SP2 in the presence of 1 equiv.of Fe3+,Cr3+,and Cu2+.This may be ascribed to the stronger coordination of the metal ions with the ligands as is already clear from the absorption spectra analysis.Finally,the bingding mode of the complex was studied by Jobʹs plot analysis.Fig.3a shows the typical UV-Vis spectroscopic responses of a SP2 ethanolic solution containing Zn2+with the increasing concentrations.The absorbance at 576 nm decreased and the absorbance at 350 and 497 nm increased with the concentration increase.The stoichiometry of the zinc complex has been investigated via Jobʹs method(Figs.3b and S9)and it has been found to be 1:1.Note that the detection of metal ions should be also performed in water or other polar solvents since SP1-SP4 could be readily soluble in these solvents with aid of ethanol.
3.3 Combinational logic circuits
As mentioned above,SP2 and SP4 response to the stimuli of metal ions and visible light,accompanying significant changes in physical and chemical properties.By taking use of these features,logic gates and combinational logic circuits can be constructed.It is well known that,the three basic types of logic gates are NOT,AND,and OR gates.The NOT gate is often called inverter which can converts the input signal(I)of 1 into the output signal(O)of 0 and vice versa.In the instant of AND gate,output O is 1 only when both inputs I1and I2are 1.The OR gate also combines the two inputs I1and I2into the output O,when I1and/or I2is 1,O is 1.Combinational logic circuits are assembled connecting NOT,AND,and OR gates.The inhibit(INH)gates are basicAND gates with one of the inputs inverted through a NOT function.Several examples of INH gate based on molecules have been reported in recent years.48-50Fig.4 illustrates that SP2(or SP4)can work as an INH logic gate upon the stimulation of metal ions and visible light.The two inputs signals are I1(Zn2+)and I2(Vis)and the output is O, the absorbance maxima of the complex(A497)or the fluorescence emission intensity at 600 nm(F600).According to the results of the spectral study,the increase in the absorbance maxima of the complex or the emission intensity at 600 nm is observed only in the presence of Zn2+and the absence of visible light.That is to say,only when I1=1 and I2=0,the output signal O=1.O is always 0 in other cases.
In particular,using the new photochromic compounds SP2 and SP4,we can develop more complicated combinational logic circuits to convert three inputs into two outputs.In the case of the combinational logic circuit shown in Fig.5,the three inputs signals are I1(Zn2+),I2(Ni2+),and I3(Vis)and the two outputs are O1(A497)and O2(F600).Binary digits can be encoded on each signal applying positive logic conventions(low=0,high= 1).Consequently,SP2(or SP4)can read a string of three binary inputs and write two specific optical outputs.The corresponding truth table and equivalent logic circuit are demonstrated in Fig.5.One portion of this logic circuit converts the three inputs I1,I2,and I3into the output O1through OR,NOT, and AND operations.The other fragment transduces the inputs of I1and I3into the output O2through NOT and AND operations.The optical output O1is high(O1=1)when only the input I1is applied(I1=1,I2=0,I3=0)or when only the input I2is ap-plied(I1=0,I2=1,I3=0)or when only the input I3is not applied (I1=1,I2=1,I3=0)(Fig.S10a).The optical output O2is high(O2= 1)when only the input I1is applied(I1=1,I2=0,I3=0)or when only the input I3is not applied(I1=1,I2=1,I3=0).The combinational logic circuit shows that all three inputs determine the output O1,while only I1and I3control the value of O2.

Fig.2 (a)Fluorescence emission spectra of SP1-SP4/MC1-MC4;(b)fluorescence emission spectra(λex=493 nm)of SP2(5.0×10-5mol·L-1, ethanol,293 K)upon addition of 1 equiv.of metal ions(Zn2+,Fe3+,Cr3+,Cu2+,Ni2+,Co2+,Cd2+,Ca2+,Mg2+,and Pb2+);(c)changes in fluorescence emission spectra(λex=493 nm)of SP2(5.0×10-5mol·L-1,ethanol,293 K)upon addition of different concentrations of Zn2+; (d)detection selectivity of SP2(5.0×10-5mol·L-1,ethanol,293 K)in the presence of various metal ions(λex=493 nm):(black bars) fluorescence emission intensity at 600 nm in the presence of 1 equiv.of Fe3+,Cr3+,Cu2+,Pb2+,Ni2+,Co2+,Cd2+,Ca2+,Mg2+,and Zn2+; (red bars)fluorescence emission intensity at 600 nm after further addition of 1 equiv.of Zn2+
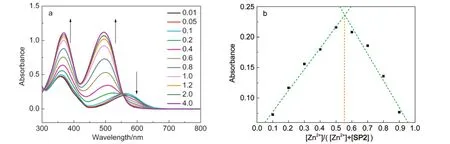
Fig.3 (a)UV-Vis spectroscopic response of SP2 ethanolsolution containing Zn2+with the increasing concentrations; (b)Jobʹs plot of SP2 with Zn2+in ethanol solution(b)Total concentration of[SP2]+[Zn2+]was kept constant.The absorbance at 497 nm was used.
As mentioned above,in the combinational logic circuit illustrated in Fig.5,one of the output O2was not affected by the input I2,while in the instance of the logic circuit shown in Fig.6, both the outputs O1and O2are dependent on the three inputs I1, I2,and I3.The combinational logic circuit also consists of three inputs,two of which are chemical inputs I1(Zn2+)and I2(Cu2+) and the other is optical input I3(Vis),the two outputs are O1(A497)and O2(F600).When positive conventions are applied to all signals,the two independent optical outputs(O1and O2)can be modulated by stimulating the molecular switch(SP2 or SP4) with the three terminal inputs(I1,I2,and I3).According to the fluorescence spectral study,addition of Cu2+to the solution of SP2(or SP4)containing 1 equiv.Zn2+can cause the fluorescence quenching.Then,the output O2is closely related to the presence and the absence of Cu2+.Therefore,the output O2is high when only the input I1is applied(I1=1,I2=0,I3=0),and both I2and I3are inhibiting factors to O2.As for the other output O1,the absorbance maximum of the complex is high upon addition of Zn2+and 1 equiv.Cu2+in the absence of visible light (Fig.S10b).Accordingly,O1is high(O1=1)when only I1is applied(I1=1,I2=0,I3=0)or when only I2is applied(I1=0,I2=1,I3= 0)or when only I3is not applied(I1=1,I2=1,I3=0).It can be seen from the truth table and the corresponding logic circuit, the inputs I1,I2and I3are transmitted into output O1through OR,NOT,and AND operations while the output O2through NOT andAND operations.
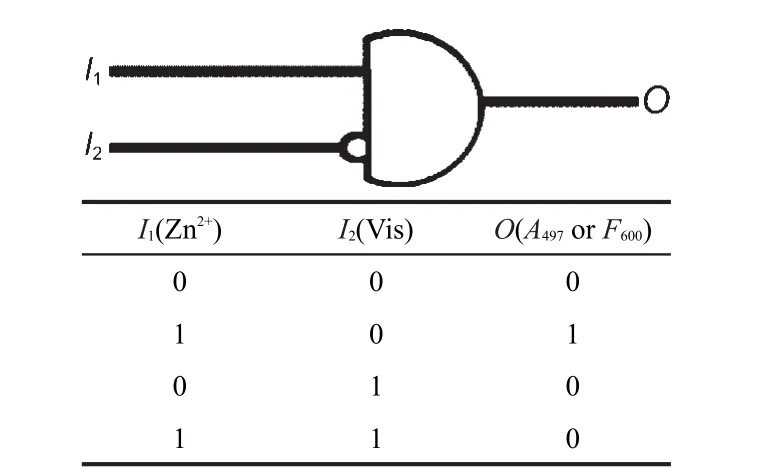
Fig.4 Truth table(bottom)and INH logic gate with two inputs and one output(up)
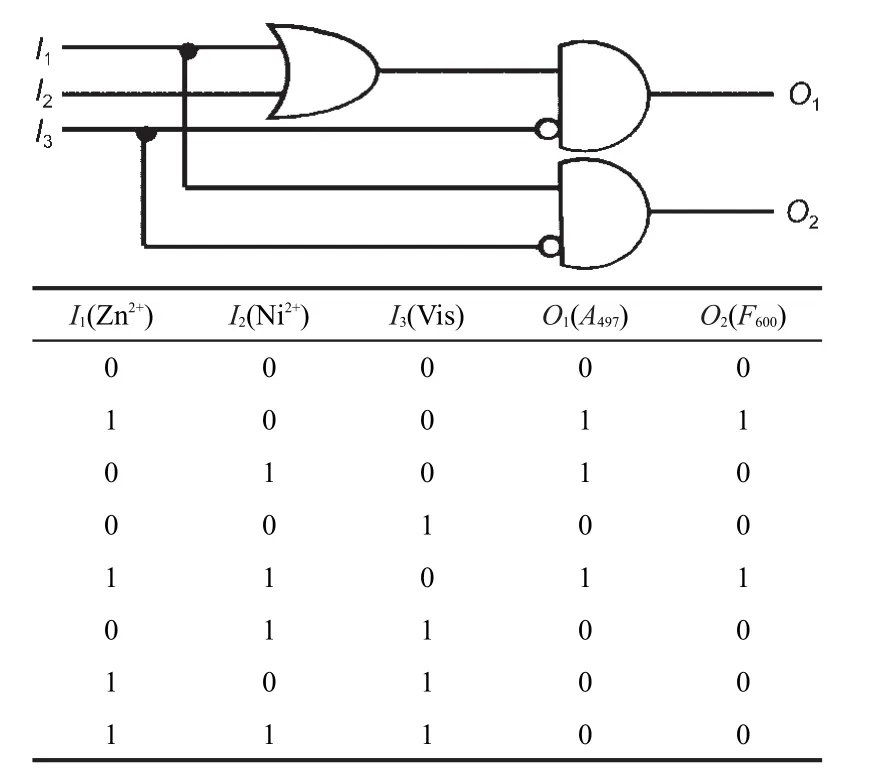
Fig.5 Truth table(bottom)and corresponding combinational logic circuits with three inputs and two outputs(up)The three inputs are I1(Zn2+),I2(Ni2+),and I3(Vis)and the two outputs are O1(A497)and O2(F600).
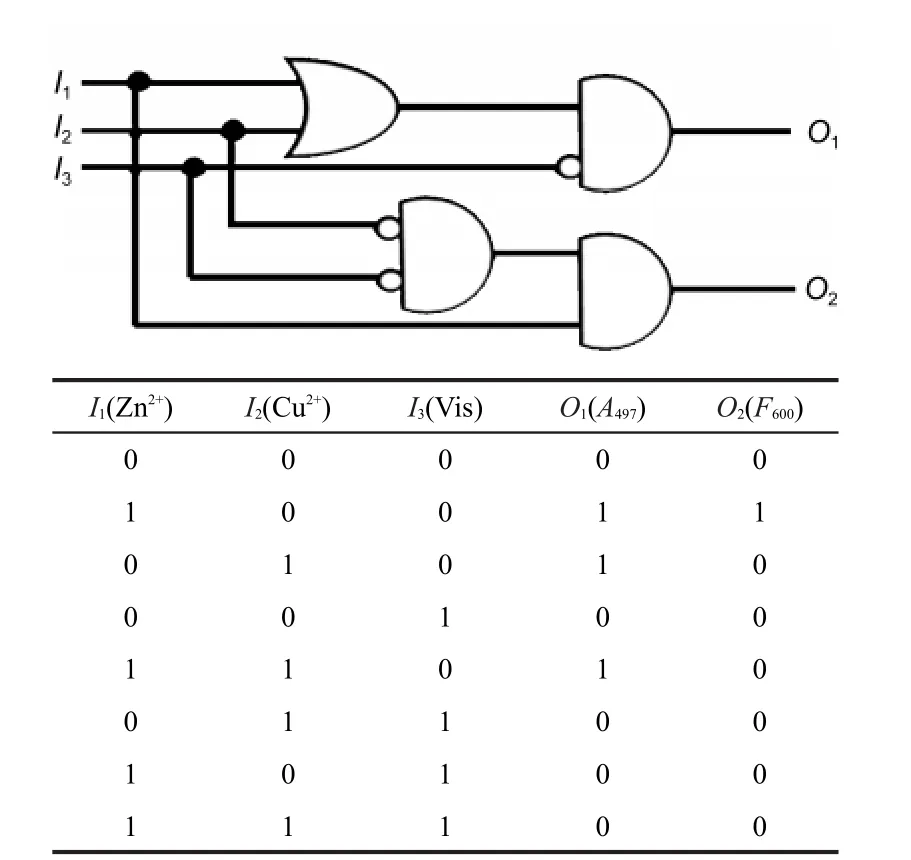
Fig.6 Truth table(bottom)and the equivalent logic circuits based on SP2 or SP4(up)The three inputs are I1(Zn2+),I2(Cu2+)and I3(Vis)and the two outputs are O1(A497)and O2(F600).
4 Conclusions
In this work,we demonstrate the use of spiropyran derivatives incorporating the chelating sites,such as the pyridine or quinoline moiety and methoxy group,into their backbones for creating chemical sensors with high sensitivity and selectivity and combinational logic gates for information processing at the molecular level.The coordination of metal ions with―OMe and pyridine or quinoline moiety facilitates the photoisomerization of spiropyran molecules from the closed form to the open merocyanine form with and even without UV irradiation,accompanying with the significant changes in their chemical and physical properties.UV-Vis absorption studies demonstrated that SP2 and SP4 showed the metal ion-dependent reversible binding affinities that led to the different hypsochromic shifts of the absorption of MC-Mn+complexes with different colors. The color changes can be recognized by a naked eye,thus offering an easy colorimetric method for metal ion detection.On the other hand,the fluorescence measurements proved the unique property of SP2 and SP4 for selectively detecting Zn2+with high sensitivity.In combination with both UV-Vis absorption and fluorescence changes under external stimuli,molecular systems based on SP2 and SP4 have been configured to mimic the functions of several integrated logic gates,suggesting attractive prospects in detecting,elaborating,and transmitting signals at the molecular level or even future molecular computing.
(1) Irie,M.Chem.Rev.2000,100,1685.doi:10.1021/cr980069d
(2) Irie,M.;Fukaminato,T.;Sasaki,T.;Tamai,N.;Kawai,T.Nature 2002,420,759.doi:10.1038/420759a
(3) de Silva,A.P.;Gunaratne,H.Q.N.;Gunnlaugsson,T.;Huxley, A.J.M.;McCoy,C.P.;Rademacher,J.T.;Rice,T.E.Chem. Rev.1997,97,1515.doi:10.1021/cr960386p
(4) Kocer,A.;Walko,M.;Meijberg,W.;Feringa,B.L.Science 2005,309,755.doi:10.1126/science.1114760
(5) Berkovic,G.;Krongauz,V.;Weiss,V.Chem.Rev.2000,100, 1741.doi:10.1021/cr9800715
(6) Guo,X.;Zhang,D.;Zhu,D.Adv.Mater.2004,16,125.doi: 10.1002/adma.200306102
(7) Kawata,S.;Kawata,Y.Chem.Rev.2000,100,1777.doi: 10.1021/cr980073p
(8) Delaire,J.A.;Nakatani,K.Chem.Rev.2000,100,1817.doi: 10.1021/cr980078m
(9)Tamai,N.;Miyasaka,H.Chem.Rev.2000,100,1875.doi: 10.1021/cr9800816
(10)Guo,X.;Zhang,D.;Yu,G.;Wan,M.;Li,J.;Liu,Y.;Zhu,D. Adv.Mater.2004,16,636.doi:10.1002/adma.200305792
(11) Shen,Q.;Wang,L.;Liu,S.;Cao,Y.;Gan,L.;Guo,X.; Steigerwald,M.L.;Shuai,Z.;Liu,Z.;Nuckolls,C.Adv.Mater. 2010,22,3282.doi:10.1002/adma.201000471
(12)Guo,X.;Huang,L.;OʹBrien,S.;Kim,P.;Nuckolls,C.J.Am. Chem.Soc.2005,127,15045.doi:10.1021/ja054335y
(13) Lee,H.Y.;Diehn,K.K.;Sun,K.;Chen,T.;Raghavan,S.R. J.Am.Chem.Soc.2011,133,8461.doi:10.1021/ja202412z
(14) Vlassiouk,I.;Park,C.D.;Vail,S.A.;Gust,D.;Smirnov,S. Nano Lett.2006,6,1013.doi:10.1021/nl060313d
(15) Guo,X.;Zhang,D.;Tao,H.;Zhu,D.Org.Lett.2004,6,2491. doi:10.1021/ol0494111
(16) Zhang,H.;Guo,X.;Hui,J.;Hu,S.;Xu,W.;Zhu,D.Nano Lett. 2011,11,4939.doi:10.1021/nl2028798
(17) Jiang,G.;Song,Y.;Guo,X.;Zhang,D.;Zhu,D.Adv.Mater. 2008,20,2888.doi:10.1002/adma.200800666
(18)Shao,N.;Zhang,Y.;Cheung,S.;Yang,R.;Chan,W.;Mo,T.;Li, K.;Liu,F.Anal.Chem.2005,77,7294.doi:10.1021/ac051010r (19)Sakamoto,H.;Takagaki,H.;Nakamura,M.;Kimura,K.Anal. Chem.2005,77,1999.doi:10.1021/ac048642i
(20)Inouye,M.;Akamatsu,K.;Nakazumi,H.J.Am.Chem.Soc. 1997,119,9160.doi:10.1021/ja9707668
(21) Shiraishi,Y.;Adachi,K.;Itoh,M.;Hirai,T.Org.Lett.2009,11, 3482.doi:10.1021/ol901399a
(22)Takase,M.;Inouye,M.Chem.Commun.2001,2432.
(23) Shao,N.;Jin,J.Y.;Cheung,S.M.;Yang,R.H.;Chan,W.H.; Mo,T.Angew.Chem.Int.Edit.2006,45,4944.doi:10.1002/ anie.200600112
(24)Andersson,J.;Li,S.;Lincoln,P.;Andréasson,J.J.Am.Chem. Soc.2008,130,11836.doi:10.1021/ja801968f
(25) de Silva,A.P.;Gunaratne,H.Q.N.;McCoy,C.P.Nature 1993, 364,42.doi:10.1038/364042a0
(26)de Silva,A.P.;Gunaratne,H.Q.N.;McCoy,C.P.J.Am.Chem. Soc.1997,119,7891.doi:10.1021/ja9712229
(27)de Silva,A.P.;McClenaghan,N.D.J.Am.Chem.Soc.2000, 122,3965.doi:10.1021/ja994080m
(28) Guo,X.;Zhang,D.;Zhou,Y.;Zhu,D.J.Org.Chem.2003,68, 5681.doi:10.1021/jo034243w
(29) Raymo,F.M.Adv.Mater.2002,14,401.doi:10.1002/1521-4095(20020318)14:6<401::AID-ADMA401>3.0.CO;2-F
(30) Guo,X.;Zhang,D.;Zhang,G.;Zhu,D.J.Phys.Chem.B.2004, 108,11942.doi:10.1021/jp047706q
(31)Guo,X.;Zhang,D.;Wang,T.;Zhu,D.Chem.Commun.2003, 914.
(32)Raymo,F.M.;Giordani,S.J.Am.Chem.Soc.2001,123,4651. doi:10.1021/ja005699n
(33) Hirano,M.;Osakada,K.;Nohira,H.;Miyashita,A.J.Org. Chem.2001,67,533.
(34) Guo,X.;Zhou,Y.;Zhang,D.;Yin,B.;Liu,Z.;Liu,C.;Lu,Z.; Huang,Y.;Zhu,D.J.Org.Chem.2004,69,8924.doi:10.1021/ jo0487799
(35) Shao,N.;Jin,J.;Wang,H.;Zheng,J.;Yang,R.;Chan,W.; Abliz,Z.J.Am.Chem.Soc.2009,132,725.
(36) Natali,M.;Soldi,L.;Giordani,S.Tetrahedron 2010,66,7612. doi:10.1016/j.tet.2010.07.035
(37) Fries,K.H.;Driskell,J.D.;Samanta,S.;Locklin,J.Anal. Chem.2010,82,3306.doi:10.1021/ac1001004
(38) Paramonov,S.V.;Lokshin,V.;Fedorova,O.A.J.Photochem. Photobiol.C:Photochem.Rev.2011,12,209.doi:10.1016/j. jphotochemrev.2011.09.001
(39) Poonia,N.S.;Bajaj,A.V.Chem.Rev.1979,79,389.doi: 10.1021/cr60321a002
(40)Abdullah,A.;Roxburgh,C.J.;Sammes,P.G.Dyes and Pigments 2008,76,319.doi:10.1016/j.dyepig.2006.09.002
(41) Ipe,B.I.;Mahima,S.;Thomas,K.G.J.Am.Chem.Soc.2003, 125,7174.doi:10.1021/ja0341182
(42) Nolan,E.M.;Lippard,S.J.Accounts Chem.Res.2008,42,193.
(43) Huang,S.;Clark,R.J.;Zhu,L.Org.Lett.2007,9,4999.doi: 10.1021/ol702208y
(44) Kowalczyk,T.;Lin,Z.;Voorhis,T.V.J.Phys.Chem.A 2010, 114,10427.
(45)Winkler,J.D.;Bowen,C.M.;Michelet,V.J.Am.Chem.Soc. 1998,120,3237.doi:10.1021/ja974181p
(46) Zhao,J.;Nelson,D.J.J.Inorg.Biochem.2005,99,383.doi: 10.1016/j.jinorgbio.2004.10.005
(47)Torrado,A.;Walkup,G.K.;Imperiali,B.J.Am.Chem.Soc. 1998,120,609.doi:10.1021/ja973357k
(48) Saghatelian,A.;Völcker,N.H.;Guckian,K.M.;Lin,V.S.Y.; Ghadiri,M.R.J.Am.Chem.Soc.2002,125,346.
(49)Qu,D.H.;Ji,F.Y.;Wang,Q.C.;Tian,H.Adv.Mater.2006,18, 2035.doi:10.1002/adma.200600235
(50) de Sousa,M.;Kluciar,M.;Abad,S.;Miranda,M.A.;de Castro, B.;Pischel,U.Photochem.Photobiol.Sci.2004,3,639.doi: 10.1039/b406415a
March 31,2012;Revised:May 14,2012;Published on Web:May 15,2012.
New Spiropyran Derivatives:Ion Sensing and Information Processing at the Molecular Level
LI Ying-Ruo1ZHANG Hong-Tao2QI Chuan-Min1,*GUO Xue-Feng2,3,*
(1Key Laboratory of Radiopharmaceuticals,College of Chemistry,Beijing Normal University,Beijing 100875,P.R.China;2Beijing National Laboratory for Molecular Sciences,State Key Laboratory for Structural Chemistry of Unstable and Stable Species, College of Chemistry and Molecular Engineering,Peking University,Beijing 100871,P.R.China;3Department of Advanced Materials and Nanotechnology,College of Engineering,Peking University,Beijing 100871,P.R.China)
We have designed and synthesized a new class of spiropyran derivatives(SP1-SP4)with functional chelating groups,such as pyridine or quinoline moieties and a methoxy group(―OMe),for use in metal ion sensing and information processing at the molecular level.It is notable that metal ions can favor coordination with chelating groups and facilitate the photoisomerization of spiropyran molecules from the closed form to the open merocyanine form without UV irradiation,thus leading to significant changes in their chemical and physical properties.UV-Vis absorption studies indicated that SP2 and SP4 exhibited metal ion-dependent reversible binding affinities that result in different hypsochromic shifts for the MC-Mn+complexes.These changes in color can be recognized by eye,thus offering an easy colorimetric method for metal ion detection.Further emission studies distinguished them as promising candidates for Zn2+detection with good sensitivity and selectivity.Moreover,on the basis of their absorption and fluorescence spectra,several combinational logic gates were constructed for information processing at the molecular level.These results demonstrate that spiropyran derivatives with desired functionalities show great potential not only for chemical or environmental sensors,but also for future molecular computing.
Spiropyran;Chemical sensor;Logic gate;UV-Vis absorption spectrum;Fluorescent spectrum
10.3866/PKU.WHXB201205155
∗Corresponding authors.GUO Xue-Feng,Email:guoxf@pku.edu.cn.QI Chuan-Min,Email:qichuanmin@bnu.edu.cn.
The project was supported by the National Key Basic Research Program of China(973)(2009CB623703,2012CB921404),National Natural Science Foundation of China(20833001,51121091,2112016,21071022),and Foundation for theAuthor of National Excellent Doctoral Dissertation of Higher Education,China(2007B21).
国家重点基础研究发展规划项目(973)(2009CB623703,2012CB921404),国家自然科学基金(20833001,51121091,2112016,21071022)及全国高等学校优秀博士论文作者专项基金(2007B21)资助
O641
