Gemini表面活性剂乙烷基-α,ω-双十四烷基二甲基溴化铵产生的高稳定泡沫
2012-11-30吴晓娜邹文生赵剑曦
吴晓娜 邹文生 赵剑曦
(福州大学化学化工学院,胶体与界面化学研究所,福州350108)
Gemini表面活性剂乙烷基-α,ω-双十四烷基二甲基溴化铵产生的高稳定泡沫
吴晓娜 邹文生 赵剑曦*
(福州大学化学化工学院,胶体与界面化学研究所,福州350108)
报道了由gemini表面活性剂乙烷基-α,ω-双十四烷基二甲基溴化铵(14-2-14)产生的高稳定泡沫体系.泡沫塌陷到初始高度一半所对应的时间(t1/2)用来表征泡沫的稳定性.测得14-2-14体系的t1/2高达961 min,远大于乙烷基-α,ω-双十二烷基二甲基溴化铵(12-2-12)产生泡沫的t1/2(754 min),表明带有一根短联接链和两条长尾链的gemini表面活性剂是高效的泡沫稳定剂.为了揭示界面弹性与泡沫稳定性之间的关联,测量了表面活性剂吸附膜的扩张流变行为.在指定的表面过剩量下,吸附膜的高频极限弹性再一次被发现与泡沫稳定性相关,较大的极限弹性很好地对应更加稳定的泡沫.
泡沫稳定剂;Gemini表面活性剂;短联接链;长尾链;高界面弹性
1 Introduction
Recently we reported stable foam systems generated by cationic gemini surfactants,2-hydroxyl-propanediyl-α,ω-bis(dimethyldodecylammonium bromide),2-hydroxyl-butanediyl-α, ω-bis(dimethyldodecylammonium bromide)or 2,3-hydroxylbutanediyl-α,ω-bis(dimethyldodecylammonium bromide)(referred to as 12-3(OH)-12,12-4(OH)-12,and 12-4(OH)2-12,respectively).1These surfactants all contained a hydroxyl-substituted spacer and thus produced the intermolecular hydrogen bonding for those adsorbed at the air/water interface,2which made the packing of molecules tighter and increased the cohesion of surfactant within the monolayer and enhanced the foam stability.1,3Among these surfactants,12-3(OH)-12 can more efficiently stabilized the foam than 12-4(OH)-12 or 12-4(OH)2-12.This result suggests that even though there exists the intermolecular hydrogen bonding,the spacer length may still dominate the packing of surfactant at the air/water interface.Indeed,ethanediyl-α,ω-bis(dimethyldodecylammonium bromide)(12-2-12) has been found to efficiently stabilize soap film even at very low concentrations.4Illuminated by these results,we expanded the study to ethanediyl-α,ω-bis(dimethyltetradecyl-ammonium bromide)(14-2-14).14-2-14 has longer alkyl tails than 12-2-12 and therefore is expected to increase the cohesion of surfactant within the monolayer and better to stabilize foam.In the present work,the foam stability generated by 14-2-14 and the dilational rheology of the adsorption films were studied in detail. For comparison,12-2-12 was also examined.
2 Experimental
2.1 Materials
Gemini surfactants,ethanediyl-α,ω-bis(tetradecyldimethylammonium bromide)and ethanediyl-α,ω-bis(dodecyldimethylammonium bromide),the chemical structures of which are shown in Scheme 1,were synthesized in our laboratory according to the method reported by Zana et al.5The products were confirmed by1H NMR and elemental analysis.All the solutions were prepared with Milli-Q water(resistivity:18.2 MΩ· cm).
2.2 Measurements
Foam stability was determined using a setup reported in literature.1The test solution of 5 mL was filled in a cylindrical glass container(25-mm internal diameter,140-mm height),at the bottom of which a porous glass disc was fixed.The cylindrical glass container was put in a water bath with a constant temperature of(25.0±0.1)°C.The solution was bubbled by air with a constant flow rate of 68 mL·min-1,and foam was generated until a designed 40 mm height that corresponded to a volume of 20 cm3and then the valve was shut immediately.Foam stability was characterized by time,t1/2,needed for the collapse of foam to half its initial height.The experiments were repeated at least three times,and the average value was adopted as t1/2.

Scheme 1 Chemical structure of the gemini surfactants
Interfacial dilational rheology was measured in an optical angle meter OCA-20 with oscillating drop accessory ODG-20. The equilibrated interface as indicated by a constant surface tension value was disturbed by sinusoidal oscillations produced by a function generator,which resulted in interfacial periodic expansion and contraction.The accessible frequency range was 0.01-1 Hz and the variation of the relative area(A) was~6%.These conditions followed the range of linear viscoelasticity.The changes in drop shape were monitored by CCD camera with a minimum of 50 frames per second.At the end of the experiment,the software retrieved the images and calculated the changes in both interface tension(dγ)and area(dA), which gave the interface dilatational modulus ε*=dγ/AdA.Using a Fourier transform analysis,the phase angle(θ)was determined.Thus,the dilational elasticity ε and dilational viscosity η could be calculated by the following relations:

where ω=2πv,v is the frequency of sinusoidal oscillation.
3 Results and discussion
3.1 Foam stability
Fig.1 shows the apparent pictures where both 12-2-12 and 14-2-14 generate foams with many gas bubbles separated by liquid films.Fig.2 illustrates the semi-logarithmic plots of the foam stability,represented as the time t1/2needed for the collapse of foam to half its initial height,as a function of surfactant concentration.As the surfactant concentration increases,t1/2rises rapidly to a plateau at respective critical micelle concentration (cmc)as shown by arrow.The maximum tmax1/2for 12-2-12(754 min)is greatly larger than that of 12-3(OH)-12(406 min)as reported previously,1verifying our inference that the gemini with a short spacer could more efficiently stabilize the foams. Significantly,14-2-14 produces tmax1/2as high as 961 min.This tmax1/2is 4-folds that of traditional cationic surfactant,tetradecyltrimethylammonium bromide(C14TABr,240 min),which is generally considered as the corresponding monomer of 14-2-14, and is also considerably larger than that of 14-3(OH)-14(660 min).6This means that a highly stable foam system is found.

Fig.1 Apparent pictures of the foam systems generated by gemini surfactants(a)ethanediyl-α,ω-bis(dodecyldimethylammonium bromide)(12-2-12), (b)ethanediyl-α,ω-bis(tetradecyldimethylammonium bromide)(14-2-14)
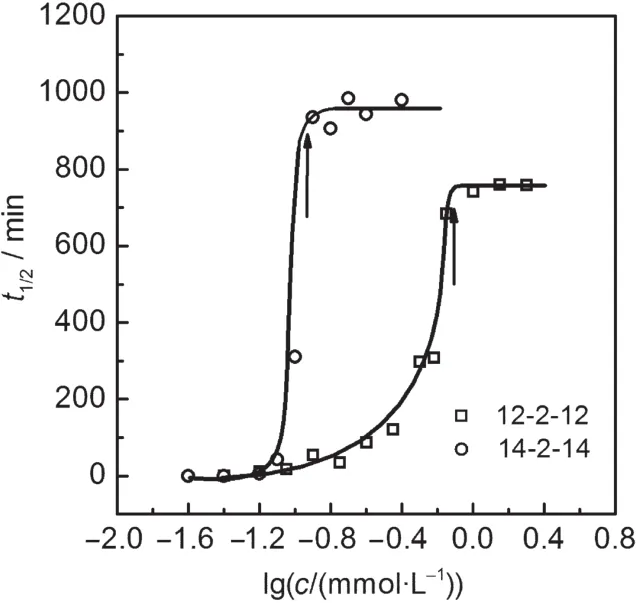
Fig.2 Semi-logarithmic plots of foam decay time(t1/2)for foam height to fall by 50%as a function of m-2-m concentration(c) at 25°CThe arrow shows respective cmc.
Here we would like to mention another homologue,ethanediyl-α,ω-bis(hexadecyldimethylammonium bromide)(16-2-16) which has longer alkyl tails than 14-2-14.Unfortunately,16-2-16 cannot form normal foams due to the reason of adsorption kinetics analogous to 16-3(OH)-16 as discussed in our previous study.6
3.2 Interfacial dilational rheology
In general,foam stability is relevant to the interfacial elasticity of the adsorption film.3,7-13In this section,we discuss the dilational rheology of the adsorption films.Fig.3 shows the frequency-dependent variation of interfacial dilational elasticity (ε)and dilational viscosity(η)at different 14-2-14 concentrations(the similar results of 12-2-12 are not shown).At a fixed working frequency,both ε and η clearly exhibit concentration dependence(Fig.4)analogous to the observations for other surfactant systems.7,14The maximum in the concentration-dependent plot of ε has been explained by different effects induced by increased surfactant concentration,7which results in gradual increase in the surface excess Γ and noticeable enhancement in the molecular exchange between bulk and surface.The fast exchanges can even the surface tension gradient out rapidly. Thus,at low concentrations ε is governed by increased surface excess,whereas at high concentrations the molecular exchange plays a dominant role.The competition of the both determines a maximum in the ε(c)curve.
Lucassen-van den Tempel(LVT)model describes the viscoelastic behavior of soluble monolayer over the range of low frequencies.15,16This model assumed that the material transport involved in the adsorption kinetics is governed only by diffusion without energy barriers and considered the instantaneous cou-pling between the interface rheology and the adsorption kinetics.The model predicted the viscoelastic moduli through the following equations:

Fig.3 Experimental plots of(a)interfacial elasticity(ε)and(b)interfacial viscosity(η)as a function of frequency(v)in 14-2-14 aqueous solutions at 25°Clg(c/(mmol·L-1)):(□)-1.60,(○)-1.41,(△)-1.20,(▽)-1.10,(◇)-1.00,(◁)-0.80,(▷)-0.70,(☆)-0.50
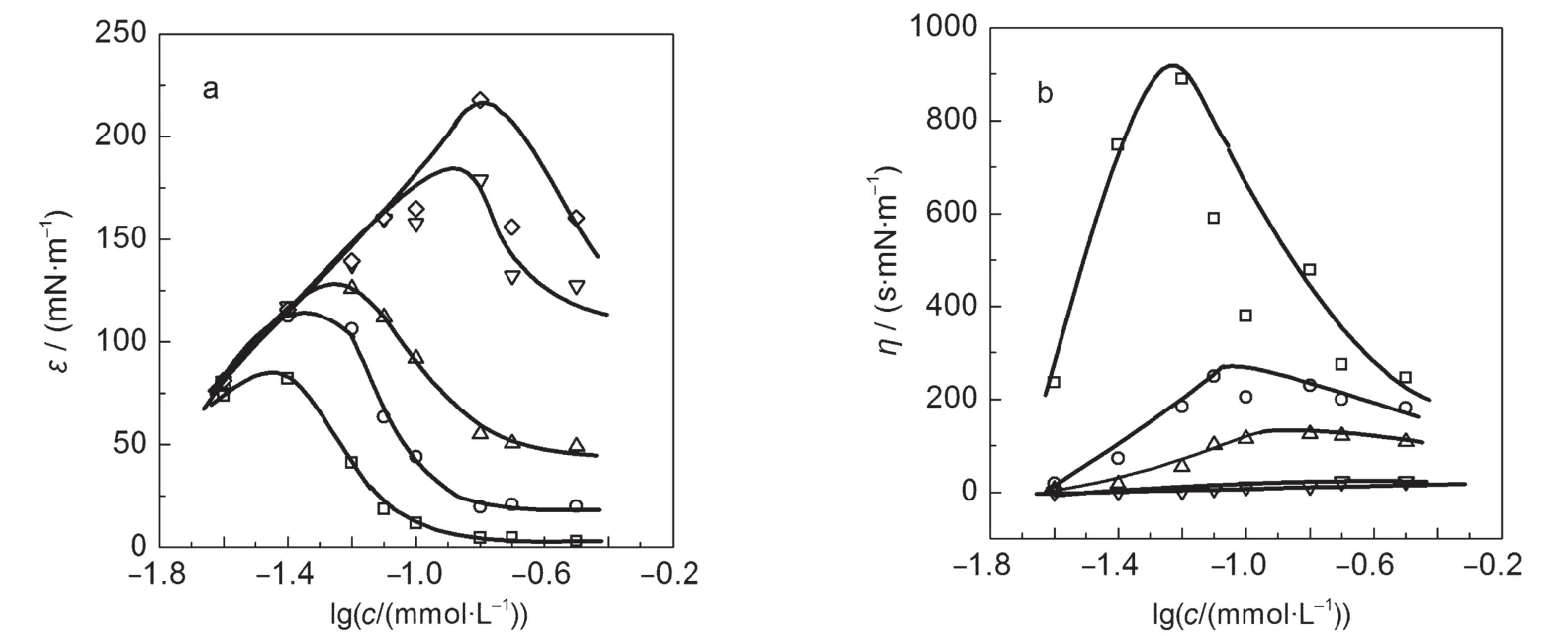
Fig.4 Semi-logarithmic plots of(a)dilational interfacial elasticity and(b)interfacial viscosity of 14-2-14 as a function of the concentration at different frequenciesν/Hz:(□)0.01,(○)0.046,(△)0.1,(▽)0.464,(◇)1.00

with

where ε0is the theoretical high-frequency limit of the surface elasticity and ω0is the molecular exchange parameter.Fig.5 shows some typical results for 14-2-14 fitted by Eqs.(3)and (4),in which the LVT model well describes the experimental data.Since the high-frequency limit is not experimentally included,the fitting values of ε0,fitwere adopted as ε0by the procedure suggested by Stubenrauch and Miller,7in which the couples of ε0,fitand ω0,fitthat best describe both the experimental ε(v,c)and η(v,c)curves,were determined.Similarly,ε0,fitis also concentration-dependent as seen in Fig.6.

Fig.5 Fitting results of(a)dilational interfacial elasticity and(b)interfacial viscosity in terms of LVT model for 14-2-14 aqueous solutions at 25°Clg(c/(mmol·L-1)):(□)-1.60,(○)-1.41,(△)-1.20

Fig.6 Concentration-dependent plots of high-frequency limit elasticity(ε0,fit)

Fig.7 Frumkin adsorption isotherms of 12-2-12 and 14-2-14 at air/water interface
In our recent study,we stressed that the relation between foam stability and interfacial elasticity of adsorption film is tenable only on the basis of high-frequency limit ε0(or ε0,fit)which excludes the influence of frequency as well as of identical surface excesses rather than the same bulk concentrations.1Analogous to the previous work,here we also adopted 80%of the surface excess as the comparison level for 12-2-12 and 14-2-14.The corresponding ε0,fitcan be obtained by combining Fig.6 and Fig.7,the latter represents the adsorption isotherms of Frumkin form obtained through fitting the surface tension data by Szyszkowski formula and then calculating the surface excesses by Gibbs equation.17Thus,the ε0,fitat 80%surface excess is 117.2 mN·m-1for 12-2-12 and 145.3 mN·m-1for 14-2-14. This result well indicates that high foam stability generated by 14-2-14 mirrors in high interfacial-elasticity of its adsorption film,being consistent with the previous conclusion.1
4 Conclusions
This paper reports a highly stable foam system generated by the gemini surfactant,14-2-14,which has two tetradecyl tails and an ethylene spacer linking the two quaternary ammonium headgroups.The present result suggests that the gemini surfactant with a short spacer and relatively long alkyl tails may be a good foam stabilizer,in which the short spacer can produce a columnar-like molecular geometry,while long alkyl tails increases the cohesion between surfactant molecules within the adsorption monolayer.The both factors are favorable to the tight arrangement of the molecules adsorbed at the interface and result in the high-elasticity film.
(1) Wu,X.N.;Zhao,J.X.;Li,E.J.;Zou,W.S.Colloid Polym.Sci. 2011,289,1025.
(2) Pei,X.M.;You,Y.;Zhao,J.X.;Deng,Y.S.;Li,E.J.;Li,Z.X. J.Colloid Interface Sci.2010,351,457.
(3) Bergeron,V.Langmuir 1997,13,3474.
(4) Espert,A.;Klitzing,R.V.;Poulin,P.;Colin,A.;Zana,R.; Langevin,D.Langmuir 1998,14,4251.
(5) Zana,R.;Benrraou,M.;Rueff,R.Langmuir 1991,7,1072.
(6)You,Y.;Wu,X.N.;Zhao,J.X.;Ye,Y.Z.;Zou,W.S.Colloids Surf.A 2011,384,164.
(7) Stubenrauch,C.;Miller,R.J.Phys.Chem.B 2004,108,6412.
(8) Santini,E.;Ravera,F.;Ferrari,M.;Stubenrauch,C.;Makievski, A.;Krägel,J.Colloids Surf.A 2007,298,12.
(9) Wang,L.;Yoon,R.H.Int.J.Miner.Process.2008,85,101.
(10) Georgieva,D.;Cagna,A.;Langevin,D.Soft Matter 2009,5, 2063.
(11)Acharya,D.P.;Gutiérrez,J.M.;Aramaki,K.;Aratani,K.; Kunieda,H.J.Colloid Interface Sci.2005,291,236.
(12) Wang,L.;Yoon,R.H.Int.J.Miner.Process 2008,85,101.
(13) Sonin,A.A.;Bonfillon,A.;Langevin,D.J.Colloid Interface Sci.1994,162,323.
(14)Li,Y.M.;Xu,G.Y.;Xin,X.;Gao,X.R.;Wu,D.Carbohydr. Polym.2008,72,211.
(15) Lucassen,J.;van den Tempel,M.Chem.Eng.Sci.1972,27, 1283.
(16) Lucassen,J.;van den Tempel,M.J.Colloid Interface Sci.1972, 41,491.
(17) Rosen,M.J.Surfactants and Interfacial Phenomena;Wiley: New York,1988.
December 20,2011;Revised:February 27,2012;Published on Wed:March 5,2012.
Highly Stable Foams Generated by the Gemini Surfactant Ethanediyl-α,ω-bis(tetradecyldimethylammonium bromide)
WU Xiao-Na ZOU Wen-Sheng ZHAO Jian-Xi*
(Institute of Colloid and Interface Chemistry,College of Chemistry and Chemical Engineering,Fuzhou University, Fuzhou 350108,P.R.China)
This paper reports a highly stable foam system generated by the gemini surfactant ethanediylα,ω-bis(tetradecyldimethylammonium bromide)(referred to as 14-2-14).The time measured for the collapse of the foam to half its initial height was used to characterize the foam stability;it was as high as 961 min for this system and significantly longer than that(754 min)for ethanediyl-α,ω-bis(dodecyldimethylammonium bromide)(12-2-12)foams.Thus the gemini surfactant structure of a short spacer together with two long tails enabled it to be a highly efficient foam stabilizer.The dilational rheology of the adsorbed films revealed the relationship between the interfacial elasticity and the foam stability.The high-frequency limit of elasticity of the adsorbed film at a specific surface coverage was again found to indicate foam stability.A larger limit of elasticity indicated a more stable foam.
Foam stabilizer;Gemini surfactant;Short spacer;Long tail;High interfacial-elasticity
10.3866/PKU.WHXB201203053
O646
∗Corresponding author.Email:jxzhao.colloid@fzu.edu.cn;Tel:+86-591-22866338;Fax:+86-591-22866152.
The project was supported by the National Natural Science Foundation of China(20673021,20873024)and Natural Science Foundation of Fujian Province,China(2010J01038).
国家自然科学基金(20673021,20873024)及福建省自然科学基金(2010J01038)资助项目