飞秒时间分辨的光电子影像技术研究2-氯吡啶超快内转换动力学
2012-11-30GHAZALAhmedYousif邱学军秦朝朝龙金友
GHAZALAhmed-Yousif 邱学军 秦朝朝 龙金友
布玛丽亚·阿布力米提 张 冰*
(中国科学院武汉物理与数学研究所,波谱与原子分子物理国家重点实验室,武汉430071;中国科学院研究生院,北京100049)
飞秒时间分辨的光电子影像技术研究2-氯吡啶超快内转换动力学
GHAZALAhmed-Yousif 邱学军 秦朝朝 龙金友
布玛丽亚·阿布力米提 张 冰*
(中国科学院武汉物理与数学研究所,波谱与原子分子物理国家重点实验室,武汉430071;中国科学院研究生院,北京100049)
利用时间分辨的飞秒光电子影像技术结合时间分辨的质谱技术,研究了2-氯吡啶分子激发态的超快过程.实时观察到了2-氯吡啶分子第二激发态(S2)向第一激发态(S1)高振动能级的超快内转换过程,该内转换的时间常数为(162±5)fs.实验结果表明,通过S2/S0(基态)和S1/S0的锥形交叉衰减到基态的衰减通道也是退布居的重要通道,其时间尺度为(5.5±0.3)ps.
光电子影像;超快过程;内转换;2-氯吡啶
1 Introduction
Femtosecond time-resolved photoelectron spectroscopy is a novel experimental means to probe excited state dynamics in isolated polyatomic molecules.Because of the sensitivity of photoelectron spectroscopy to both electronic configurations and vibrational dynamics,it is well suited to the study of the ultrafast nonadiabatic processes.1As a variant of femtosecond time-resolved photoelectron spectroscopy,femtosecond timeresolved photoelectron imaging(TR-PEI)spectroscopy has the advantage of measuring the time evolution of both energy and angular distributions simultaneously.Associated with resonance-enhanced multiphoton ionization(REMPI),TR-PEI is a powerful experimental means for probing radiationless transitions in polyatomic molecules.2-10Nitrogen-containing aromatic compounds have received a great deal of attention as benchmark systems for the investigation of electronic dephasing processes.11-16In particular,pyrazine is the best-studied molecule of an intermediate coupling cases.11,12,14,15The pyridine and substituted pyridine such as 2-chloropyridine,17have received great attention because of its potential environmental and health threats.It may enter the environment as a consequence of its extensive use as an insecticide and herbicide in agriculture and through industrial activities associated with pharmaceutical and textile manufacture and chemical synthesis.
Nonadiabatic dynamics is caused,due to the breakdown of Born-Oppenheimer approximation,by rapid vibrational motion at a conical intersection or the seam of crossing between two electronic states.Ultrafast electronic relaxation processes18play a central role in photochemistry and photobiology,internal conversion(IC)induced by electronic nonadiabaticity and intersystem crossing(ISC)induced by spin-orbit coupling being the two major nonradiative pathways.In polyatomic molecules excited to higher electronic states,internal conversion to vibronic levels of lower electronic states is one of the dominant mechanisms for deactivation.It has been established19,20that in pyridine and halogenated pyridines,there are at least one singlet-singlet(nπ*)electronic transition and one singlet-singlet(ππ*)electronic transition.
For a study on the electronic transition of 2-fluoropyridine, 2-chloropyridine,and 2-bromopyridine,Stephenson19observed solvent effects and concluded that the nπ*transition of 2-chloropyridine shifted into the spectral region of the ππ*transition or further.Medhi and Medhi21also observed the absorption spectrum of 2-fluoropyridine in the vapor phase.They showed that the lowest singlet state was the ππ*state,and the nπ*state lay closely above the ππ*state in 2-fluoropyridine.
In the present study,we explored the ultrafast dynamics on the corresponding processes of 2-chloropyridine as an example of a system with low symmetry.From a series of time-resolved photoelectron images,we can obtain photoelectron energy distributions.Photoelectron energy distributions reflect the ionization mechanism in which different photoelectrons are ionized from different states.The decay of photoelectron signals shows the population decay of the states from which the lifetimes of different states are determined.The internal conversion processes of the S2(the second excited state)state excited by the absorption of one photon of 266 nm have been directly observed in real-time.
2 Experimental methods
The time-resolved photoelectron imaging setup employed in this study has been described in detail elsewhere.22,23Briefly, the molecular beam apparatus consists of a beam source and a main chamber,both of which are pumped by turbo-molecular pumps.The liquid sample(2-chloropyridine,99.9%purity), 5%seeded in helium buffer gas at a background pressure of 2.02×10-5Pa,was expanded through a pulsed valve to generate a pulsed molecular beam.The molecular beam was skimmed and introduced into the ionization chamber where it was intersected perpendicularly by the laser beam.The electrons thus produced were accelerated parallel to the molecular beam,and then projected onto a position-sensitive imaging detector.The imaging detector consisted of a microchannel plate(MCP),a phosphor screen,and an intensified charge-coupled device (CCD)camera.In order to observe the total photoelectron and ion intensity,the emission from the phosphor screen was monitored with a photomultiplier tube(PMT).The field-free region (360 mm)of the time-of-flight(TOF)spectrometer was shielded with aµ-metal tube to avoid external magnetic fields that might otherwise deflect the electron trajectories.
The femtosecond laser system has been described in detail elsewhere.24Briefly,the seed beam was generated by a commercial Ti:sapphire oscillator pumped by a continuous wave (CW)second harmonic of an Nd:YVO4 laser,and then amplified by an Nd:YLF pumped regenerative amplifier to generate a 1 kHz pulse train centered at 800 nm of 45 fs pulse width with maximum energy of 1 mJ·pulse-1.The second harmonic pulse(400 nm)was generated in a 0.5 mm thick BBO crystal, and the third harmonic pulse(266 nm)was generated in a 0.2 mm thick BBO crystal by sum frequency mixing of the second harmonic and the fundamental.The pump and probe beams with vertical polarization were combined using a dichroic mirror,and then directed into the molecular beam chamber.The time delay between the pump and probe pulses was accurately monitored by a computer-controlled linear translation stage (PI,M-126.CG1).
3 Results and discussion

Fig.1 Typical time-of-flight mass spectrum of C5H4NCl following absorption of 266 nm photons and further probing with 800 nm laser pulses at zero time delay
The mass spectrum of 2-chloropyridine was obtained when the pump and probe beams overlapped.Two peaks are presented,corresponding to the C5H4NCl+parent ion at m/e of 113 and the C5H4N+ion at m/e of 78,as shown in Fig.1.The ratio of the area of the parent ion to fragment ion is 9:1.The spectrum shows the parent ion peak is dominant,while the other one is relatively weaker.The production of C5H4N+by ionizing C5H4N produced in the dissociation process is negligible at the employed probe intensities since it requires at least 7 photons of 800 nm in the probe step(ionization energy is 10.68 eV).25Moreover,the time-dependent ion signal of the fragment ions has a trend similar to that of the parent ion.Therefore,it is believed that most of the fragment ions are from the dissociation of C5H4NCl+parent ions.The contribution of the generation of fragment ions to the total photoelectron signal can be neglected.26No van der Waals cluster ions,such as(C5H4NCl)n,can be observed under our experimental conditions.There is also no Cl+existed in a single laser alone(pump or probe)or two beams together during our experiment.The ionization energy (IE)of Cl is 12.96 eV,27maybe the intensity of our laser is not enough to make the Cl and other fragments absorb three 266 nm photons or eight 800 nm photons and ionize.
2-Chloropyridine has a broad absorption peak from 220 to 300 nm and the absorption at 266 nm is relatively strong.28Considering the large spectral width of the femtosecond pump pulse in our experiment,the pump pulse excites 2-chloropyridine to its S2(nπ*)state.The polarizations of both the pump laser at 266 nm and the probe laser at 800 nm are vertical,and are parallel to the face of the detector.All measurements in this work were performed under conditions that minimized the ionization signals from either the 266 nm pump or 800 nm probe laser alone.
Fig.2 shows the time-resolved total ion signal of the parent ion formed after one photon excitation of the S2state at 266 nm as a function of the pump-probe delay.The ion signal reflects the excitation and decay of the excited states of 2-chloropyridine.It reveals that a single exponential model does not adequately reproduce the observed time profiles.In order to extract the complex dynamics starting at the S2state of 2-chloropyridine,at least two time components were expected to fit the time profile in Fig.2.However,the extensive fitting by two time components could not reproduce the observed signal very well,leaving the fitting uncertainties being even bigger than the fitted values.We performed curve fitting of the observed time profiles using a single exponential decay function,exp(-t/ τ).29The time constant τ of 2-chloropyridine estimated to be (162±5)fs,which reflects the excitation and decay of the initially excited S2state.The unsatisfactory fittings with two or three exponential functions are probably due to the extremely short lifetimes of the S2,S1(the first excited state),and S3(the third excited state)which are largely restricted by our instrument response function of 165 fs.The dynamics of those decays will be discussed later by energy analysis of the corresponding photoelectrons released from the probe laser pulse.
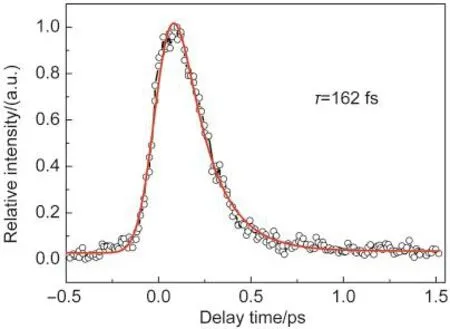
Fig.2 Time-resolved parent ion signal as a function of the delay timeThe circles are the experimental result and the solid red line is the fitted result.
In Fig.3,the typical photoelectron images were observed with a pump and probe laser wavelengths of 266 and 800 nm, respectively.Raw photoelectron images were reconstructed by the basis-set expansion method(BASEX).30The BASEX-inverted photoelectron images represent a section of the threedimensional(3D)photoelectron scattering distribution.Four concentric peaks appear in the observed images.The inner rings correspond to the lower kinetic energy electrons,while the outer rings are associated with the higher energy electrons. The corresponding time-resolved photoelectron kinetic energy (PKE)distributions which are extracted from images are shown in Fig.4.In Fig.4,we observe four peaks centered at 0.12,0.33,0.45,and 0.86 eV.The ionization potential31to the zero vibrational level of the cation(D0)of 2-chloropyridine was determined to be 9.68 eV,thus when the excited molecule absorbs four ionization photons,it reaches an energy of the ionization potential.The arrows in Fig.4 indicate the photoelectron energies 1.1,0.68,and 0.1 eV that we expected for the ionization31to the D0(9.68 eV),D1(10.18 eV),and D2(10.77 eV) by four-photon absorption of probe pulse.Hence,the observed signals are probably due to absorption of four photons from the ionization pulse by the excited 2-chloropyridine.The four peaks centered at 0.12,0.33,0.45,and 0.86 eV are assigned as the first,the second,the third,and the fourth peaks,respectively.

Fig.3 Aseries of time-resolved BASEX-inverted photoelectron imagesThe linear polarizations of the pump and probe lasers are aligned to be vertical in the plane of the figure.
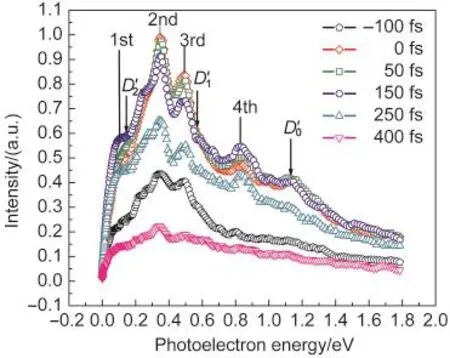
Fig.4 PKE distributions extracted from the imagesThe arrows indicate the photoelectron energy(1.1,0.68,and 0.1 eV)expected for the ionization to the zero vibrational level of the cation indicated by D0ʹ,D1ʹ,andD2ʹ,by four-photon absorption of probe pulse,respectively.Each of the PKE distributions has been normalized to the total photoelectron counts.
The energy levels of the two lowest excited electronic singlet states S1(ππ*)and S2(nπ*)of 2-chloropyridine were estimated to be 4.6 eV and≥4.6 eV,respectively.19It was found that the nπ*transitions of 2-chloropyridine,2-fluropyridine, and 2-bromopyridine lie higher than the ππ*transitions in energy.19Medhi and Medhi21also observed the absorption spectrum of 2-fluropyridine in the vapor phase.These authors have assigned the peak at 38047 cm-1to the origin of the lowest singlet ππ*state.These observations show that the lowest singlet state is the ππ*state,and the nπ*state shifts into the spectral region of the ππ*state in chloropyridine.19,20On the other hand, it was suggested by Sidman32that the nπ*shifts due to the electron donating resonance effect of the halogen substituent which increases the energy of the excited state relative to the energy of the ground state.
In 2-chloropyridine,it is known that the ionic state D0(9.68 eV)and D2(10.77 eV)have π-hole character and D1(10.18 eV) has n-hole character.31According to the Franck-Condon principle,the first peak in the PKE distributions can be assigned to the D2←S1ionization processes by four-photon absorption at 800 nm.Because the expected values can be calculated using the following equation,(Ee=nhv-D0-ΔEgap),which is about 0.1 above the 2-chloropyridine adiabatic D2,and very close to first peak value in Fig.4.The second and third peaks in the PKE distributions can be assigned to the D1←S2ionization processes by four-photon absorption at 800 nm.The expected values should be,0.68 eV,above the 2-chloropyridine adiabatic D1. The fourth peak(0.86)can be assigned to the D0←S1ionization processes by four-photon absorption at 800 nm.The expected value is 1.1 eV,so the S2which has nπ*character correlates with D1state,and S1which has ππ*character correlates with D0and D2states through Koopmansʹtheory.1As can be seen in
Fig.4,the photoelectron spectrum of the S1state has two peaks at about 0.12 and 0.86 eV,and the photoelectron spectrum of the S2state has two peaks at about 0.33 and 0.45 eV.Hence,the energy resolution allows the photoelectron spectra of S2and S1to be separated.This is also concluded by Satzger et al.33where the time-resolved photoelectron spectroscopy was used to reassign the four lowest lying cationic states of adenine and 9-methyl adenine.
As shown in Fig.4,the experimental PKE distributions during the first 150 fs can be characterized by a rapid decay of electrons from the second and third peaks and soft growth in the electrons from the first and fourth peaks.
After the first 150 fs of the delay time,all photoelectron peaks decrease slowly together at the same rate,as shown in
Fig.4.As demonstrated in the ion counts of the PKE distributions presented later,the slow decay corresponds to the first and fourth components with a time around of(5.5±0.3)ps.
To get more understanding into the changes of the four peaks with the pump-probe delay time,we show in Fig.5 the ion counts of the area of the second peak and those of the third peak as we think they are coming from the S2state.In the same way,we show the ion counts of the area of the first peak as we think that it is one of the S1peaks.We fit the ion counts of each peak in the raw images at different delays.An exponential fit directly reveals the decay times of the second and third peaks decay to be(204±5)fs and(279±5)fs,respectively,and there is no serious difference between the two fits,which is in excellent agreement with the time-dependence of the ion signal (162±5)fs.The approximate decay times of these two peaks suggest that these rings are due to ionization from the same state.In the same way,an exponential fit directly reveals the decay times of the first and fourth peaks to be about(5.3±0.3) ps and(5.8±0.3)ps,respectively.Therefore,the approximate decay times of these two peaks also suggest that these rings are due to ionization from the same state.
An exponential fit directly reveals that the decay time of the first peak was different from the ones of the second and third peaks.On the other hand,the different decay times of these peaks also suggest that these rings are due to ionization from the different states.Fig.5 clearly shows that the second and third peaks(Fig.5(a))exhibit a sharp rise,while the first peak (Fig.5(b))exhibits a slow rise.Thus,the second and third peaks are due to ionization of the populated S2state which may relax down to the vibrationally excited S1and S0states by rapid internal conversion.Since the ground state molecule will have 4.66 eV of vibrational excitation,which is essentially unavailable to the ionization process due to very small Franck-Condon factors to energetically accessible ion state,the appearance of the first and fourth peaks are due to population of the S1state by internal conversion from the S2state.
The results of PKE distributions contain more information than the overall internal conversion time.It can be seen from
Fig.4 that the relative intensities of the second and third photoelectron peaks corresponding to S2state decay very strongly while the ones of the first and fourth photoelectron peaks corresponding to S1state grow very slightly.In the same way,the integrated area values of the first and fourth peaks change with the time from 0 to 400 fs and the same case has been seen in the second and third peaks.This can be explained by proposing that the decay process of the populated S2state is attributed to simultaneous internal conversion from the S2state to the higher vibrational levels of S1and S0states with the later process being the dominant mechanism,if 2-chloropyridine has no other relaxation processes such as photochemical reaction from the vibronic level in the excited state.

Fig.6 Schematic description of the photoionization of 2-chloropyridineKoopmans correlations allow for a mapping of different excited states onto different ionic states.The known character of the excited states permits assignment of the various photoelectron peaks observed.The figure shows the assignment given in the text.
As shown in Fig.6,the pump laser pulse at 266 nm excites the molecule into S2state with the nπ*character.When the probe laser pulse ionizes the S2state,the ionic state should have n-hole ionic state according to Koopmansʹcorrelations.1However,after the internal conversion from S2to S1which has ππ*character,the ionic state should have a π-hole ionic state. The following dynamics requests that the S2/S0and S1/S0conical intersections,which lead to the internal conversion to the ground state from the S2and S1states.Anyway,the corresponding Franck-Condon factors between the ground state with high vibrational levels and the cation states are completely poor. The secondary population of the ground state has not been observed in our pump-probe experiments.Nibu et al.34measured the fluorescence excitation,multiphoton ionization,and dispersed fluorescence spectra of some substituted pyridines like 2-fluoropyridine.They concluded that the fact that the relaxation pass way to the triplet state is not the reason for the quantum yield decreases in the vibronic level of the ππ*state. Hence,the main reason for the quantum yield decrease in the excited state is attributed to the relaxation from S1to the ground state as the case of the S1state in pyridine.20,35,36Therefore,we adopt the same order of levels as for 2-chloropyridine. It was concluded that the constancy of fluorescence emissions yield value of 2-fluropridine34can be related to a much smaller energy gap between the S1and S2states.Therefore,HCTH/ 6-311+G*basis set was used for the quantum-chemical calculations using the Gaussian 03 package.37We found that the energy difference between the first and second states is about 0.13 eV which is very small.In this way,and according to the small energy gap between the S1and S2states in our experiment,it is possible that the slow decay component is the superposition of IC from S1and S2to S0.
4 Conclusions
We have used femtosecond time-resolved pump-probe photoelectron imaging to study real-time observation of ultrafast processes in optically excited states of 2-chloropyridine.2-Chloropyridine was excited by the absorption of one photon of 266 nm to the optically bright S2state and the internal conversion from S2state to S1state is observed by time-resolved PKE distributions in real time.The time scale of internal conversion from the S2to S1state was determined to be(162±5)fs according to the decay of the ion signals.Following dynamics involves the coupling of S2/S0and S1/S0conical intersections, which lead to the internal conversion to the ground state from the S2and S1states,the time constant of which has been estimated to be about(5.5±0.3)ps.According to the quantum-chemical calculations,the difference between the first and the second states is about 0.13 eV which is very small.
(1)Stolow,A.;Bragg,A.E.;Neumark,D.M.Chem.Rev.2004, 104,1719.doi:10.1021/cr020683w
(2) Suzuki,T.Annu.Rev.Phys.Chem.2006,57,555.doi:10.1146/ annurev.physchem.57.032905.104601
(3) Neumark,D.M.Annu.Rev.Phys.Chem.2001,52,255.doi: 10.1146/annurev.physchem.52.1.255
(4) Seideman,T.Annu.Rev.Phys.Chem.2002,53,41.doi:10.1146/ annurev.physchem.53.082101.130051
(5) Reid,K.L.Annu.Rev.Phys.Chem.2003,54,397.doi:10.1146/ annurev.physchem.54.011002.103814
(6) Tang,Y.;Ji,L.;Tang,B.F.;Zhu,R.S.;Zhang,S.;Zhang,B. Acta Phys.-Chim.Sin.2004,20,344.[唐 颖,姬 磊,唐碧峰,朱荣淑,张 嵩,张 冰.物理化学学报,2004,20,344.] doi:10.3866/PKU.WHXB20040402
(7)Tsubouchi,M.;Whitaker,B.J.;Wang,L.;Kohguchi,H.; Suzuki,T.Phys.Rev.Lett.2001,86,4500.doi:10.1103/ PhysRevLett.86.4500
(8)Matsumoto,Y.;Kim,S.K.;Suzuki,T.J.Chem.Phys.2003, 119,300.doi:10.1063/1.1578062
(9) Zhang,F.;Cao,Z.Z.;Qin,X.;Liu,Y.Z.;Wang,Y.M.;Zhang, B.Acta Phys.-Chim.Sin.2008,24,1335.[张 锋,曹振洲,覃 晓,刘玉柱,王艳梅,张 冰.物理化学学报,2008,24, 1335.]doi:10.1016/S1872-1508(08)60055-8
(10) Suzuki,T.;Wang,L.;Kohguchi,H.J.Chem.Phys.1999,111, 4859.doi:10.1063/1.479822
(11) Frad,A.;Lahmani,F.;Tramer,A.;Tric,C.J.Chem.Phys.1974, 60,4419.doi:10.1063/1.1680920
(12) Lahmani,F.;Tramer,A.;Tric,C.J.Chem.Phys.1974,60,4431. doi:10.1063/1.1680921
(13)Yamazaki,I.;Murao,T.;Yamanaka,T.;Yoshihara,K.Faraday Discuss.Chem.Soc.1983,75,395.doi:10.1039/dc9837500395
(14) Knee,J.L.;Doany,F.E.;Zewail,A.H.J.Chem.Phys.1985, 82,1042.doi:10.1063/1.448572
(15) De Lange,P.J.;Drabe,K.E.;Kommandeur,J.J.Chem.Phys. 1986,84,538.doi:10.1063/1.450125
(16) Zhong,D.;Diau,E.W.-G.;Bernhardt,T.M.;Feyter,S.D.; Roberts,J.D.;Zewail,A.H.Chem.Phys.Lett.1998,298,129. doi:10.1016/S0009-2614(98)01160-9
(17) Vlastosa,D.;Skoutelisa,C.G.;Theodoridisa,I.T.;Stapletonb, D.R.;Papadakia,M.I.J.Hazard Mater.2010,177,892.doi: 10.1016/j.jhazmat.2009.12.117
(18)Tsubouchi,M.;Whitaker,B.J.;Suzuki,T.J.Phys.Chem.A 2004,108,6823.doi:10.1021/jp0484985
(19) Stephenson,H.P.J.Chem.Phys.1954,22,1077.doi:10.1063/ 1.1740268
(20) Ram,K.;Pandeya,B.R.;Tripathib,R.S.Spectrosc.Lett.1977, 10,893.doi:10.1080/00387017708065026
(21) Medhi,K.C.;Medhi,R.N.Spectrochim.Acta A 1990,46,1169. doi:10.1016/0584-8539(90)80193-3
(22)Liu,Y.Z.;Qin,C.C.;Zhang,S.;Wang,Y.M.;Zhang,B.Acta Phys.-Chim.Sin.2011,27,965. [刘玉柱,秦朝朝,张 嵩,王艳梅,张 冰.物理化学学报,2011,27,965.]doi:10.3866/ PKU.WHXB20110404
(23)Qin,C.C.;Liu,Y.Z.;Zhang,S.;Wang,Y.M.;Tang,Y.;Zhang, B.Phys.Rev.A 2011,83,033423.doi:10.1103/PhysRevA. 83.033423
(24) Liu,Y.Z.;Tang,B.F.;Zhang,S.;Zhang,B.Opt.Express 2010, 18,5791.doi:10.1364/OE.18.005791
(25)Yim,M.K.;Choe,J.C.J.Phys.Chem.A 2011,115,3087.doi: 10.1021/jp110074r
(26)Kadi,M.;Davidsson,J.;Tarnovsky,A.N.;Rasmusson,M.; Akesson,E.Chem.Phys.Lett.2001,350,93.doi:10.1016/ S0009-2614(01)01283-0
(27) Kimura,K.;Yamazaki,T.;Achiba,Y.Chem.Phys.Lett.1978, 58,104.doi:10.1016/0009-2614(78)80326-1
(28)Brown,H.C.;McDaniel,D.H.J.Am.Chem.Soc.1955,77, 3752.doi:10.1021/ja01619a022
(29) Suzuki,Y.;Horio,T.;Fuji,T.;Suzuki,T.J.Chem.Phys.2011, 134,184313.doi:10.1063/1.3586809
(30) Dribinski,V.;Ossadtchi,A.;Mandelshtam,V.A.;Reisler,H. Rev.Sci.Instrum.2002,73,2634.doi:10.1063/1.1482156
(31) Modelli,A.;Distefano,G.J.Electron Spectrosc.Relat.Phenom. 1981,23,323.doi:10.1016/0368-2048(81)80037-0
(32) Sidman,J.W.Chem.Rev.1958,58,689.doi:10.1021/ cr50022a004
(33) Satzger,H.;Townsend,D.;Stolow,A.Chem.Phys.Lett.2006, 430,144.doi:10.1016/j.cplett.2006.08.122
(34)Nibu,Y.;Okabe,C.;Ohsaki,T.;Shimada,H.J.Phys.Chem.A 2006,110,6047.doi:10.1021/jp057314z
(35) Bumaliya,A.;Zhu,R.;Long,J.;Xu,Y.;Liu,Y.,Ghazal,A.Y.; Yang,M.;Zhang,B.J.Chem.Phys.2011,134,234301.doi: 10.1063/1.3600334
(36) Qiu,X.;Zhu,R.;Xu,Y.;Bumaliya,A.;Zhang,S.;Zhang,B. Chin.J.Chem.Phys.2011,24,551.doi:10.1088/1674-0068/24/ 05/551-556
(37) Frisch,M.J.;Trucks,G.W.;Schlegel,H.B.;et al.Gaussian 03, Revision B.01;Gaussian Inc.:Pittsburgh,PA,2003.
June 26,2012;Revised:August 13,2012;Published on Web:August 13,2012.
Ultrafast Internal Conversion Dynamics of 2-Chloropyridine by Femtosecond Time-Resolved Photoelectron Imaging
GHAZALAhmed-Yousif QIU Xue-Jun QIN Chao-Chao LONG Jin-You ABULIMITI Bumaliya ZHANG Bing*
(State Key Laboratory of Magnetic Resonance and Atomic and Molecular Physics,Wuhan Institute of Physics and
Mathematics,Chinese Academy of Sciences,Wuhan 430071,P.R.China;Graduate University of Chinese Academy of Sciences, Beijing 100049,P.R.China)
Ultrafast internal conversion dynamics of 2-chloropyridine were studied by femtosecond time-resolved photoelectron imaging spectroscopy coupled with time-resolved mass spectroscopy.The ultrafast internal conversion from the second excited state(S2)to the first excited state(S1)via an adjacent conical intersection within(162±5)fs was clearly observed from the time-dependence of the photoelectron spectra.The subsequent deactivations involved the coupling of S2/S0(the ground state)and S1/S0conical intersections,which occurred on a timescale of about(5.5±0.3)ps,and led to the internal conversion to the ground state from the S2and S1states.
Photoelectron imaging;Ultrafast process;Internal conversion;2-Chloropyridine
10.3866/PKU.WHXB201208135
O644
∗Corresponding author.Email:bzhang@wipm.ac.cn;Tel:+86-27-87197441;Fax:+86-27-87198491. The project was supported by the National Natural Science Foundation of China(91121006).
国家自然科学基金(91121006)资助项目
