Y2O3-MgO Composite Nano-ceramics Prepared from Core-shell Nano-powders
2021-07-23JIANGHongtaoQINHaimingFENGShaoweiCHENHongbingJIANGJun
JIANG Hong-tao, QIN Hai-ming, FENG Shao-wei, CHEN Hong-bing, JIANG Jun
(1. School of Materials Science and Chemical Engineering, Ningbo University, Ningbo 315201, China;2. Ningbo Institute of Materials Technology and Engineering, Chinese Academy of Sciences, Ningbo 315201, China;3. University of Chinese Academy of Sciences, Beijing 100049, China;4. National Engineering Laboratory for Hydrometallurgical Cleaner Production Technology, CAS Key Laboratory of Green Process and Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China)
Abstract: Y2O3-MgO composite nano-ceramics are regarded as a significant candidate of infrared transparent ceramics on account of excellent optical and mechanical properties. Nevertheless, a huge challenge remains regarding the critical optical scattering and needless absorption in the near- and mid-infrared bands, which hinders its applications in extreme harsh environments. In present work, Y2O3-MgO core-shell structure nano-powders were prepared via urea precipitation method before that Y2O3-MgO composite nano-ceramics were prepared under spark plasma sintering. Thermogravimetric and differential scanning calorimetry(TG/DSC), X-ray diffraction and scanning electron microscope were performed to analyze as prepared core-shell structure nano-powders and composite nano-ceramics. The size of Y2O3-MgO core-shell structure nano-powders is about 250 nm, and average grain size of the prepared ceramics is approximately 360 nm. The transmittance is 57% at 6 μm, and the Vickers hardness is 820 HV. The powder synthesis method accomplished in present work offers a novel solution for composite nano-ceramics, which easily regulate particle size and proportion of different components.
Key words: Y2O3-MgO composite nano-ceramics; core-shell structure nano-powder; urea precipitation method; spark plasma sintering
关 键 词:Y2O3-MgO复相纳米陶瓷; 核壳结构纳米粉体; 尿素沉淀法; 放电等离子烧结
1 Introduction
In recent years, infrared window materials are brought into focus due to widely used in infrared tracking, identification, search, guidance, navigation, and thermal imaging[1-7]. In order to cope with various application environments, the performance of infrared window materials need to meet the following requirements: infrared transparency, high mechanical strength, high thermal conductivity, and resistance to thermal shock and erosion[5-6]. However, it is not realistic to improve the operational properties of infrared materials(ZnS, Al2O3and MgAl2O4) that have been widely used in extreme environments and under severe loads, such as aerospace applications[7]. Recently, progress of the composite ceramic as a competitive candidate in this area brings new driving power.
Among all nano-composite ceramics, the Y2O3-MgO nano-composite ceramics are presentative infrared transparent ceramic, since the Y2O3-MgO nano-composite ceramics process outstanding thermal and mechanical properties for extreme environments[8-10]. The most commonly approach to sintering Y2O3-MgO nano-composite ceramics is the spark plasma sintering(SPS). SPS regarded as a rapid solidification sintering method is an especially efficient technique, through which the sintering time can be massively decreased owing to the rapid heating rate at a speed higher than 100 ℃/min compared with conventional sintering methods. For the pressureless sintering, the higher sintering temperature and the longer sintering time lead to dramatically grain growth, particularly in the final period of the densification. For another, SPS enhances the driving force of sintering by dynamically activating plastic deformation and diffusion processes, which is efficacious to restrict the grain growth under a lower sintering temperature and high intensity of pressure. In 2010, Jiangetal.[11]prepared Y2O3-MgO nano-composite ceramics with the grain size less than 100 nm by SPS sintering under a load of 80 MPa. Liuetal.[12]used SPS to sinter the powders after ultrasonic horn treatment to prepare the Y2O3-MgO nano-composite ceramics at 50 MPa. Huangetal.[13]prepared the Y2O3-MgO nano-composite ceramics with a grain size of 100-200 nm through SPS sintering with 100 MPa. Then, Xuetal.[14]prepared the Y2O3-MgO nano-composite ceramics with a grain size of 100-300 nm through SPS sintering under 50 MPa, which got the nano-powder through the esterification sol-gel route. Recently, Safronovaetal.[15]explored the influence of temperature on the Y2O3-MgO nano-composite ceramics during SPS sintering. At the same time, Liuetal.[16]and Maetal.[17]independently explored the influence of pressure and LiF sintering aid on grain growth of Y2O3-MgO ceramics.

In present work, the homogenous Y2O3-MgO core-shell structure nano-powders were prepared through urea precipitation approach. In order to obtain high sinterability powders, the calcination temperature, powder morphology and size of Y2O3-MgO core-shell structure nano-powders were studied compared with that of single-phase Y2O3. Y2O3-MgO composite nano-ceramics were sintered by SPS using core-shell powders as the beginning powders. This core-shell nano-powders preparation method and SPS procedure are simple and inexpensive, which provide a novel way to fabricate Y2O3-MgO composite nano-ceramics.
2 Experimental and Characterizations
Y2O3-MgO core-shell structure nano-powders were prepared by urea precipitation. The raw materials were Y2O3(5N), nitric acid(AR), urea(99%), and MgO(99.9%, 50 nm). Firstly, 0.015 mol Y(NO3)3solution was prepared by dissolving 0.007 5 mol Y2O3in a certain number of HNO3. Next, Y(NO3)3solution was added together with 0.5 mol urea into a three-necked flask of 2 000 mL. Then, MgO was weighted with a volume ratio of 1∶1 to Y2O3into the container through stirring and dispersing sufficiently. After that, MgO was transferred to the solution in the three-necked flask. Currently, there were about 1 300 mL of solution in the three-necked flask. Finally, the three-necked flask was placed in a heating mantle to heat the solution temperature from room temperature to (85±1) ℃ in about 40 min. At the same time, an electric stirrer was used to stir at a rate of 500 r/min. When the solution was obviously turbid, the reaction was maintained in this state for 2 h. After two hours, the resulting suspension was obtained by suction filtration, and then the suspension was placed in an oven and dried at 80 ℃ for 24 h. The dried precursors were put into the muff furnace and calcined at the selected temperature for 1 h, after which 0.25% LiF(99%) was added and the powders were ground, and then screened with a 140-mesh sieve.
Transparent Y2O3ceramics powders were also prepared by urea precipitation method, and then ground and screened with 140 mesh, and then compressed intoφ10 discs by a powder tablet machine. The ceramic tablets were compacted at 200 MPa using a cold isostatic press. The sintering method was vacuum sintering, the temperature was 1 750 ℃, and the holding time was 4 h.
The powders obtained above were sintered into ceramics through SPS(LABOX-1575, SinterL and Inc., Japan). The powder samples loaded into the graphite mold were heated from room temperature to a pre-set temperature(1 200 ℃) at the heating rate of 100 ℃/min under vacuum(10 Pa) and the dwell time was 8 min with the pressure of 50 MPa. The sintered Y2O3-MgO composite nano-ceramics were annealed in the air at 1 000 ℃ for 15 h to eliminate oxygen vacancies, carbon and residual stress. When measuring infrared transmittance, the sample is polished on both sides to a thickness of~0.9 mm.
XRD patterns were measured by a Bruker D8 X-ray diffractometer with Cu Kα radiation(λ=0.154 056 nm) at 40 kV and 40 mA. Thermal analysis of the precursors was measured by thermogravimetric/differential scanning calorimetry(TG/DSC, STA 449F3, NETZSCH, Germany) at a heating rate of 10 K/min in air. A thermal field emission scanning electron microscope(TFE-SEM, Thermo Scientific Verios G4 UC) was used to observe the microscopic morphology of the powders. The particle size distributions of the powders were measured by Laser particle size analyzer(HELOS-OASIS, Sympatec GmbH, Germany). The grain and grain boundary morphology of the ceramic were measured by a field emission scanning electron microscope(FE-SEM, Hitachi S4800, Japan). The transmittance in the wavelength range ofλ=0.25-2.5 μm was conducted by using a spectrometer(Lambda 950, Perkin Elmer Co., USA). Fourier transform infrared spectroscopy(NICOLET 6700, Thermo Co, USA) was used to measure the transmittance of the mirror polished samples at a range of 2.5-10 μm. An image analysis microhardness tester(HV-1000/S, SIOMM, Shanghai) was used to carry out a 10 s, 100 N load test to obtain the Vickers hardness result.
3 Results and Discussion
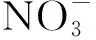

Fig.1 TG-DSC curves of the Y2O3-MgO core-shell structure nano-powder precursors
Fig.2 shows XRD patterns of MgO nano-powders, Y2O3nano-powder precursors, Y2O3-MgO core-shell structure nano-powder precursors and Y2O3-MgO core-shell structure nano-powders calcined at different temperatures. Compared with Y2O3nano-powder precursors, Y2O3-MgO core-shell structure nano-powder precursors appear weaker MgO peaks. The other peaks may be caused by the formation of the coating structure and some changes in the disordered structure of the outer layer. According to thermal analysis, the powders have reached its crystallization temperature at 650 ℃. After reaching 750 ℃, the powders have been completely crystallized, and no obvious heat absorption and exotherm were observed, and the quality almost no longer changes. As a result, 750 ℃ is the optimum calcination temperature for the powders. The XRD of core-shell structure nano-powders calcined at 650, 750, 850 ℃are consistent with that of Y2O3and MgO, and there is no obvious change between them, except that the peak shape gradually becomes sharp with the increase of temperature, indicating that the powders have basically formed phase at 650 ℃. Therefore, the calcined temperature of Y2O3-MgO core-shell structure nano-powder precursors is higher about 100 ℃ than precursor crystallization temperature in order to remove possible traces of carbon- and nitrogen-containing compounds.

Fig.2 XRD patterns of MgO nano-powders, Y2O3 nano-powder precursors, Y2O3-MgO core-shell structure nano-powder precursors and Y2O3-MgO core-shell structure nano-powders calcined at different temperatures.
Fig.3 shows the micromorphology of MgO nano-powders, Y2O3powders, Y2O3-MgO core-shell structure nano-powder precursors and Y2O3-MgO core-shell structure nano-powders. The powder size of MgO nano-powders in Fig.3(a) is about 50 nm, and the powder morphology are relatively uniform. Fig.3(b) shows the prepared Y2O3powders by the urea precipitation method. The size of Y2O3powders with good sphericity and good monodispersity is about 200-300 nm. The micrograph of Y2O3-MgO core-shell structure nano-powder precursors (Fig.3(c)) is much more similar to that of MgO nano-powders due to the nucleation process starting on the surface of MgO nano-powders. As shown in Fig.3(d), Y2O3-MgO core-shell structure nano-powders with soft agglomeration are composed of microcrystals. Y2O3-MgO core-shell structure nano-powders exhibit a clear interface between core and shell, which indicates the MgO nano-powders as a core are successfully cladded with Y2O3powders as a shell. The size distribution of all the powders is shown in Fig.4. Compared with the SEM images, the four kinds of powders have different degrees of agglomeration. Fig.4(a) shows the particle size distribution of MgO nano-powders is around 600 nm, which is quite different from the SEM image. This is due to the small size of MgO nano-powders and large specific surface area, which is easy to form large agglomerated particles. According to Fig.4(b), the size of Y2O3powders is mainly concentrated in 200-300 nm, which is more consistent with Fig.3(b) image. Therefore, the larger particles are ascribed to slightly agglomerating of Y2O3powders. Fig.4(c) shows that the particle size distribution of Y2O3-MgO core-shell structure nano-powder precursors is mainly 100-300 nm. Since the precursors have not undergone crystallization after calcination, the particles have not grown. From Fig.4(d), the agglomeration of Y2O3-MgO core-shell structure nano-powders nearly disappear with uniform size distribution at about 250 nm. After calcined at low temperature, the inorganic acid ions are decomposed and core-shell structure powders did not happen growing up. Fig.5 shows the EDS mapping images of Y2O3-MgO core-shell structure nano-powders calcined at 750 ℃. As represented in Fig.5, Y, Mg and O elements are evenly distributed throughout Y2O3-MgO core-shell
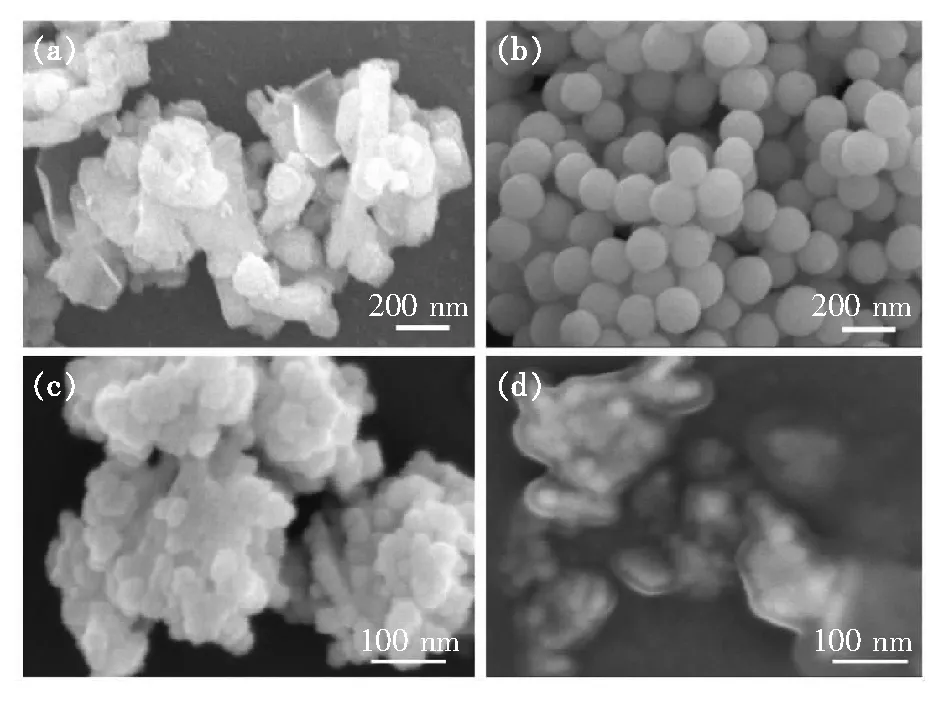
Fig.3 SEM images of MgO nano-powders(a), Y2O3 powders(b), Y2O3-MgO core-shell structure nano-powder precursors(c), Y2O3-MgO core-shell structure nano-powders(d).

Fig.4 Particle size distributions of MgO nano-powders(a), Y2O3 powders(b), Y2O3-MgO core-shell structure nano-powder precursors(c), Y2O3-MgO core-shell structure nano-powders(d).

Fig.5 EDS mapping images of Y2O3-MgO core-shell structure nano-powders calcined at 750 ℃ structure nano-powders. However, the content distribution of Y is denser than that of Mg due to Y distributing on the outer surface of core-shell structure.
Fig.6 shows the micro-morphology of Y2O3ceramics and Y2O3-MgO composite nano-ceramics, and the EDS mapping images of Y2O3-MgO composite nano-ceramics. From Fig.6(a) and (b), the average grain size of the Y2O3ceramics is about 100 μm, while the average grain size of the Y2O3-MgO composite nano-ceramics is about 360 nm. It can be seen from the image that Y2O3ceramics show larger grains and irregular grain growth, while the grains of Y2O3-MgO composite nano-ceramics are smaller and the grain growth is more uniform. Following BSE images and EDS mapping images, in Fig.6(d), the white phase and black phase are respectively Y2O3and MgO. At the same time, the grain size of MgO is generally smaller than that of Y2O3, and the larger MgO grains may be caused by the incomplete coating structure. And from the Fig.6(d), obviously, it can be seen that the black phase is surrounded by the white phase, that is, MgO is surrounded by Y2O3, which corresponds to the prepared core-shell structure nano-powders, thus confirming the construction of the core-shell structure. The core-shell structure nano-powders are beneficial to a more even distribution of the two grains, which is conducive to inhibiting growth of ceramic grains in a smaller scale. It indicates that the core-shell structure nano-powders has a certain binding effect on the grain growth during sintering, making the grains unable to grow at will.
Fig.7 shows IR transmittance spectra of Y2O3ceramics and Y2O3-MgO composite nano-ceramics.
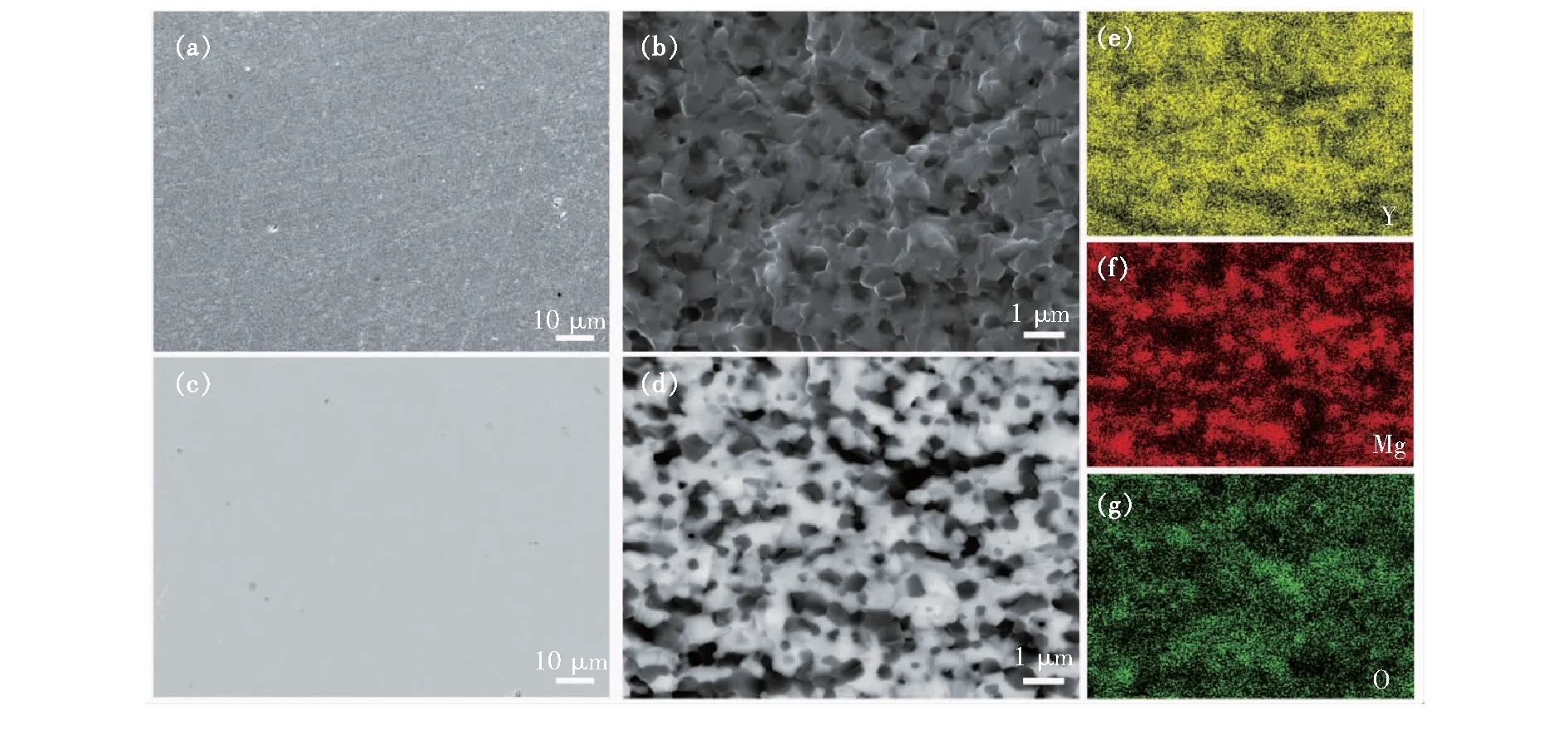
Fig.6 SEM images of Y2O3 ceramics(a) and Y2O3-MgO composite nano-ceramics(b). BSE images of Y2O3 ceramics(c) and Y2O3-MgO composite nano-ceramics(d). (e)-(g)EDS mapping images of (b).
Fig.7 IR transmittance spectra of Y2O3 ceramics and Y2O3-MgO composite nano-ceramics with the thickness of 0.9 mm. Inset: the photo of synthesized Y2O3 ceramics(left) and Y2O3-MgO composite nano-ceramics(right).
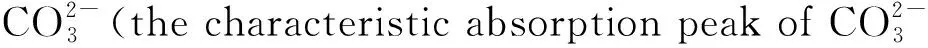
Fig.8 shows the comparison of Vickers hardness of Y2O3ceramics and Y2O3-MgO composite nano-ceramics. The Vickers hardness of Y2O3ceramics is about 780 HV, and the Vickers hardness of Y2O3-MgO composite nano-ceramics is about 820 HV. Compared with Y2O3ceramics, after adding MgO, its mechanical properties have indeed improved. However, its hardness is far from reaching the required level, so the powder preparation and sintering process still need to be improved.

Fig.8 Vickers hardness of Y2O3 ceramics and Y2O3-MgO composite nano-ceramics
4 Conclusion
Y2O3-MgO core-shell structure nano-powders with the particle size of about 250 nm were successfully prepared by urea precipitation method. Y2O3-MgO composite nano-ceramics with the average grain size of 360 nm are accomplishedviaSPS treatment. Grain size of Y2O3-MgO composite nano-ceramics is more uniform and smaller compared with Y2O3ceramics, indicating that the core-shell structure has certain binding effect on the grain growth. This core-shell structure nano-powders preparation method offers a new approach to further control the grain size of nano-composite ceramics. However, the transmittance and Vickers hardness of Y2O3-MgO composite nano-ceramics are not good enough, so the powder preparation and sintering process still need to be explored. Together with the SPS treatment, the method accomplished in this work provides a novel way to fabricate Y2O3-MgO composite nano-ceramics.
Response Letter is available for this paper at:http://cjl.lightpublishing.cn/thesisDetails#10.37188/CJL.
20210095.
