双光子敏化Eu3+高效发光活细胞成像纳米生物探针
2012-12-11符小艺邵光胜韩荣成薛富民帆付立民张建平
符小艺 邵光胜 韩荣成 马 严 薛富民 杨 帆付立民 张建平 王 远,*
(1北京分子科学国家实验室,分子动态与稳态国家重点实验室,北京大学化学与分子工程学院,北京100871; 2华南理工大学材料科学与工程学院,广州510640;3中国人民大学化学系,北京100872)
双光子敏化Eu3+高效发光活细胞成像纳米生物探针
符小艺1,2,†邵光胜1,†韩荣成1,†马 严1薛富民1杨 帆3付立民3张建平3王 远1,*
(1北京分子科学国家实验室,分子动态与稳态国家重点实验室,北京大学化学与分子工程学院,北京100871;2华南理工大学材料科学与工程学院,广州510640;3中国人民大学化学系,北京100872)
将Eu(tta)3dpbt(dpbt:2-(N,N-diethylanilin-4-yl)-4,6-bis(3,5-dimethylpyrazol-1-yl)-1,3,5-triazine;tta: thenoyltrifluoroacetonato)包埋在甲基丙烯酸甲酯-苯乙烯共聚物、正辛基三甲氧基硅及其水解缩合产物组成的杂化基质中,制备了Eu(tta)3dpbt质量分数为40%的荧光纳米粒子,其平均粒径为45 nm.所制备的发光纳米粒子在水中分散稳定性高、光稳定性好、细胞毒性低、长波敏化Eu3+发光性能优良,适宜作为生物分析的发光标记物.所制备的发光纳米粒子的可见区激发峰位于415 nm,激发峰尾部延展至475 nm,其发光量子产率为0.31 (λex=415 nm,T=23°C),最大双光子激发作用截面为5.0×105GM(λex=830 nm,1 GM=10-50cm4·s·photo-1· particle-1).以转铁蛋白修饰上述发光纳米粒子表面制备的纳米生物探针被成功应用于活的HeLa肿瘤细胞的特异性标记和双光子激发Eu3+发光成像.
发光;铕配合物;双光子激发;纳米生物探针;细胞成像
1 Introduction
Bioprobes based on two-photon-sensitized(TPS)luminescence of Eu3+have been attracting intensive attention in the fields of material science and bioanalysis because of the anticipation that two-photon excitation bioimaging based on such probes will combine the advantages of high sensitivity,high signal-to-noise ratio,deep penetration,as well as low photodamage to living organism.1-5The superior luminescent properties of europium complexes are characterized by the narrow-line emission of Eu3+in the red-light region,with acceptable transparence for many biosamples,large Stokes shifts,and long luminescence lifetimes.3-5Remarkable progresses have been achieved in the design and synthesis of Eu3+complexes with excellent two-photon excitation(TPE)luminescence properties,4,6-17and several ones have been successfully applied in the multi-photon-excitation bio-imaging of cells.8,9,18In comparison with free Eu3+complex molecules,bionanoprobes prepared by encapsulating proper Eu3+complexes into water-dispersible and biocompatible nanoparticles possess the additional benefits of markedly enhanced luminescent brightness, chemical stability and photostability,as well as lower cytotoxicity.4,19,20The previously reported complex Eu(tta)3dpbt21(dpbt:2-(N,N-diethylanilin-4-yl)-4,6-bis(3,5-dimethylpyrazol-1-yl)-1,3,5-triazine;tta:thenoyltrifluoroacetonato,Scheme S1(see Supporting Information))is one of the most proper dyes for preparing bionanoprobes with desirable two-photon-sensitized Eu3+luminescence properties in view of its high molecular TPE action cross section(δ×Φ,82 GM at 808 nm,1 GM=10-50cm4·s· photon-1·molecule-1),and the excellent TPE luminescent properties of its molecular aggregates.22,23However,the attempts to prepare desired nanoprobes encapsulating Eu(tta)3dpbt or its derivatives16,24by conventional encapsulation methods such as microemulsion polymerization25or Stöber method26failed,due to the dissociation of the complexes and loss of their intrinsic luminescence properties during the encapsulation processes.
Recently,we reported a co-precipitation-assembly method for preparing nanospheres encapsulating 10%(w)of Eu(tta)3dpbt (EuLNPs)which exhibited fine TPE Eu3+luminescence properties with a maximal δ×Φ value for the nanospheres of 1.2×105GM at 825 nm.Bionanoprobes using EuLNPs as the luminescent marker have been successfully applied in the TPE cell-selective-imaging of live cancer cells.20We believe that the two-photon excitation imaging quality based on nanoprobes encapsulating Eu(tta)3dpbt could be further improved by increasing the particlesʹTPE action cross section,which could be realized by augmenting the Eu(tta)3dpbt content of the nanospheres.However,with the previously reported experimental conditions,the preparation of nanospheres containing more than 15%(w)of Eu(tta)3dpbt produced a large amount of precipitates.
Herein we report the preparation and luminescent properties of new hybrid nanospheres with high Eu(tta)3dpbt loading (40%(w))and excellent dispersion-stability in water(EuPHS) which were prepared by a co-precipitation-condensation method.27,28The two-photon-sensitized Eu3+luminescence properties of EuPHS were measured.And the tumor cell targeting behavior of transferrin-modified EuPHS(Tf-EuPHS)was observed using a non-invasive two-photon excited(TPE)luminescence imaging.Receptor-mediated tumor cell targeting behavior of Tf-EuPHS was performed in vitro by inhibition with NaN3and 2-deoxy-D-glucose.In addition,the biocompatibility of EuPHS was also evaluated with cytotoxicity tests.
2 Materials and methods
2.1 Materials
Eu(tta)3dpbt was synthesized according to the method reported previously.21Octyltrimethoxysilane(OTS,97%)was purchased from Alfa Aesar.Poly(styrene-co-methyl methacrylate) (P(ST-co-MMA),40%styrene,Mw~100000-150000)was purchased from Aldrich.Transferrin(>98%)purchased from Sigma,cetyltrimethyl ammonium bromide(≥99%)obtained from Acros Organics,and tris(hydroxymethyl)aminomethane from Amresco were used without further purification.Other chemicals ofAR grade were used as received.
2.2 Preparation of EuPHS
A colloidal solution of EuPHS was prepared by a co-precipitation-condensation method.27,28In a typical experiment,2.5 mL of acetone solution containing OTS(1.5×10-3mol·L-1), P(ST-co-MMA)(0.16 g·L-1),and Eu(tta)3dpbt(1.3×10-4mol· L-1)was dropwise added into an aqueous solution of cetyltrimethylammonium bromide(CTAB)(7.0 mL,1.4×10-3mol· L-1)under stirring at room temperature.The mixture was further stirred for about 15 min to produce a yellow colloidal solution.To remove large particles,the as-prepared colloidal solution was centrifuged at 10000 g.The supernate was then treated by centrifuging at 40000 g,and the obtained precipitation was re-dispersed in water of 8 mL to produce a colloidal solution of EuPHS.This process was repeated to remove most of CTAB and produce a stable colloid solution of EuPHS(about 52.5 mg·L-1,corresponding to a yield of EuPHS of 30%(w), see Supporting Information for details)with an average diameter of 45 nm as measured by transmission electron microscopy (TEM).A colloidal solution of nanoparticles(EuHS)was also prepared by the aforementioned processes except for the addition of P(ST-co-MMA).
2.3 Preparation of bionanoprobes
The obtained colloidal solution of EuPHS(5 mL)was mixed with a solution of transferrin(0.2 mg in 0.2 mL water),and the mixture was incubated at 25°C for 2 h in a thermomix shaker shaking at 300 r·min-1.After being separated by centrifuging (40000 g,5 min)and washed with Tris-HCl buffer(10 mmol· L-1,pH 7.8)twice,the resulting EuPHS modified with transferrin(Tf-EuPHS)were dispersed in 5 mL of Tris-HCl buffer to obtain a colloid solution which was stored at 4°C for use.
2.4 Cytotoxicity test
The cytotoxicity measurements were performed using MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide)assay.Briefly,HeLa cells were trypsinized and resuspended in Dulbeccoʹs modified Eagleʹs medium(DMEM)containing 10%(V/V)fetal bovine serum(FBS)and 80 U·mL-1gentamycin sulfate.The cells were seeded at a density of 0.2-1.0 million cells per well in a 96-well plate.After 24 h of incubation at 37°C in 5%CO2,the cells were washed 3 times with phosphate buffered solution(PBS,pH 7.4).Colloidal solutions of EuPHS or Tf-EuPHS with different concentrations (100 μL)were added to the wells.After 20 h of incubation at 37°C in 5%CO2,a solution of MTT(20 μL,5.0 mg·mL-1) was added to each well.After 4 h of incubation at 37°C in 5% CO2,the medium was discarded,and the intracellular precipitate of formazan was collected by dimethylsulfoxide(DMSO) (100 μL).The absorbance at 570 nm was measured on a Bio-Rad Model 550 microplate reader.Each data point was collected by averaging the absorbance values of six wells,and the untreated cells were used as controls.Statistical significance was assessed by the two-sample Studentʹs t-test using SPSS 13.0;P values(level of significance)(<0.01)were considered statistically significant.
2.5 Cellular uptake
The effects of NaN3and 2-deoxy-D-glucose on the cellular uptake of Tf-EuPHS were examined.The cells were pre-incubated in PBS buffer solution and supplemented with 10 mmol· L-1NaN3and 50 mmol·L-12-deoxy-D-glucose for 30 min at 37°C followed by incubation in a solution of Tf-EuPHS.
2.6 Cell culture and imaging
HeLa cells were propagated in Dulbeccoʹs modified Eagleʹs medium(DMEM)supplemented with 10%(V/V)fetal bovine serum and 80 U·mL-1gentamycin sulfate.Then the cultured cells were trypsinized and re-suspended in this DMEM at a concentration of about 7.5×105mL-1.The cell suspension(100 μL)was transferred to a confocal dish(35 mm).After incubation for 24 h at 37°C in 5%CO2,the cells were carefully rinsed with PBS solution(pH 7.4).Then a colloidal solution of Tf-EuPHS(100 μL,0.2 mg·mL-1)was added.After 3 h incubation at 37°C in 5%CO2,the dish was rinsed three times with PBS solution(pH 7.4)and then 1 mL of fresh serum-free medium was added.The plates were incubated for another 10 min at 37°C and then directly imaged on an upright confocal microscope(Leica TCS SP5)equipped with a femtosecond Ti:sapphire laser and a 20×water immersion objective(Carl Zeiss). The two-photon-excitation luminescence images were taken upon femtosecond 800 nm irradiation(δ×Φ800nm=2.5×105GM) with an average laser power of 10 mW,and integration of Eu3+luminescence in the spectral range from 590 to 620 nm.For comparison,all of the confocal images were taken at the same setting.Transmitted light differential interference contrast (DIC)images were taken on the same instrument.
2.7 Characterizations
Transmission electron microscopy(TEM)and high-resolution transmission electron microscopy(HRTEM)images were taken on a transmission electron microscope(JEM 2000FX,Hitachi,Japan)and a field emission microscope(Tecnai F30, FEI,Netherlands),respectively.Energy dispersive X-ray spectroscopy(EDX)measurements and elemental mapping were carried out on a field emission transmission microscope(Tecnai G2F20 U-TWIN,Netherlands)with energy dispersive X-ray spectroscope(EDX,EPMA-1600,Shimadzu,Japan). UV-Vis absorption and photoluminescence measurements were carried out on an absorption spectrometer(CARY 1E,Varian, German)and a fluorescence spectrophotometer(F-4500,Hitachi,Japan).The photoluminescence decay kinetics of EuPHS colloidal nanoparticles was measured by FLS920(Edinburgh Instruments,German).Luminescence quantum yield(Φ)of the prepared nanoparticles was determined according to the method described by Demas and Grosby,29using DCM(4-dicyanomethylene-2-methyl-6-p-dimethylaminostyryl-4H-pyran)in npropanol(Φ=0.57±0.02)as the reference.The photobleaching experiments were carried out on a fluorescence spectrophotometer using a 150 W xenon lamp as an excitation source.Colloidal solutions of the nanoparticles were continuously irradiated, and the emission intensity was recorded at 8 s intervals.The measurements of TPE action cross sections on the colloidal solution of EuPHS were conducted according to the method reported previously,22using Rhodamine B(RhB)as a standard with known two-photo-absorption cross sections.
3 Results and discussion
3.1 Preparation and characterization of EuPHS
A colloidal solution of luminescent nanoparticles encapsulating Eu(tta)3dpbt was prepared by adding dropwise of an acetone solution containing P(ST-co-MMA),OTS,and Eu(tta)3dpbt to an aqueous solution of CTAB under stirring.The prepared colloidal solution was centrifuged to get a precipitate of Eu-PHS which was re-dispersed in water or a Tris-HCl buffer solution(10 mmol·L-1,pH 7.8)to form colloidal solutions.The finally obtained colloidal solutions were very stable and no precipitate was observed after standing for months(see Experimental Section).The prepared EuPHS were composed of 40% (w)of Eu(tta)3dpbt,10%(w)of OTS,35%(w)of poly(octylsi-loxane),and 15%(w)of P(ST-co-MMA)as estimated from the results of elemental analysis,gas chromatography,and inductively coupled plasma atomic emission spectroscopy (ICP-AES)(see Supporting Information for details).The Eu(tta)3dpbt content of EuPHS is 4 times as high as that in the previously reported EuLNPs nanospheres.20
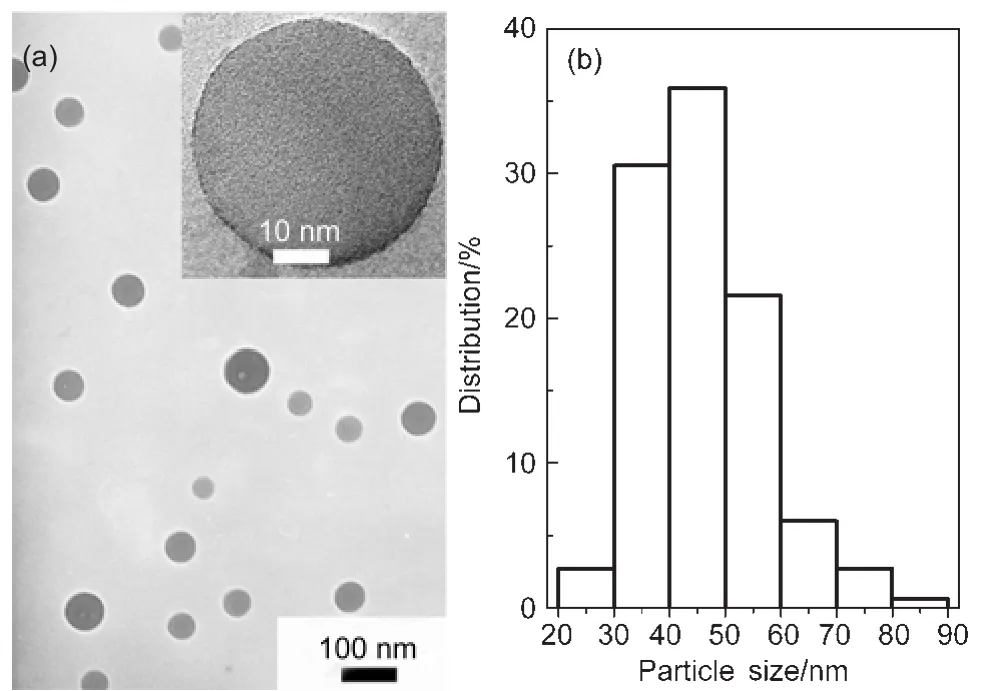
Fig.1 Representative TEM image(a)and the size distribution(b) of EuPHS(N=334)The inset shows a higher resolution image of one of EuPHS.
Fig.1 shows the TEM,HRTEM images(inset),and the size distribution of EuPHS which are spherical and 45 nm in diameter.EDX measurements on individual EuPHS revealed that they were composed of C,Si,Eu,and S(Fig.S1(see Supporting Information)).Elemental mapping conducted by EDX in a scanning transmission electron microscope(STEM)indicated that the C and Eu distributions among the nanospheres were homogeneous,while Si was enriched in the surface layer of the nanoparticles(Fig.2).These results suggest that Eu(tta)3dpbt molecules or small molecular aggregates are well dispersed in the matrix of the encapsulation materials,and hydrolysis products of octyltrimethoxysilane(poly(octylsiloxane))are enriched in the surfaces of the nanoshperes.
Since the precipitation of P(ST-co-MMA)in the solution was a quick process,while the hydrolysis of OTS in the given conditions was a slow one,we believe that the formation process of the EuPHS consists of two steps.When the acetone solution was added into the aqueous solution of CTAB,nanoparticles of the hydrophobic polymer P(ST-co-MMA)containing hydrophobic Eu(tta)3dpbt and OTS quickly formed by precipitation.Then the hydrolysis of OTS around the surfaces of the preformed nanoparticles occurred,which was accompanied by a condensation reaction of the hydrolysis products to form poly(octylsiloxane)shells covered on the cores.As measured by gas chromatography and ICP-AES(see Supporting Information for details),the molar ratio of OTS to the hydrolyzed OTS in EuPHS was about 1:4.Therefore,it is reasonable to deduce that the cores of EuPHS nanoparticles are mainly composed of P(ST-co-MMA),Eu(tta)3dpbt,and OTS.It was found that OTS played a key role in the formation of the spherical nanoparticles.The nanoparticles prepared in the absence of OTS were random in shape as shown in their TEM images(Fig.S2(see Supporting Information)).
3.2 Luminescence properties of EuPHS
Fig.3 shows the excitation and luminescence spectra of Eu(tta)3dpbt in toluene and in EuPHS nanospheres dispersed in an aqueous solution.The excitation peak(λem=614 nm)of Eu(tta)3dpbt in toluene,related to the sensitization effect of coordinated dpbt,locates at 402 nm,while that in EuPHS red-shifts to 415 nm,with an edge of this band extending up to 475 nm.The luminescence spectrum of the toluene solution of Eu(tta)3dpbt displays a small peak centered at 440 nm which is the emitting band of free dpbt derived from the dissociation of Eu(tta)3dpbt.21In contrast,this fluorescence signal of dpbt could not be observed in the luminescence spectrum of EuPHS, indicating the intactness of coordination structure between Eu3+and dpbt in EuPHS.
The photoluminescence decay kinetics at the probing wavelength of 614 nm could be accounted by a two-exponential decay model function,which yielded the apparent decay time constants of 51µs(8%)and 508µs(92%)(Fig.S3(see Supporting Information)).The phenomena of red-shift in the excitation peak and the multi-exponential decay were also observed in the previously reported nanosized Eu(tta)3dpbt congeries prepared by a precipitation method,22which could be explained by the formation of J-type aggregates of the polar Eu(tta)3dpbt molecules.
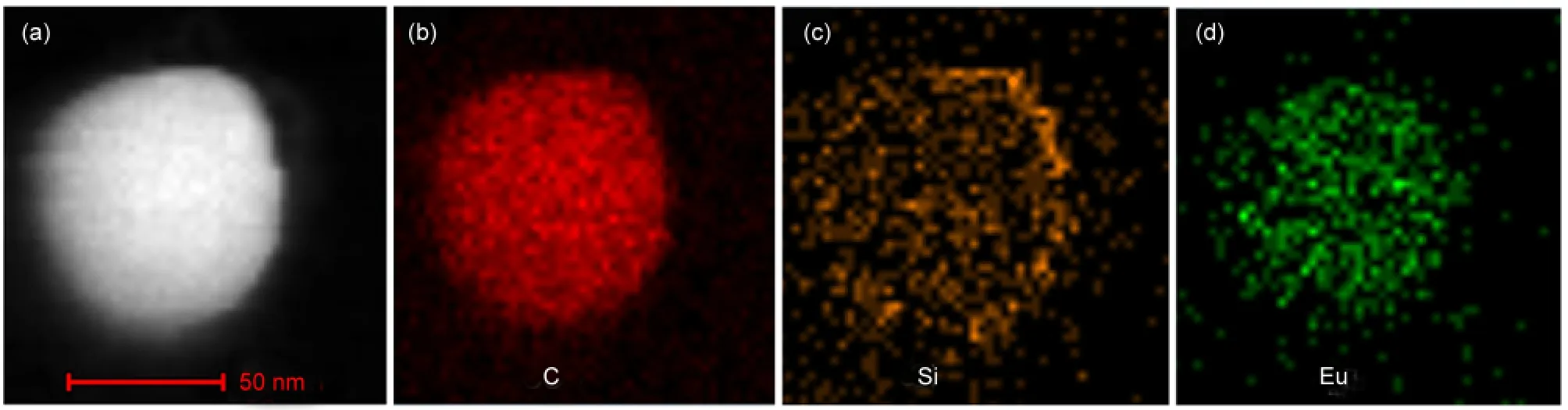
Fig.2 STEM image(a)and the elemental mapping of C(b),Si(c),and Eu(d)of one of EuPHS
As shown in Fig.4,the UV-Vis absorption spectrum of EuPHS in the colloidal solution shows a maximal absorption peak centered at 415 nm with a molar extinction coefficient(ε) of 2.4×104mol-1·L·cm-1.The luminescence quantum yield(Φ) for the Eu3+emission of the prepared nanoparticles was mea-sured to be 0.31±0.03 upon the excitation at 415 nm at 23°C. Assuming that the nanoparticles have a diameter of 45 nm and the density of the nanoparticles is equal to~1.0 g·cm-3(the densities of P(ST-co-MMA),OTS,and Eu(tta)3dpbt are 1.05, 0.91,and 0.92 g·cm-3,respectively),it is estimated that each nanoparticle contains about 10000 Eu(tta)3dpbt molecules(see Supporting Information for details).The luminescent brightness of EuPHS(defined as ε×Φ)is estimated to be 7.4×107mol-1·L·cm-1under excitation at 415 nm,suggesting a remarkable signal amplification capability of EuPHS in bioanalysis.
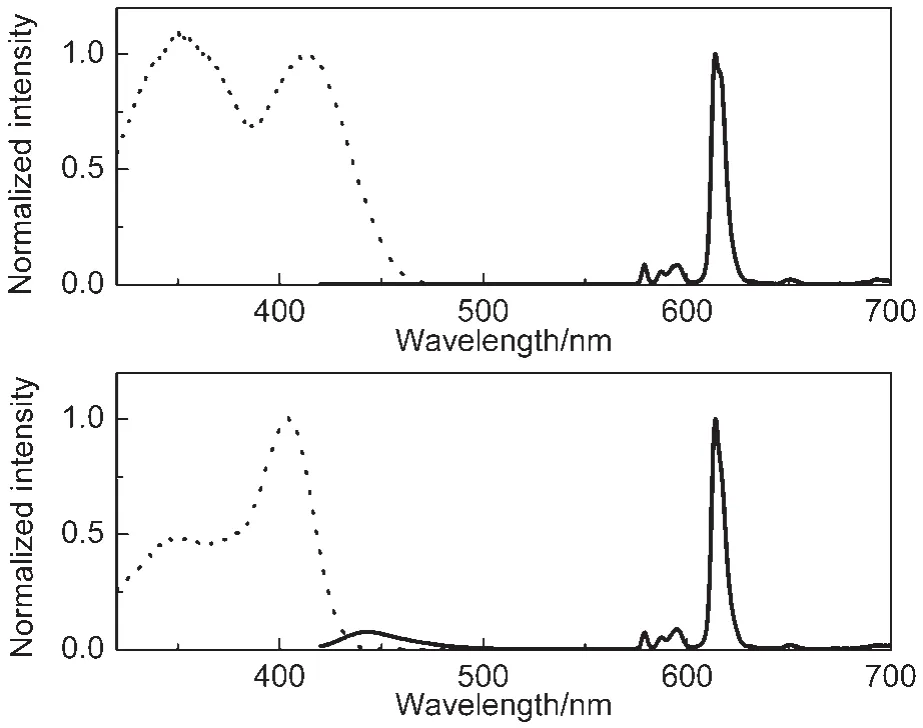
Fig.3 Normalized excitation(dashed line,λem=614 nm)and emission(solid line,λex=415 nm)spectra of Eu(tta)3dpbt in a colloidal solution of EuPHS(upper)and in toluene(lower)
The maximal TPE action cross section(δ×Φ)of the europium complex molecules in EuPHS was measured to be 50 GM at 23°C at 830 nm(Fig.5).This δ×Φ value was about 62%of that of Eu(tta)3dpbt molecules in toluene,6indicating that the excellent two-photon-sensitized luminescence properties of Eu(tta)3dpbt molecules were maintained mostly in EuPHS. Considering the number of europium complex molecules (~10000)in each nanoparticle having the average diameter,the maximal TPE action cross section of EuPHS(dav=45 nm)was roughly estimated to be 5×105GM,which was about 10 times higher than the highest value reported for CdSe/ZnS core-shell quantum dots(dav≈4.5 nm)determined by a similar method,30and was about 4 times higher than that of our previously reported EuLNPs nanoparticles(52 nm in average diameter).20
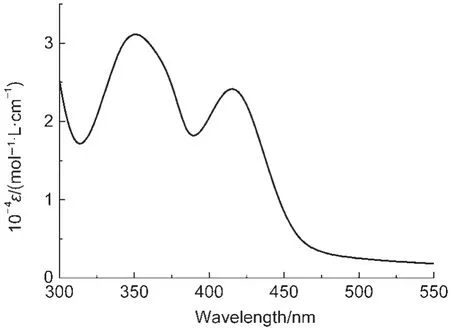
Fig.4 UV-Vis absorption spectrum of EuPHS in water
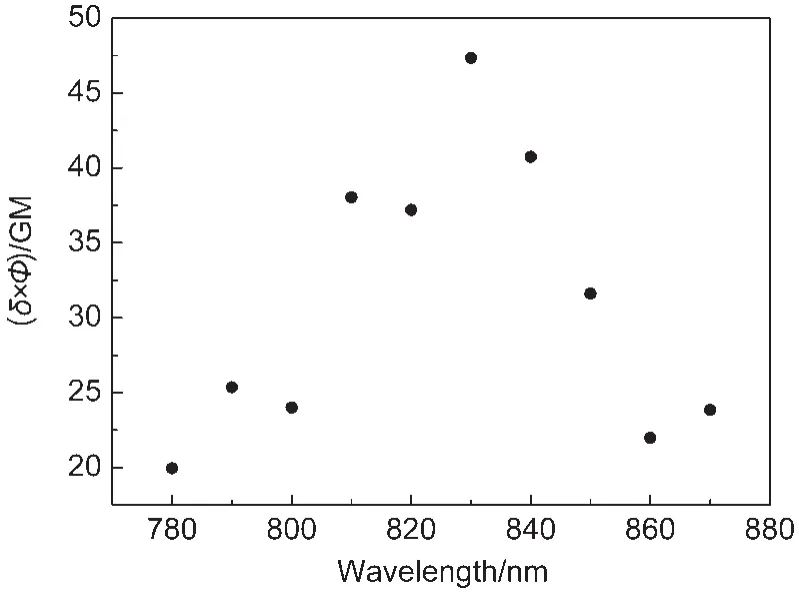
Fig.5 Two-photon excitation action cross sections(δ×Φ)of Eu(tta)3dpbt in EuPHSThe experimental uncertainty is about 15%. 1 GM=10-50cm4·s·photon-1·molecule-1
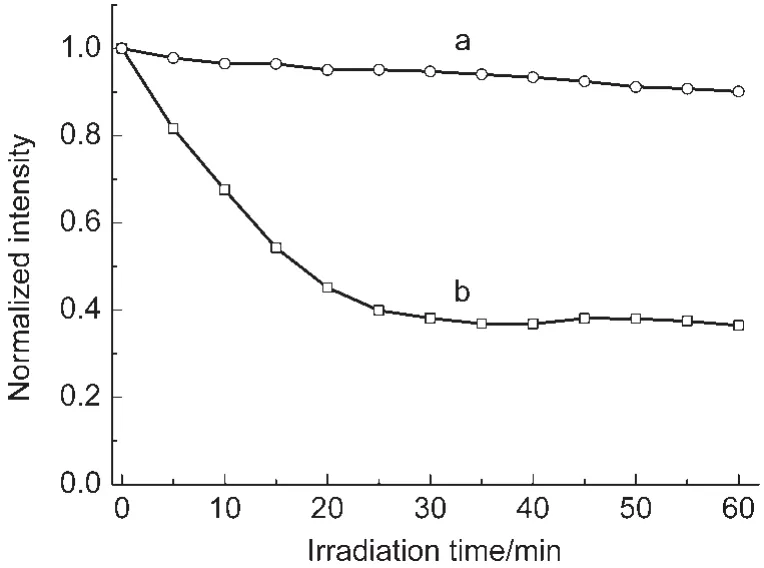
Fig.6 Photobleaching curves of colloidal solutions of EuPHS(a)and nanoparticles prepared in the absence of P(ST-co-MMA(EuHS)(b)
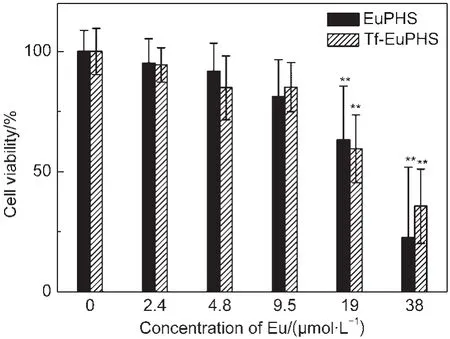
Fig.7 Cytotoxicity tests of EuPHS and Tf-EuPHS with HeLa cellsData are represented as mean±SD(standard deviation)(n=6).The untreated cells were used as the control.Statistical significance(Studentʹs t-test)compared to the control is indicated:**P<0.01
Photostability is important for luminescent probes.In order to evaluate the photostability of the prepared nanoparticles, photobleaching measurements were carried out on EuPHS and nanoparticles prepared in the absence of the polymer(EuHS) for comparison,using a 150 W xenon lamp as an excitation source(Fig.6).The emission intensities of the EuPHS and EuHS nanoparticles decreased by 10%and 64%,respectively, after irradiation with the lamp for 1 h.The good photostability of EuPHS was derived from the hybrid encapsulation material acting as a barrier to protect Eu(tta)3dpbt molecules from photoreactions with species in the colloidal solution.The photostability of the EuHS nanoparticles was much lower than that of EuPHS,indicating that the polysiloxane in the EuHS nanoparticles could not provide sufficient shield for Eu(tta)3dpbt molecules from the outside environment.
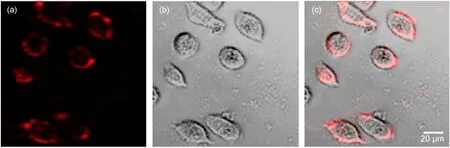
Fig.8 Images of live HeLa cells incubated with Tf-EuPHS for 3 h at 37°C(a)two-photon-excited luminescence images.The excitation wavelength was fixed at 800 nm with an average laser power of~10 mW on the focal plane,and the luminescence was collected in the spectral range from 590 to 620 nm;(b)differential interference contrast(DIC)images;(c)the overlay of the corresponding luminescence and DIC images.Images shown in panels of a-c have the same scale bar.
3.3 Cytotoxicity of EuPHS
The cytotoxicity of EuPHS was evaluated by the methyl thiazolyl tetrazolium assay(see Experiment Section for details). Cell viability of HeLa carcinoma cells was record after exposed to EuPHS for 24 h.EuPHS did not show statistically significant toxicity(P<0.01)to HeLa cells at the dosage lower than 25.0 μg·mL-1(9.5 μmol·L-1of Eu(tta)3dpbt)(Fig.7).
The cytotoxicity of EuPHS is similar to that of previously reported EuLNPs nanoparticles which showed no apparent cytotoxicity to HeLa and Lewis cells when the concentration of EuLNPs was less than 100.0 μg·mL-1(7.4 μmol·mL-1Eu(tta)3dpbt),20while much lower than that of an unpacked complex(Eu(tta)2Cldpbt)labeling material which killed 30%of HeLa cells at a Eu(tta)2Cldpbt concentration of 1.0 μg·mL-1(0.9 μmol·mL-1).18The efficient shielding of Eu(tta)3dpbt molecules by the matrix in EuPHS may be a cause of the low cytotoxicity of EuPHS to the cells.Nanoprobes(Tf-EuPHS)prepared by adsorbing transferrin on the surfaces of EuPHS exhibited the similar behavior in the cytotoxicity tests(Fig.7).It should be mentioned that in the preparation of the present bionanoprobes,most of CTAB added for preparing EuPHS nanospheres has been removed.Therefore, the contribution of CTAB to the cytotoxicity is negligible in the aforementioned experiments.
3.4 Two-photon-excitation imaging of live cell
Transferrin receptor,over-expressed in many cancer types, provides an opportunity for designing receptor-targeted approaches for cancer cell labeling and imaging.31Tf-EuPHS were used as luminescent labels in the two-photon-excitation imaging of live HeLa cells.As shown in Fig.8,the receptor-mediated endocytosis of Tf-EuPHS by the cells occurred smoothly and high quality two-photon-excitation imaging of the labeled cells were achieved due to the high Eu3+luminescence capacity(δ×Φ)of EuPHS upon TPE.
It is well documented that transferrin can be internalized through clathrin-dependent endocytosis.32We performed the control experiments,in which the cells were pre-treated with NaN3and 2-deoxy-D-glucose that can block the clathrin-dependent endocytosis.33,34As shown in Fig.S4(see Supporting Information),this pretreatment drastically reduced the uptake level of Tf-EuPHS.These results confirmed that the internalization of Tf-EuPHS occurred through a transferrin receptor-mediated clathrin-dependent pathway,implying that other biomolecules (such as antibody and peptide)with receptor-target recognizability could also be used to conjugate with EuPHS for preparing target-recognizable TPE nanoprobes.The high TPE action cross section(δ×Φ)and the characteristic Eu3+emission,the good dispersibility in water,high photostability,and excellent biocompatibility of EuPHS make them promising for a wide variety of potential biomedical applications based on two-photon excitation or visible light excitation imaging of live cells.
4 Conclusions
In summary,luminescent nanospheres EuPHS(dav=45 nm) with a markedly enhanced TPE Eu3+luminescence capacity have been prepared by encapsulating Eu(tta)3dpbt in an in situ formed hybrid material composed of P(ST-co-MMA),OTS, and poly(octylsiloxane)through a co-precipitation-condensation encapsulation method.EuPHS containing 40%(w)of Eu(tta)3dpbt exhibited good stability and dispersibility in aqueous solutions,high photostability,and low cytotoxicity.The Eu3+luminescence excitation peak centered at 415 nm with a red-edge extending up to 475 nm,and the quantum yield for Eu3+luminescence of 0.31 under excitation at 415 nm of EuPHS reflect their excellent visible-light-excitation luminescence properties.The high TPE action cross section of EuPHS (5×105GM at 830 nm and 23°C)is promising for the application in bioimaging in vivo.
Supporting Information: Experimental details for the EuPHS composition analysis and estimation of the molecule number of Eu(tta)3dpbt in one of EuPHS with the average diameter,molecular structure of Eu(tta)3dpbt(Scheme S1),EDX analysis of EuPHS(Fig.S1),TEM image of the nanoparticles prepared in the absence of OTS(Fig.S2),the photoluminescence decay curve of EuPHS(Fig.S3).Images of Hela cells pre-treated with NaN3and 2-deoxy-D-glucose(Fig.S4).The Information is available free of charge via the internet at http:// www.whxb.pku.edu.cn.
(1) Helmchen,F.;Denk,W.Nat.Methods 2005,2,932.doi: 10.1038/nmeth818
(2) Denk,W.;Strickler,J.H.;Webb,W.W.Science 1990,248,73. doi:10.1126/science.2321027
(3) Eliseeva,S.V.;Bunzli,J.C.G.Chem.Soc.Rev.2010,39,189. doi:10.1039/b905604c
(4)Ma,Y.;Wang,Y.Coord.Chem.Rev.2010,254,972.doi: 10.1016/j.ccr.2010.02.013
(5)Andraud,C.;Maury,O.Eur.J.Inorg.Chem.2009,4357.
(6)Fu,L.M.;Wen,X.F.;Ai,X.C.;Sun,Y.;Wu,Y.S.;Zhang,J.P.; Wang,Y.Angew.Chem.Int.Edit.2005,44,747.doi:10.1002/ (ISSN)1521-3773
(7) Piszczek,G.;Maliwal,B.P.;Gryczynski,I.;Dattelbaum,J.; Lakowicz,J.R.J.Fluoresc.2001,11,101.doi:10.1023/A: 1016673300913
(8) Picot,A.;DʹAleo,A.;Baldeck,P.L.;Grichine,A.;Duperray,A.; Andraud,C.;Maury,O.J.Am.Chem.Soc.2008,130,1532.doi: 10.1021/ja076837c
(9) Eliseeva,S.V.;Aubock,G.;van Mourik,F.;Cannizzo,A.; Song,B.;Deiters,E.;Chauvin,A.S.;Chergui,M.;Bunzli,J.C. G.J.Phys.Chem.B 2010,114,2932.doi:10.1021/jp9090206
(10)Shi,M.;Ding,C.R.;Dong,J.W.;Wang,H.Z.;Tian,Y.P.;Hu, Z.J.Phys.Chem.Chem.Phys.2009,11,5119.
(11) Lakowicz,J.R.;Piszczek,G.;Maliwal,B.P.;Gryczynski,I. ChemPhysChem 2001,2,247.doi:10.1002/(ISSN)1439-7641
(12)Werts,M.H.V.;Nerambourg,N.;Pelegry,D.;Le Grand,Y.; Blanchard-Desce,M.Photochem.Photobiol.Sci.2005,4,531. doi:10.1039/b504495b
(13) Picot,A.;Malvolti,F.;Le Guennic,B.;Baldeck,P.L.;Williams, J.A.G.;Andraud,C.;Maury,O.Inorg.Chem.2007,46,2659. doi:10.1021/ic062181x
(14) DʹAleo,A.;Picot,A.;Baldeck,P.L.;Andraud,C.;Maury,O. Inorg.Chem.2008,47,10269.doi:10.1021/ic8012975
(15) DʹAleo,A.;Allali,M.;Picot,A.;Baldeck,P.L.;Toupet,L.; Andraud,C.;Maury,O.C.R.Chimie 2010,13,681.doi: 10.1016/j.crci.2010.01.008
(16)Xue,F.M.;Ma,Y.;Fu,L.M.;Hao,R.;Shao,G.S.;Tang,M. X.;Zhang,J.P.;Wang,Y.Phys.Chem.Chem.Phys.2010,12, 3195.
(17) Palsson,L.O.;Pal,R.;Murray,B.S.;Parker,D.;Beeby,A. Dalton Trans.2007,5726.
(18)Law,G.L.;Wong,K.L.;Man,C.W.Y.;Tsao,S.W.;Wong,W. T.J.Biophotonics 2009,2,718.doi:10.1002/jbio.v2:12
(19)Wu,J.;Ye,Z.Q.;Wang,G.L.;Jin,D.Y.;Yuan,J.L.;Guan,Y. F.;Piper,J.J.Mater.Chem.2009,19,1258.doi:10.1039/ b815999h
(20)Shao,G.S.;Han,R.C.;Ma,Y.;Tang,M.X.;Xue,F.M.;Sha, Y.L.;Wang,Y.Chem.Eur.J.2010,16,8647.doi:10.1002/ chem.201001367
(21)Yang,C.;Fu,L.M.;Wang,Y.;Zhang,J.P.;Wong,W.T.;Ai,X. C.;Qiao,Y.F.;Zou,B.S.;Gui,L.L.Angew.Chem.Int.Edit. 2004,43,5010.doi:10.1002/(ISSN)1521-3773
(22)Wen,X.F.;Li,M.Y.;Wang,Y.;Zhang,J.P.;Fu,L.M.;Hao,R.; Ma,Y.;Ai,X.C.Langmuir 2008,24,6932.doi:10.1021/ la800903s
(23)Shao,G.S.;Xue,F.M.;Han,R.C.;Tang,M.X.;Wang,Y.Acta Phys.-Chim.Sin.2010,26,2031.[邵光胜,薛富民,韩荣成,汤敏贤,王 远.物理化学学报,2010,26,2031.]doi:10.3866/ PKU.WHXB20100715
(24)Hao,R.;Li,M.;Wang,Y.;Zhang,J.;Ma,Y.;Fu,L.;Wen,X.; Wu,Y.;Ai,X.;Zhang,S.;Wei,Y.Adv.Funct.Mater.2007,17, 3663.doi:10.1002/(ISSN)1616-3028
(25) Arriagada,F.J.;Osseo-Asare,K.J.Colloid Interface Sci.1999, 211,210.doi:10.1006/jcis.1998.5985
(26) Stöber,W.;Fink,A.;Bohn,E.J.Colloid Interface Sci.1968,26, 62.doi:10.1016/0021-9797(68)90272-5
(27)Wang,Y.;Fu,X.Y.;Shao,G.S.Photoluminescent nanoparticle, preparation,and application thereof.CN Patent, 200910203407.3,2009-05-05.[王 远,符小艺,邵光胜.荧光纳米粒子及其制备方法和应用:中国,CN200910203407.3[P] 2009-05-05.]
(28) Peng,H.S.;Stich,M.I.J.;Yu,J.B.;Sun,L.N.;Fischer,L.H.; Wolfbeis,O.S.Adv.Mater.2010,22,716.doi:10.1002/adma. v22:6
(29) Demas,J.N.;Crosby,G.A.J.Phys.Chem.1971,75,991.doi: 10.1021/j100678a001
(30) Larson,D.R.;Zipfel,W.R.;Williams,R.M.;Clark,S.W.; Bruchez,M.P.;Wise,F.W.;Webb,W.W.Science 2003,300, 1434.doi:10.1126/science.1083780
(31)Chan,W.C.W.;Nie,S.M.Science 1998,281,2016.doi: 10.1126/science.281.5385.2016
(32) Richard,J.P.;Melikov,K.;Brooks,H.;Prevot,P.;Lebleu,B.; Chernomordik,L.V.J.Biol.Chem.2005,280,15300.doi: 10.1074/jbc.M401604200
(33) Kam,N.W.S.;Liu,Z.A.;Dai,H.J.Angew.Chem.Int.Edit. 2006,45,577.doi:10.1002/(ISSN)1521-3773
(34) Zhang,B.L.;Li,Y.Q.;Fang,C.Y.;Chang,C.C.;Chen,C.S.; Chen,Y.Y.;Chang,H.C.Small 2009,5,2716.doi:10.1002/ smll.v5:23
June 20,2012;Revised:August 16,2012;Published on Web:August 16,2012.
Nanoprobes with Enhanced Two-Photon-Sensitized Eu3+Luminescence Properties for Live Cell Imaging
FU Xiao-Yi1,2,†SHAO Guang-Sheng1,†HAN Rong-Cheng1,†MA Yan1XUE Fu-Min1YANG Fan3FU Li-Min3ZHANG Jian-Ping3WANG Yuan1,*
(1Beijing National Laboratory for Molecular Sciences,State Key Laboratory for Structural Chemistry of Unstable and Stable Species,College of Chemistry and Molecular Engineering,Peking University,Beijing 100871,P.R.China;2School of Materials Science and Engineering,South China University of Technology,Guangzhou 510640,P.R.China;3Department of Chemistry,Renmin University of China,Beijing 100872,P.R.China)
Luminescent nanospheres(EuPHS,dav=45 nm)containing 40%(w)of Eu(tta)3dpbt(tta= thenoyltrifluoroacetonato;dpbt=2-(N,N-diethylanilin-4-yl)-4,6-bis(3,5-dimethylpyrazol-1-yl)-1,3,5-triazine) were prepared by encapsulating Eu(tta)3dpbt in a hybrid matrix formed in situ from poly(styrene-co-methyl methacrylate),octyltrimethoxysilane,and poly(octylsiloxane).The EuPHS are promising luminescent markers for bioanalysis because of their good dispersibility in aqueous solutions,high photostability,low cytotoxicity,and bright Eu3+luminescence under excitation at long wavelengths.EuPHS exhibited excellent visible light-sensitized and near-infrared two-photon-sensitized Eu3+luminescence properties,with a visible-light excitation peak at 415 nm and an excitation window extending up to 475 nm.The quantum yield for Eu3+luminescence was 0.31(λex=415 nm,T=23°C),and the two-photon excitation(TPE)action cross section was 5.0×105GM(1 GM=10-50cm4·s·photon-1·particle-1)at 830 nm.Bionanoprobes prepared by adsorbing transferrin on the surfaces of EuPHS were successfully applied in target-specific labeling and two-photon-excitation imaging of live HeLa cells.
Luminescence;Europium complex;Two-photon excitation;Bio-nanoprobe; Cell Imaging
10.3866/PKU.WHXB201208161
∗Corresponding author.Email:wangy@pku.edu.cn;Tel:+86-10-62751491.†These authors contributed equally to this work.
The project was supported by the National Natural Science Foundation of China(21073002,21133001,21227803,51121091)and National Key Basic Research Special Foundation of China(2011CB808702).
国家自然科学基金(21073002,21133001,21227803,51121091)及国家重点基础研究专项经费(2011CB808702)资助
O648
