蟾蜍甾烯14β-artebufogenin的核磁共振光谱信号全归属
2012-11-23裴月湖贾继明孟作环
乔 莉,裴月湖,贾继明,宋 剑,孟作环
1石家庄以岭医药研究院,石家庄050035;2沈阳药科大学中药学院,沈阳110016
蟾蜍甾烯14β-artebufogenin的核磁共振光谱信号全归属
乔 莉1,2*,裴月湖2,贾继明1,宋 剑1,孟作环1
1石家庄以岭医药研究院,石家庄050035;2沈阳药科大学中药学院,沈阳110016
本论文利用柱色谱法从中药蟾酥中分离得到一个蟾蜍甾烯化合物,利用二维核磁共振和其它化学及光谱学手段对其结构进行了鉴定,并首次给出了其完整的核磁信号归属。
蟾酥;蟾蜍甾烯;1H和13C核磁共振;14β-artebufogenin;二维核磁共振
Introduction
Bufadienolides represent a type of steroids with A/B cis and C/D cis structures and a β-2-pyrone ring at the 17-position.These compounds possess potent cardiotonic,blood pressure-stimulating,antiviral,and local anesthetic activities.More than 300 bufadienolides have been isolated from natural sources including plants and animals,which have been reported to have significant antitumor activities[1].Chinese traditional drug Chansu is usually used as a cardiac and it composed mostly by over thirty bufadienolides such as resibufogenin,cinobufagin,and bufalin.In this paper,a bufadienolide named 14β-artebufogenin(1)was isolated from Chansu(Venenum Bufonis).The present study reports the isolation and characterization of it and the spectral data of compound 1 were reported for the first time.
Materials and Methods
General experimental procedures
Melting point was measured with a Yanako MS-S3 (Yanaco Co.Ltd,Kyoto,Japan)micro melting point apparatus and was uncorrected.UV spectrum was measured on a Shimadzu UV-1601.IR spectrum was measured on a Bruker IFS 55 spectrometer.All the NMR spectra were taken on a Bruker-ARX-600 spectrometer(1H at 600 MHz and13C at 150 MHz).Column chromatography was performed on silica gel G (200-300 mesh,Qingdao Haiyang Chemical Factory) and C-18 preparative HPLC(Shimadzu).
Materials
Chansu was obtained in Anguo Folk-Medicinal Market,Hebei province,China,in March of 2005,and identified as dried secretion of Bufo bufo gargarizans Cantor by Prof.SUN Qi-shi.
Extraction and isolation
Chansu(500 g)was ground into a rough powder and extracted with chloroform in a Soxhlet's apparatus.The extract was concentrated under reduced pressure,and the residue(150 g)was subjected to silica gel column chromatography,eluting with petroleum ether-acetone (increasing acetone 0-100%).Fifteen fractions(fractions I-XV)were obtained and fraction IV(petroleum ether-acetone 100∶15,100 mg)was rechromatographed on preparative HPLC.Compound 1(5 mg)was obtained(methanol-water 63∶27).
Results and Discussion
Compound 1 was obtained as colorless crystal (CH3OH),and positive to Liberman-Burchard reaction,which indicated 1 to be a steroid.The positive ESIMS showed a pseudo molecular ion at m/z 407[M+ Na]+and the molecular formula was established as C24H32O4by HRFABMS at m/z 385.2322[M+H]+combined with1H and13C NMR data.The UV and IR absorption spectra suggested the presence of 2-pyrone ring(296 nm and 1635 cm-1)and ketone group(1712 cm-1)[2].In the1H NMR spectrum,H-21,H-22,and H-23 signals(at δ7.57,7.68 and 6.28,respectively) were characteristic of the 2-pyrone ring of bufadienolide[2].All the information above showed that Compound 1 was a bufadienolide.In the HMBC spectrum,the correlations between the proton signal at δ2.69 and 120.9(C-20),δ149.8(C-21),and δ146.5(C-22) indicated that the proton at δ2.69 was assigned to H-17.The methyl proton signal at δ0.79 was assigned to H-18 according to its HMBC correlations with C-17 (δ42.6),C-12(δ40.1),C-13(δ42.2),and C-14 (δ55.8).Therefore,the other methyl proton signal at δ0.81 was assigned to H-19.In the HMBC spectrum the correlation between H-14(δ2.13),H-16(δ2.79,2.24),and C-15(δ217.8)indicated that C-15 was a carbonyl carbon.

Fig.1 The structure of compound 1
The relative stereochemistry of Compound 1 was determined by the analysis of NOESY spectrum(Fig.2).The NOESY correlation of H-18/H-8,H-8/H-14,and H-18/H-14 indicated that H-14 was β-oriented.So the structure of Compound 1 was determined as 14β-artebufogenin,which probably derivatized from resibufogenin by the solvent chloroform in an acid environment.Compound 1 was first reported in 1959 by Linde Horst[3]but the author did not give any experimental data.In this paper,the spectral data of Compound 1 were reported for the first time.
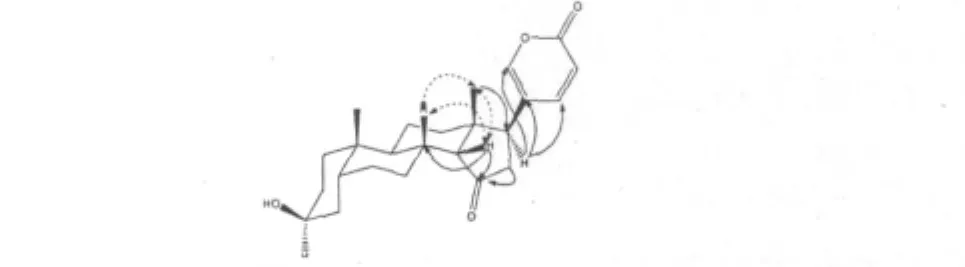
Fig.2 Key HMBC and NOESY correlations of compound 1
Compound 1 Colorless plate(MeOH);mp 190-192℃;UV(MeOH)λmaxnm:298,203;IR(KBr)νmax: 3400,2869,1712,1635,1243,1152,1031,956 cm-1;1H and13C NMR spectral data see table 1;

Table 1 The NMR spectral data of compound 1 600 MHz for1H NMR,150 MHz for13C NMR,DMSO-d6

1.54(1H,m) 34.7 C-8,C-11 10 33.2 11 1.65(1H,m),1.54(1H,m) 22.6 C-9,C-12,C-13 12 2.51(1H,m),2.48(1H,m) 40.1 C-9,C-13 13 42.2 C-12,C-17,C-19 14 2.13(1H,brs) 55.8 C-8,C-13,C-15,C-16 15 217.8 16 2.79(1H,dd,9.9,4.8),2.24(1H,d,9.9) 41.4 C-14,C-15,C-17 17 2.69(1H,brd,4.8) 42.6 C-13,C-16,C-20,C-21,C-22 18 0.79(3H,s) 21.0 C-12,C-13,C-14,C-17 19 0.81(3H,s) 23.7 C-1,C-5,C-9,C-10 20 120.9 21 7.68(1H,d,2.1) 149.8 C-20,C-22 22 7.58(1H,dd,9.6,2.1) 146.5 C-20,C-21,C-23 23 6.28(1H,d,9.6) 115.0 C-20,C-22 24 161.1 3β-OH 4.13(1H,s) 9
Acknowledgements
The authors thank Mr.Sha Yi and Ms.Li Wen for the measurements of NMR spectra.
1 Ye M,Han J,Guo HZ,et al.Structural determination and complete NMR spectral assignments of a new bufadienolide glycoside.Mag Res Chem,2002,40:786-788.
2 Nogawa T,Kamano Y,Yamashita A,et al.Isolation and structure of five new cancer cell growth inhibitory bufadienolides from the Chinese traditional drug Ch'an Su.J Nat Prod,2001,64:1148-1152.
3 Linde H,Meyer K.Toad poisons.XVII.Constitution of resibufogenin.Helv Chim Acta,1959,42:807-826.
October 29,2010;Accepted March 14,2011
This research project was supported by the National Key Scientific and Technological Project in 11th Five-year Plan (2009ZX09102-140)
Complete NMR Spectral Assignments of Bufadienolide 14β-Artebufogenin
QIAO Li1,2*,PEI Yue-hu2,JIA Ji-ming1,SONG Jian1,MENG Zuo-huan11Yiling Medical Research Academy,Shijiazhuang 050035,China;2School of Traditional Chinese Materia Medica,Shenyang Pharmaceutical University,Shenyang 110016,China
In this paper we studied the chemical constituents from traditional Chinese drug Chansu.One bufadienolide which named as 14β-artebufogenin was separated by column chromatography and its structure was elucidated based on chemical and spectral analysis.The complete assignments of its1H and13C NMR spectral signals were assisted by 2D NMR spectra methods.
Chansu;bufadienolides;1H and13C NMR;14β-artebufogenin;2D NMR
1001-6880(2012)05-0624-03
*Corresponding author Tel:86-311-85901304;E-mail:ql200207@yahoo.com.cn
Q284.2
A
