Tumor-Imaging Core-Shell Nano-Models for Catalase
2012-11-09YANGXiaCHENQiuYunSONGJingBao
YANG Xia CHEN Qiu-Yun SONG Jing-Bao
(School of Chemistry and Chemical Engineering,Jiangsu University,Zhenjiang,Jiangsu 212013,China)
Tumor-Imaging Core-Shell Nano-Models for Catalase
YANG Xia CHEN Qiu-Yun*SONG Jing-Bao
(School of Chemistry and Chemical Engineering,Jiangsu University,Zhenjiang,Jiangsu212013,China)
Microwave synthesis approach has been developed for preparing Mn-silica core-shell nano-complexes with fluorescent imaging by assembling the Mn(Ⅱ)complexes of bis(2-pyridylmethyl)amino-2-propionic acid(Adpa) on the surface of silica core-shell nanoparticles.IR,UV,TEM were used to characterize the structure of nanocomplexes.The results of H2O2disproportionation show that Mn-silica core-shell nano-complexes have good analog characteristics of catalase as a new kind of nano-models for catalase.Cell fluorescence image in vitro indicates that these Adpa modified nanoparticles locate outside of tumor cells,in contrast,Mn-silica core-shell nano-complexes could enter tumor cells which enables simultaneous tumor-targeting and good fluorescent imaging as a new tumor imaging agent.
core-shell nanosphere;nano-complex;imaging,cancer
0 Introduction
Multifunctionalnanoparticles with a core-shell architecture,which combine many functionalities such as imaging,targeting,drug delivery,and therapy in a single stable construct,show greatpromise in biomedical applications[1-3]. Silica is a hydrophilic material that is photophysically inert and the surface of the organic-modified silica nanoparticles can be functionalized with a variety of active groups such as amine and carboxyl groups for specific function[4-5]. Rhodamine B isothiocyanate doped silica-coated coreshell fluorescent dye nanoparticles can specifically recognize early-stage apoptotic cells through the binding of surface conjugated Annexin V to the phosphatidylserine on the outer membrane of apoptoticcells[6].Manganese(Ⅱ) ions are the required co-factor for many ubiquitous enzymes and mitochondria can accumulate Mn(Ⅱ) ions through an ATP-dependent Ca transporter[7].The complex of Mn(Ⅱ)-dpa (dpa=di (picolyl)amines)modified SiO2@Mn(Ⅱ)-dpa nanoparticles was used to targetcancer cells through intracellular Ca2+signaling mitochondria accumulating in vivo formagnetic resonance imaging (MRI) detection[8].In addition to attenuation of the absorption of calcium in mitochondria,most transition metal complexes of di(picolyl)amine were reported as models of non-heme dioxygenase[9].Manganese(Ⅱ) complexes [Mn2(μ-O2CCH3)3L2]+(L=bis(pyridylmethyl)amine)and aminopyridine manganese(Ⅱ) complexes were studied for their ability to disproportionate H2O2and to produce highly value intermediates[10].These mean that manganese(Ⅱ)complexes with the derivatives of bis(2-pyridylmethyl)amine could disproportionate H2O2or react with H2O2to produce oxidant.Cellular levels of H2O2play directly orindirectly a key role in maglignant transformation and sensitize cancer cells to death.Overpression of H2O2-detoxifying enzymes or human catalase in vivo can result in H2O2concentration decreasing and cancer cells reverting to normalappearance[11].Recently,we found that carboxylate-bridged dimanganese(Ⅱ)systems were good models for catalase and exhibited good inhibition on the proliferation ofU251 and HeLa cells.The inhibiting activity of these manganese(Ⅱ) complexes on thetumorcellsin vitroisrelated totheir disproportionating H2O2activity[12].It is reported that nano-particles with size of 1~100 nm can accumulate in cancer cells selectively[13-14].Here,we report a class of core-shell architecture to assemble the Mn(Ⅱ)complexes of Adpa(Adpa=bis(2-pyridylmethyl)amino-2-propionicacid)on thesurface ofsilica shell nanoparticles and nano complexes with imaging and mimics of catalase.
1 Experimental
1.1 Instruments and Materials
Rhodamine B isothiocyanate (RBITC),Triton X-100, 3-aminopropyltrimethoxysilane (APTS), ammonium hydroxide,N,N-dicyclohexylcarbodiimide (DCC),N-hydroxysuccinimide (NHS),cetyltrimethylam-monium bromide (CTAB)and solvents were of analytical grade.Water was purified with a Millipore Milli-Q system (25℃:18.2 MΩ·cm,7.20×10-2N· m-1).Rhodamine B isothiocyanate doped silica-coated fluorescent nanoparticles (RBITC@SiO2)and bis(2-pyridylmethyl)amino-2-propionic acid (Adpa)were prepared according to the reported method[5,7].TEM was performed at room temperature on a JEOL JEM-200CX transmission electron microscope using an accelerating voltage of 200 kV.FTIR characterizations were performed using a Nicolet Nexus 470 FTIR spectrophotometer in the spectral range of 4 000~400 cm-1.The electronic absorption spectrum was recorded using a UV-2450 UV-Vis spectrophotometer at room temperature.Photoluminescent emission spectraweremeasured on Varian CarryEclipse spectrofluorometer.The content of Mn(Ⅱ) ions in the nanoparticle wasmeasured by TAS-986 Atomic absorption spectrophotometer.
1.2 Synthesis of RBITC@SiO2-Adpa and RBITC @SiO2-AdpaMn
The Adpa molecules were covalently linked with 3-aminopropyltriethoxysilane(APTS)to form an APTSAdpa conjugate.DCC was used to ensure that the carboxylic group of Adpa selectively reacts with the amino group of APTS.Adpa (0.1 mL,0.35 mmol), DCC (22.5 mg,0.11 mmol)and NHS (40.3 mg,0.35 mmol)were sequentially added into a 5.0 mL N,N-dimethyl-formamide(DMF)solution.The mixture was stirred for 12 h at room temperature.After the filtration,85.3 mg(0.38 mmol)of APS was added into the solution and the solution was stirred for 24 h at room temperature.The obtained solution was directly used without further treatment.
0.044 8 g Rhodamine B isothiocyanate doped silica-coated fluorescent nanoparticles(RBITC-SiO2) was dispersed in 25 mL of toluene,0.1 mL of asprepared Adpa-APTS conjugates and 20 μL APS were added into the above solution.After the solution was transferred into a flask under nitrogen for 10 minutes, the reaction was allowed to proceed for 1.5 h at 70℃ in microwave reactor.After centrifuged and washed with ethanol,nanoparticles(RBITC@SiO2-Adpa)were then obtained.
The obtained RBITC@SiO2-Adpa (0.485 g)was dispersed in ethanol(25 mL),MnAc2·4H2O(0.068 g, 0.014 mmol)was added into the solution and the solution was stirred for 2 h at room temperature. Nanoparticles(RBITC@SiO2-AdpaMn)were obtained after centrifuging and washing with methanol.The amount of Mn(Ⅱ) ions in RBITC@SiO2-dpa-Mn was evaluated by atomic absorption spectra after the nanoparticles were cooked with excess amount of nitric acids at 70℃for 3 h.The content of Mn(Ⅱ) in the nanoparticles is 1.13%.
1.3 Catalase-like Activity
All of the reactions between the nano-complexes and dihydrogen peroxide were performed in buffered (Tris/Tris-HCl,0.1 mol·L-1,NaClO40.1 mol·L-1,pH= 7.1)solutions at 0℃ and 37℃.The reactivity of the complexes with H2O2was first investigated in buffered solutions via UV-Vis spectroscopy titration at 0℃and 37℃.After the solution (5 mL)of nano-complexes (0.1 mol·L-1)was stirred at 0℃ and 37℃ for 30 min,0.5 mL of H2O2aqueous solution (30%)was added,and the spectra were recorded at 10 min intervals at 0℃ or 2 min intervals at 37℃.The volumetric measurements of the evolved dioxygen produced during the reaction of the complexes with H2O2were performed in triplicate as follows:a 10 mL round-bottom flask containing a nano-complex(3×10-4mol·L-1,3.0 mL)in a buffered system was placed in an ice (273.0±0.1 K)bath.The flask was closed with a rubber septum,and a cannula was used to connect the reaction flask to an inverted graduated pipet,filled with water.While the solution containing the nanocomplex was stirred,a solution of 0.5 mL of H2O2aqueous solution was added through the septum using a microsyringe.The volume of oxygen produced was measured in the pipet.The kinetic measurements for nano-complexes were performed in Tris/Tris-HCl solution at37 ℃.Differentconcentrations of dihydrogen peroxide were prepared by diluting the 30% H2O2aqueous solution with Tris/Tris-HCl solution.The optimum reaction order of the substrate with respect to the complexes was determined by reacting different concentrations of complexes with a constant concentration of substrate.Similarly,the optimum reaction order of the complexes with respect to the substrate was determined by reacting different concentrations of substrate with a constant concentration of complexes.
1.4 Cellular uptake and imaging
Human cancer cell line HeLa was obtained from Cancer Cell Repository (Shanghai cell bank).Cells were maintained in RPMI-1640 medium and DMEM medium (Gibco,USA)supplemented with 10%(V/V) heat-inactivated fetal bovine serum,antibiotics(100 U·mL-1penicillin and 100 U·mL-1streptomycin),at 37°C in a humidified atmosphere of 5%CO2.HeLa cells(2.4×104)were seeded into 24-well plates(Every plate was 100 μL)and cultured for 24 h,then nanoparticles (testnanoparticles (2.0 mg)were dispersed in H2O and diluted with culture media)were added and incubated for 3 h.At last,cells were washed with PBS twice to remove the free nanoparticles.Nanoparticles uptake and imaging of HeLa cells were observed using Nikon Ti-E2000 microscope with live cell system (LCS)which can provide CO2,temperature control and position fixing. The bright and fluorescence imaging of cells(ex.555 nm)were recorded and analyzed.
2 Results and discussion
2.1 Synthesis and characterization
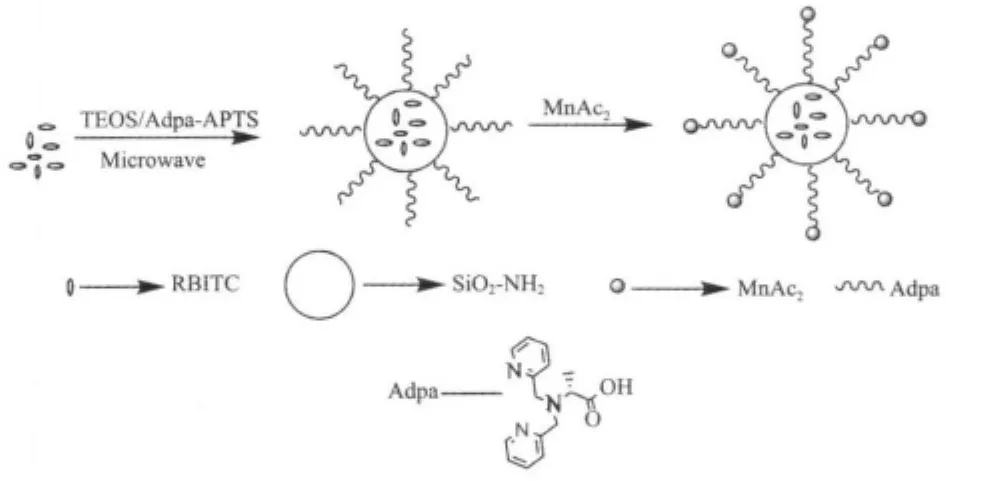
Scheme 1 Synthetic route of RBITC@SiO2-AdpaMn
Scheme 1 sketches this synthesis procedure from fluorescent dye molecules towards fluorescence coreshell nano-models forcatalase.Normally,reverse microemulsion method was used to prepare rhodamine B isothiocyanate doped silica-coated(RBITC@SiO2) and furthermodification ofAPS derivativeswas carried out by refluxing for 24 h in toluene[5].Although the process is useful,it takes long reaction time and produces by-products thatlimitbioapplications.Here higher yielded grafted nanomodels were obtained through the microwave synthesis.These indicate that microwave assisted synthesis approach is effective and fast to the modification of silica.
FTIR spectra (Table 1)ofthe two silica nanoparticles confirmed the existence of amino in the purified nanoparticles,showing characteristic peaks around 3 417 and 2 928 cm-1that correspond to stretching bands of N-H and C-H.The IR spectra show pyridyl ring bands of νas(C=N)at approximately 1 609 cm-1and the δ(CH)vibration of the pyridyl ring at 794 cm-1.The peaks at 1 659 cm-1indicates the existence of-CO-NH of Adpa.The strong peaks around 1 100 cm-1in Table 1 confirm the existence of SiO2which corresponds to stretching band of Si-O.

Table 1 IR bands(cm-1)of RBITC@SiO2-Adpa and RBITC@SiO2-AdpaMn
The bands at 278 nm of pyridyl rings dominate the UV spectra for RBITC@SiO2-AdpaMn nanoparticles and RBITC@SiO2-Adpa nanoparticles confirming the existence of Mn(Ⅱ)-Adpa grafted silica nanoparticles.558 nm ofπ-π* transition bands belongs to rhodamine B isothiocyanate(Fig.1).
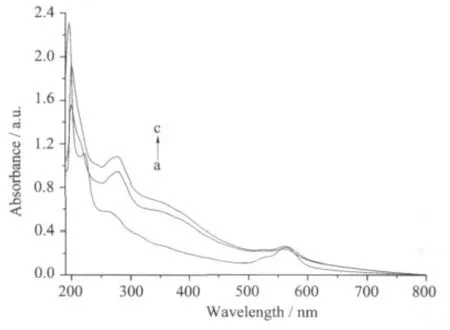
Fig.1 UV spectra for RBITC@SiO2(a,2.1 mg), RBITC@SiO2-AdpaMn(b,3.3 mg), RBITC@SiO2-Adpa(c,2.9 mg)dispersed in Tris-HCl(pH=7.1)(10 mL)
The content of surface modified Adpa in the nano-models is 0.08 mmol·L-1according to the bands at 278 nm of free Adpa.The emission bands at 582 nm show thatthe fluorescence intensity ofthe RBITC@iO2-Adpa system little decreases upon the surface modification with Mn (Fig.3 line a).The hypochromism possibly is due to the self-quench of the Mn ions because the transition-metal ions (Mn2+) have unsaturated d-layer electrons[14].However,the fluorescence intensity at 582 nm of RBITCSiO2@AdpaMn increases after addition of 0.5 mLH2O2,indicating the nano-complex could possibly bind with H2O2to produce new intermediates.The nanoparticle suspension was added drop-wise onto a carbon-coated copper membrane and dried at room temperature.Particle size wasmeasured with a Hitachi-800 transmission electron microscope.The diameters of RBITC@SiO2-Adpa and RBITC@SiO2-AdpaMn are 90~100 nm(Fig.3)
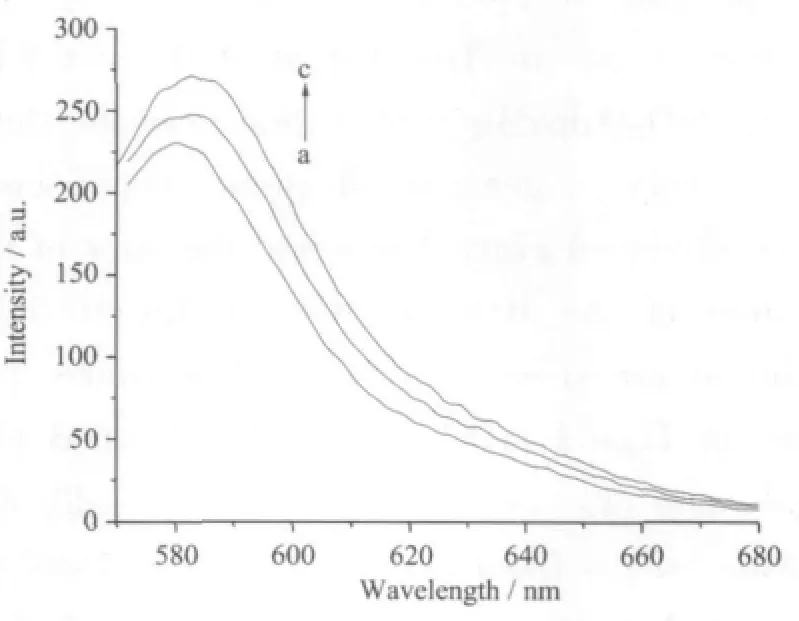
Fig.2 Fluorescence emission spectra for RBITC@SiO2-AdpaMn(a,3.3 mg),RBITC@SiO2-Adpa (b,2.9 mg)and H2O2added to RBITC@SiO2-AdpaMn(c,3.3 mg)in Tris-HCl(pH=7.1) (10 mL),the slit widths at the excitation and emission of the spectrofluorimeter is 5

Fig.3(a)TEM images for RBITC@SiO2-Adpa
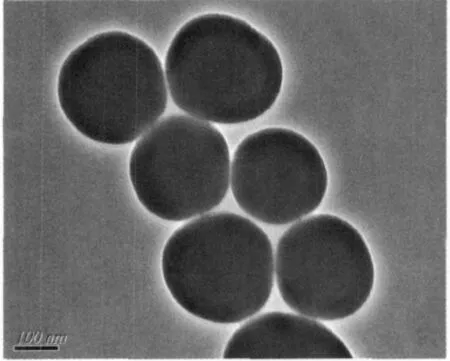
Fig.3(b)TEM images for RBITC@SiO2-AdpaMn
2.2 Catalase-likeactivitymeasuredbyO2evolution -kinetics studies
The O2volume evolution was used for the study ofkinetic ofH2O2disproportionation.The H2O2disproportionation promoted by RBITC@SiO2-AdpaMn was carried out in Tris-HCl at 0 ℃ and 37 ℃. RBITC@SiO2-AdpaMn can disproportionate dihydrogen peroxide to generate dioxygen.The volume of oxygen produced every 2 min and the rates of the O2evolution of the RBITC@SiO2-AdpaMn at different conditions are shown in Fig.4.1.The kinetic plot is shown in Fig.4.2 and Fig.4.3.The obtained plot of initialrate vs concentration ofthe dihydrogen peroxide (E)is fitted by Hill equationAccording to the equation,the value of KMis 0.2150 mmol·L-1,turnover Number Kcatcalculated from the equation Kcat=Vmax/cE,tis 0.5368 s-1[16].The value of kcat/KMis used to evaluate the catalase activity.kcat/KMof the nano-model is 2 496.7,which shows that the nano-complex has good activity.The condition of the reaction carried out at 37℃ in Tris-HClsolution is mimetic condition ofthe cell environment.So we deduce that nano-complex would also show good catalase activity in vitro.
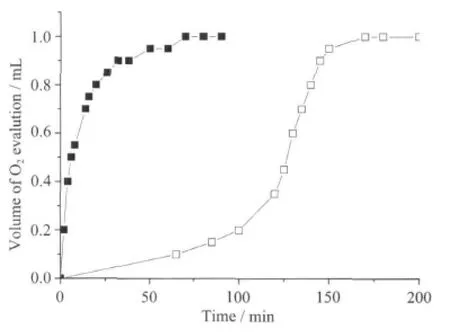
Fig.4.1 Rates of the O2evolution for the RBITC@SiO2-AdpaMn(nano-complex)at different conditions, cnano-complex=0.3 mmol·L-1,30%H2O2 aqueous solution 0.5 mL,Vsolvent=3 mL.37℃in Tris-HCl(■),0℃in Tris-HCl(□)

Fig.4.2 V0vs Concentration of H2O2plots of RBITC@ SiO2-AdpaMn(0.3 mmol·L-1)in Tris-HCl,37℃
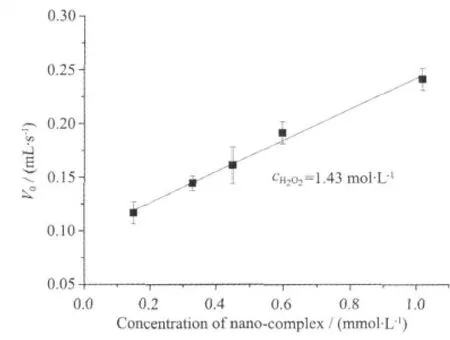
Fig.4.3 Initial rate(V0)of substrate consumption vs concentration of RBITC@SiO2-AdpaMn in Tris-HCl 37℃
2.3 Cell uptake and imaging
The fluorescence images of the RBITC@SiO2-Adpa and RBITC@SiO2-AdpaMn were assayed using HeLa cellline.For HeLa cells treated with RBITC@SiO2-Adpa, the red fluorescence image indicates these nanoparticles located outside of HeLa cells due to that the relative larger size and low water solubility result in no internalization of the nontarget control.On the contrary,the intense fluorescence image of cell is observed when HeLa cells incubate with the RBITC@SiO2-AdpaMn.The results are shown in Fig.5.A total of about 95%of cells display the RBITC signal.All emissions from the nanoparticle containing culture medium surrounding the cells are removed by washing the cells with PBS.Electron micrographs of the cells provide direct evidence that a large numberof RBITC@SiO2-AdpaMn are possibly endocytosed by the HeLa cells.The mitochondrial membrane potentials of tumor cells are higher than those of normal cells,most agents have a positively charged moiety that takes advantage of electrostatic forces in locating its target[17].These preliminary data reveal that the targeting ability of nanospheres to HeLa cells is enhanced greatly due to the Adpa-Mn complex conjugation onto the surface of the RBITC@SiO2producing the positive charged RBITC@SiO2-AdpaMn and allowing passive accumulating and mitochondrial membrane of cancer cells induced accumulation.

Fig.5 Fluorescence images of HeLa cells treated with nanoparticles
3 Conclusions
A Nano-complex (RBITC@SiO2-AdpaMn)with catalase activity was synthesized and characterized. It is found that the nano-complex could disproportionate H2O2effectively to oxygen,so it is a good mimic of catalase.Furthermore,we found that RBITC@SiO2-AdpaMn could target and imaging tumor cell by allowing passive accumulating and mitochondrialmembraneofcancercellsinduced accumulation.
[1]Sanvicens N,Marco M P.Trends Biotechnol.,2008,26(8):42-433
[2]Wu W T,Shen J,Banerjee P,et al.Biomater.,2010,31(29): 7555-7566
[3]Zhang R R,Wu C L,Tong L L,et al.Langmuir,2009,25 (17):10153-10158
[4]Bonacchi S,Genovese D,Juris R,et al.Angew.Chem.Int. Ed.,2011,50(18):4056-4066
[5]Tao G P,Chen Q Y,Yang X,et al.Colloids Surf.B:Biointerf., 2011,86(1):106-110
[6]Shi H,He X X,Wang K M,et al.Nanomed.Nanotechnol. Biol.Med.,2007,3(4):266-272
[7]Chen Q Y,Zhou D F,Huang J,et al.J.Inorg.Biochem., 2010,104(11):1141-1147
[8]Chen Q Y,Wang L Y,Zhang L R,et al.Sci.China Chem., 2010,53(8):1728-1731
[9]CHEN Qiu-Yun(陈秋云),HUANG Juan(黄娟),LI Jun-Feng (李军峰),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao).,2008,24(11):1789-1793
[10]Romero I,Dubois L,Collomb M N,et al.Inorg.Chem., 2002,41(7):1795-1806
[11]López-Lázaro M.Cancer Lett.,2007,252(1):1-8
[12]Zhou D F,Chen Q Y,Qi Y,et al.Inorg.Chem.,2011,doi. org/10.1021/ic200004y
[13]Bahadur N M,Furusawa T,Sato M,et al.J.Colloid Interf. Sci.,2011,355(2):312-320
[14]CHEN Qiu-Yun(陈秋云),WANG Lin-Yun(王玲昀),CHEN Hao(陈浩),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2010,26(10):1784-1789
[15]Groni S,Blain G,Guillot R,et al.Inorg.Chem.,2007,46(6): 1951-1953
[16]Shin B K,Kim M Y,Han J H.Polyhedron,2010,29(12): 2560-2568
[17]Jasuja K,Linn J,Melton S,et al.J.Phys.Chem.Lett., 2010,1(12):1853-1860
具有肿瘤荧光成像性能的核壳纳米过氧化氢酶模拟物
杨 霞 陈秋云*宋京宝
(江苏大学化学化工学院,镇江 212013)
运用微波法在硅核壳荧光材料的表面修饰了2-(二吡啶甲胺基)丙酸的锰配合物,获得具有荧光性能的锰-硅核壳纳米结构复合物,运用IR,UV,TEM等方法表征了纳米复合物的结构。H2O2岐化实验显示锰-硅核壳纳米复合物具有较好的过氧化氢酶模拟特性,是一种新的纳米过氧化氢酶模拟物。体外细胞荧光成像研究表明2-(二吡啶甲胺基)丙酸修饰的纳米球不能进入肿瘤细胞内,而锰-硅核壳纳米复合物能进入肿瘤细胞内,具备良好的肿瘤靶向性,显著提高肿瘤荧光成像效果,可作为新型的肿瘤成像剂。
核壳纳米球;纳米复合物;成像;肿瘤
O627.41
A
1001-4861(2012)01-0164-07
2011-07-06。收修改稿日期:2011-08-24。
国家自然科学基金(No.20971059)资助项目。
*通讯联系人。E-mail:chenqy@ujs.edu.cn
猜你喜欢
杂志排行
无机化学学报的其它文章
- 纳米结构CuO为敏感电极的阻抗谱型NO2传感器
- Synthesis,Crystal Structure and Fluorescence Property of Cu(Ⅱ)Coordination Polymer with Pyridylimidazolidinone and Bipyridine
- Hydrothermal Synthesis,Crystal Structure and Catalytic Properties of a Polyoxovanadate Organicamine
- Syntheses and Crystal Structures of Nickeand CopperComplexes with Schiff Base Ligand of 5-Chlorosalicylaldehyde
- S2-控制剂对Ag纳米产物的形貌及光学性能的影响
- Synthesis,Crystal Structure and Properties of a Manganese(Ⅱ)Complex with an Asymmetrical Substituted Triaryltriazole
