Synthesis,Crystal Structure and Properties of a Manganese(Ⅱ)Complex with an Asymmetrical Substituted Triaryltriazole
2012-11-09SHENGuoPingZHAOJianCHENLangSUNFeng
SHEN Guo-PingZHAO JianCHEN LangSUN Feng
SHEN Xuan1ZHU Dun-Ru*,1,2LIU Xiao-Qin*,1
(1College of Chemistry and Chemical Engineering,State Key Laboratory of Materials-oriented Chemical Engineering,Nanjing University of Technology,Nanjing 210009,China) (2Jiangsu Key Laboratory for Chemistry of Low-Dimensional Materials,Huaian,Jiangsu 223300,China)
Synthesis,Crystal Structure and Properties of a Manganese(Ⅱ)Complex with an Asymmetrical Substituted Triaryltriazole
SHEN Guo-Ping1ZHAO Jian1CHEN Lang1SUN Feng1
SHEN Xuan1ZHU Dun-Ru*,1,2LIU Xiao-Qin*,1
(1College of Chemistry and Chemical Engineering,State Key Laboratory of Materials-oriented Chemical Engineering,Nanjing University of Technology,Nanjing210009,China) (2Jiangsu Key Laboratory for Chemistry of Low-Dimensional Materials,Huaian,Jiangsu223300,China)
A mononuclear manganese(Ⅱ)complex,trans-[MnL2(NCS)2](L=3-(p-methoxyphenyl)-4-(p-chlorophenyl) -5-(2-pyridyl)-1,2,4-triazole),was synthesized and characterized by FTIR,ESI-MS,TG and X-ray crystallography.The complex crystallizes in monoclinic system with space group P21/c,a=1.4585(3)nm,b=0.91500(18)nm,c= 1.616 3(3)nm,β=101.70(3)°,V=2.112 2(7)nm3,Z=2,and final R1=0.058 5.The manganese atom lies in a distorted octahedral environment with two NCS-ions in the trans positions.The ligand L coordinates via one triazole nitrogen and one pyridine nitrogen atom.The TG analysis shows that the complex is stable below 260℃.Magnetic data show that the complex is paramagnetic in the range of 1.8~300 K.CCDC:833359.
Mn(Ⅱ)complex;crystal structure;1,2,4-triazole;properties
0 Introduction
During the past three decades,the substituted 1,2,4-triazole ligands have gained considerable attention in coordination chemistry.This is mainly because of the fact that some iron(Ⅱ)complexes with substituted 1,2,4-triazoles show novel structures[1-2]and intriguing spin-crossover properties which can be applied formolecular electronics,as information storageand switching materials[3-8].Recently,some symmetrical N4-substituted-3,5-di(2-pyridyl)-4H-1,2,4-triazole ligands and their metal complexes have been synthesized by our group and others[8-13].Most recently our interests of investigation have focused on preparing asymmetrical 3,4,5-trisubstituted-1,2,4-triazole ligands and their complexes[14-18].In this context,one of the asymmetrical triaryltriazole ligands,3-(p-methoxyphenyl)-4-(p-chlorophenyl)-5-(2-pyridyl)-1,2,4-triazole(L),has been synthesized and structural characterized by us[18].We report herein synthesis of its complex,trans-[MnL2(NCS)2].The single crystal structure,spectral characterization, thermal stability and magnetic property are also discussed.
1 Experimental
1.1 Materials and measurements
All chemicals used were of analytical grade.Solventswere purified by conventionalmethods.Elemental analyses(C,H,N,S)were carried out with a Thermo Finnigan Flash 1112A elemental analyzer.IR spectrum was recorded on a Nicolet Avatar 380 FTIR instrument with KBr pellets in the range of 4 000~400 cm-1.Electrospray ionization mass spectrum(ESI-MS) was recorded with an LCQ ADVANTAGE MAX mass spectrometer,with MeOH as the mobile phase;the flow rate of the mobile phase was 0.2 mL·min-1.The spray voltage,the capillary voltage,and the capillary temperature were 4 kV,40 V,and 260℃,respectively.Thermogravimetric analysis(TGA)was performed with a simultaneous NETZSCH STA 449C thermal analyzer under flowing nitrogen from 35 to 800℃ at a heating rate of 10℃·min-1.
1.2 Synthesis of trans-[MnL2(NCS)2]
To a solution of KSCN (0.4 mmol)in anhydrous MeOH(3 mL)was added a solution of MnCl2·4H2O(0.2 mmol)in MeOH (2 mL).The mixture was stirred for 15 min and filtered.The KCl precipitate was washed with 2 mL of anhydrous MeOH.The methanolic fractions containing Mn(SCN)2were collected,and then was added dropwise to a solution of the ligand L(0.4 mmol) in MeOH (3 mL).A light-yellow microcrystalline product,which formed immediately,was filtered and washed with H2O,and dried under vacuum to give 150 mg (83.7%)of the complex.The light-yellow single crystals suitable for X-ray diffraction were obtained by evaporation from an EtOH solution.Elemental analyses calcd.for C42H30Cl2MnN10O2S2(%):C 56.25,H 3.37,N 15.62,S 7.15;found(%):C 56.33,H 3.31,N 15.55,S 7.04.IR data(ν,cm-1):3009(w);2971(w);2067(s); 1 612(s);1 600(m);1 577(m);1 493(s);1 478(s);1 252 (m);1 172(m);1 093(m);1 026(m);996(m);835(m); 792(m);595(m).ESI-MS:m/z=839.50;747.42;385.58.
1.3 Crystal structure determination
The well-shaped single crystals of trans-[MnL2(NCS)2]were selected for X-ray diffraction study.The unit cell parameters and intensity data were collected at 293 K on a Bruker SMART APEX CCD diffractometer using a graphite-monochromated Mo Kα (λ=0.071073 nm)radiation.The structure was solved by direct methods and refined on F2by full-matrix least squares procedures using SHELXTL software[19].All nonhydrogen atoms were anisotropically refined.All hydrogen atoms were included in the final stage of the refinement on calculated positions bonded to their carrier atoms.CrystallographicdataaresummarizedinTable1.
CCDC:833359.
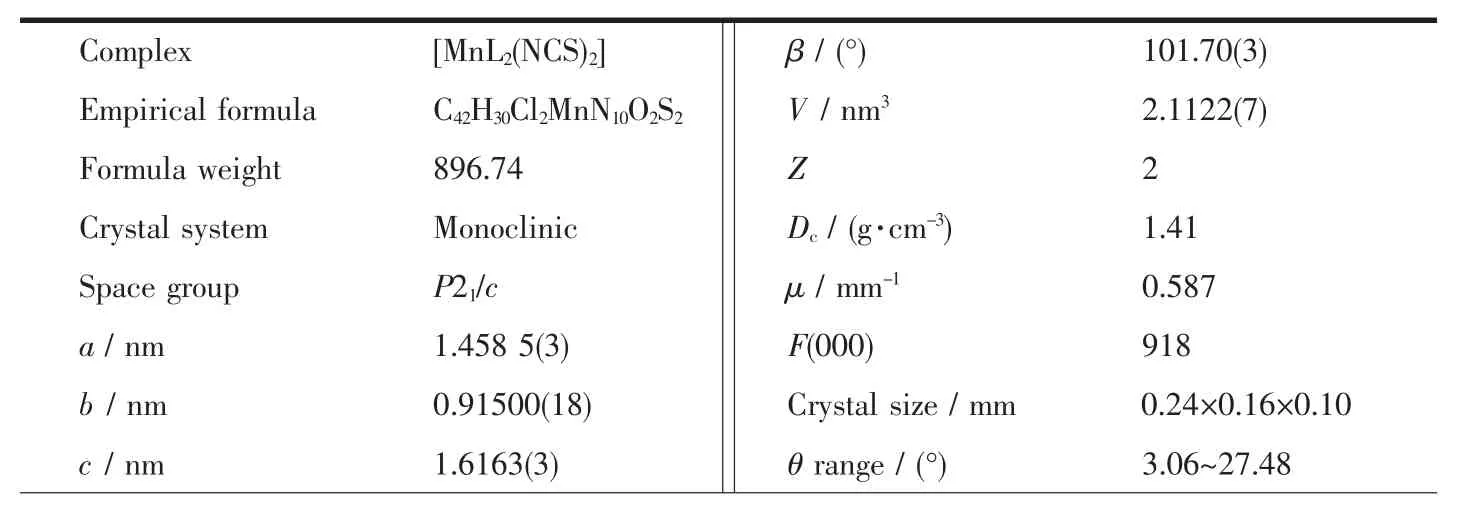
Table 1 Crystal data and structure refinement for the complex

Continued Table 1
2 Results and discussion
2.1 Crystal structure
A projection of the structure of trans-[MnL2(NCS)2] is presented in Fig.1 together with the atomic labeling system.The complex crystallizes in the monoclinic space group P21/c.Relevant interatomic distances and angles are given in Table 2.
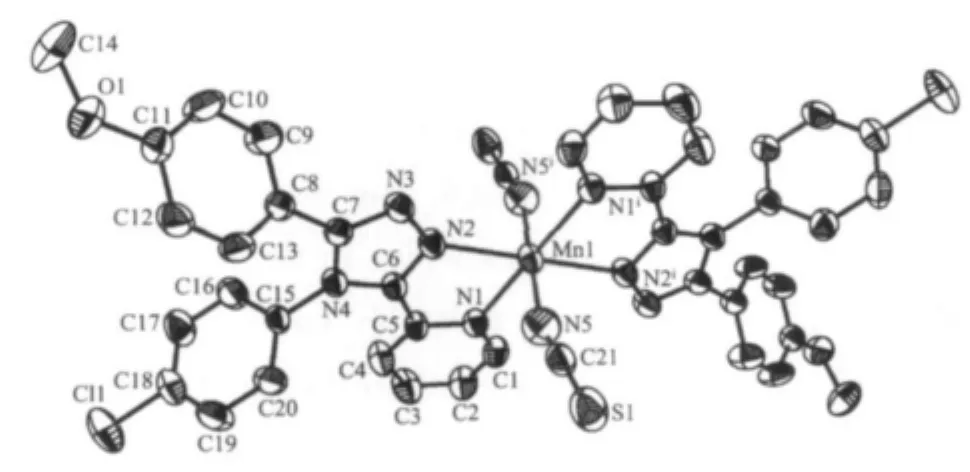
Fig.1 Projection of the structure of complex with 50% thermal ellipsoids probability
Each Mn(Ⅱ) atom adopts a distorted[MnN6] octahedral geometry coordinated by four nitrogen atoms from two L ligands in the equatorial plane and two nitrogen atoms from two NCS-ions in the axial position.Thisfeature isdifferentfrom thatfound in a mononuclear Mn(Ⅱ)complex with the asymmetrical 3,5-disubstituted 1,2,4-triazole,cis-[MnL′2(NCS)2][20](L′=3 -methyl-4-(p-bromophenyl)-5-(2-pyridyl)-1,2,4-triazole) where the two NCS-anions are in the cis arrangements.Each L ligand coordinates to Mn(Ⅱ)atom via N1 atom of the pyridyl ring and N2 atom of the triazole,which is similar to the coordination modes in the related Mn(Ⅱ)complexes[10,15].The Mn-N bond lengths are within the normal ranges observed for the octahedralMn(Ⅱ)complexes[10,15,20].However,the Mn-Ntrzbond length is 0.0031 nm shorter than the Mn-Npyone.The same feature has been observed in the analogous Mn(Ⅱ)complexes with trans-NCS-groups[10,15].The NCS-groups are almost linear(N5-C21-S1 178.7(3)°),whereas the Mn-NC (S)linkages are a little bent(Mn1-N5-C21 165.4(3)°).The pyridyl group,the substituted benzene rings and the 1,2,4-triazole moiety of L ligand in trans-[MnL2(NCS)2]do not share a common plane.The triazole ring makes dihedral angles of 13.2(2)°,53.1(2)°and 75.6(2)°with the pyridyl,the p-methoxyphenyl and the p-chlorophenyl ring,respectively.Due to the existence of a dihedral angle(77.1(2)°)between the pyridyl ring and the p-chlorophenyl ring in the L ligand,there is an intramolecular edge-to-face C-H…π interaction involving C4-H4A and the p-chlorophenyl ring(H4A…Cg1= 0.296 nm and∠C4-H4A…Cg1=149°,Cg1 is the centroid of the p-chlorophenyl ring).The crystal structure is further stabilized by weak intermolecular C-H…S and C-H…O hydrogen bonds(Fig.2 and Table 3).
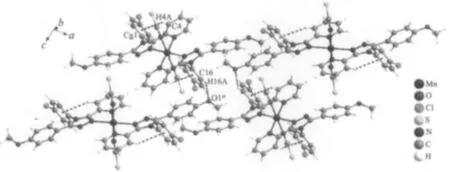
Symmetry code:iiix,1/2-y,z-1/2Fig.2 Hydrogen bonding interactions in the complex
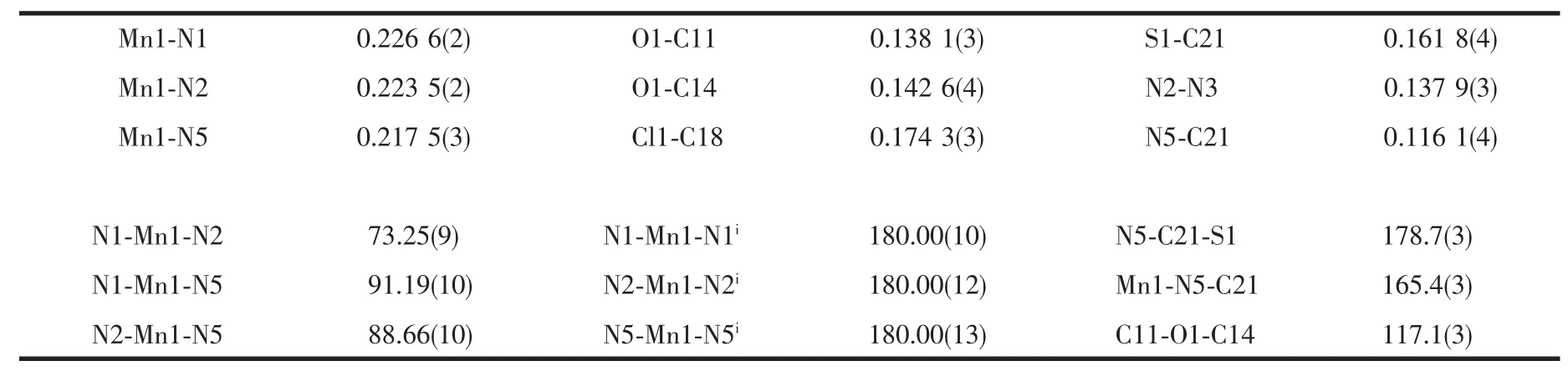
Table 2 Selected bond distances(nm)and bond angles(°)for the complex
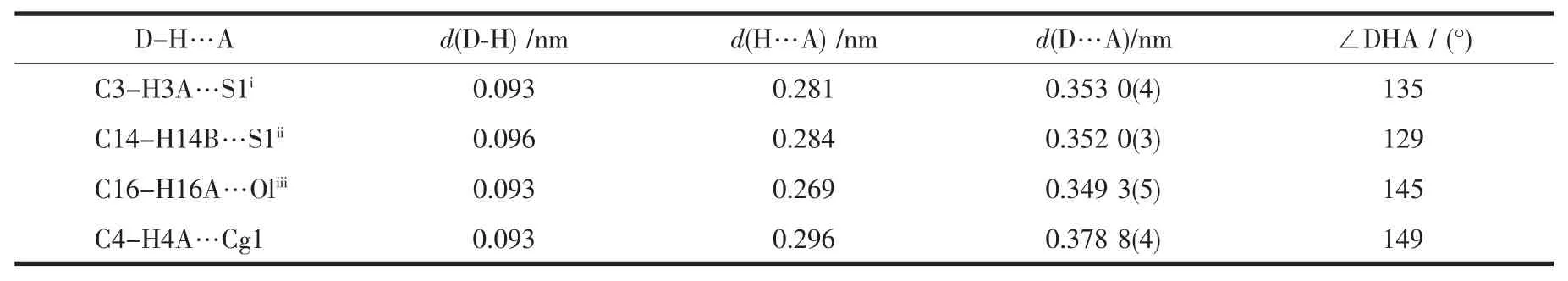
Table 3 Hydrogen bonding interactions in the complex
2.2 Spectral characterization
In the IR spectrum of the complex,there is only one sharp band at 2067(s)cm-1,attributable to the C≡N stretching vibrations of two thiocyanate groups,which shows that two NCS-ions are in a trans arrangement[21].This feature is in agreement with the results of X-ray analysis.A band at 1600 cm-1(s)can be assigned to the coordinated pyridine ring.A band at 1093 cm-1(s)can be assigned to the stretching frequency of Ar-Cl.In addition,the diagnostic asymmetrical and symmetrical stretching frequencies of C-O-C are at 1 256 and 1 026 cm-1(m),respectively.
The structure of trans-[MnL2(NCS)2]in solution was also studied by electrospray ionization mass spectrometry (ESI-MS)[22-23].The base peak at m/z 839.50 is [MnL2(NCS)]+ion.The peaks at m/z 747.42 and 385.58 are[NaL2]+and[NaL]+,respectively(Fig.3).

Fig.3 ESI-MS spectra of the complex
2.3 Thermal stability
Due to lack of any guest molecules in the complex, TGA shows a high thermal stability of the complex.Practically no weight loss was observed up to 260℃.An abrupt weight loss is only observed above 260℃because of the decomposition of the ligands.The thermal decomposition feature of the complex is in good agreement with the crystal structure.
2.4 Magnetic property
The temperature dependence of the magnetic susceptibility for the complex was measured at an applied field of 2 000 Oe and the χMand χM-1value versus T plot is shown in Fig.4.The result suggests that the complex is a high-spin species in the range of 1.8~300 K,which indicates that the cubic crystal-field splitting(Δ=10 Dq)is lower than the electron pairing energy (P).The μeffvalue (5.52 B.M.)is in the normal range observed for a high-spin manganese(Ⅱ) complex (μeff=5.6~6.1 B.M.)of Ohsymmetry.According to the Curie-Weiss law,χM=C/(T-θ),the data are in a good linear relationship between χM-1versus T.The complex is weak ferromagnetic with C=3.92(2)and θ=2.2(7)K, which is different from that found in a homologous Mn(Ⅱ)complex[10].
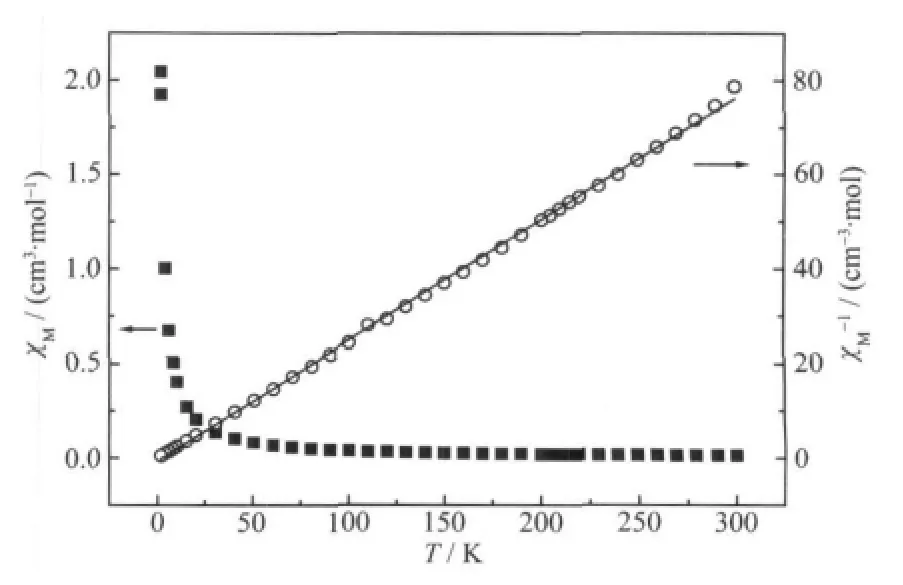
Fig.4 χM((Ⅱ))and χM-1(○)versus T plot for the complex
[1]Keij F S,de Graaff R A G,Haasnoot J G,et al.Dalton.Trans., 1984:2093-2097
[2]van Koningsbruggen P J,Gatteschi D,De Graaff R A G,et al. Inorg.Chem.,1995,34:5175-5182
[3]Kahn O,Martinez C J.Science,1998,279:44-48
[4]Gütlich P,Hauser A,Spiering H.Angew.Chem.Int.Ed.,1994, 33:2024-2054
[5]van Koningsbruggen P J.Top.Curr.Chem.,2004,233:123-149 [6]Zhu D R,Xu Y,Yu Z,et al.Chem.Mater.,2002,14:838-843
[7]Kröber J,Codjovi E,Kahn O,et al.J.Am.Chem.Soc.,1993, 115:9810-9811
[8]Kitchen J A,Brooker S.Coord.Chem.Rev.,2008,252:2072-2092
[9]Klingele M H,Brooker S.Coord.Chem.Rev.,2003,241:119-132
[10]ZHU Dun-Ru(朱敦如),WANG Tian-Wei(王天维),ZHONG Sheng-Lai(仲盛来),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2004,20(5):508-512
[11]Zhou J,Yang J,Qi L,et al.Transition Met.Chem.,2007,32: 711-715
[12]Yang J,Bao W W,Ren X M,et al.J.Coord.Chem.,2009,62: 1809-1816
[13]Matouzenko G S,Bousseksou A,Borshch S A,et al.Inorg. Chem.,2004,43:227-236
[14]Lu W,Zhu D R,Xu Y,et al.Struct.Chem.,2010,21:237-244
[15]Zhao J,Cheng H M,Shen G P,et al.J.Coord.Chem.,2011, 64:941-952
[16]Shen G P,Zhao J,Jiang J J,et al.J.Mol.Struct.,2011,1002: 159-166
[17]Chen L,Zhao J,Shen G P,et al.J.Coord.Chem.,2011,64: 3980-3991
[18]Zhao J,Shen G P,Zhang Y,et al.J.Heterocycl.Chem.,2011, DOI 10.1002/jhet.949
[19]Sheldrick G M.Acta Crystallogr.,2008,A64:112-122
[20]ZHAO Jian(赵建),LU Wei(卢伟),CHEN Lang(陈浪),et al. Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2011,27(4): 743-746
[21]Zhu D R,Xu Y,Mei Y,et al.J.Mol.Struct.,2001,559:119-125
[22]Wilson S R,Yasmin A,Wu Y.J.Org.Chem.,1992,57:6941-6945
[23]Arakawa R,Matuo T,Ito H,et al.Mass.Spectrom.,1994,29: 289-294
一个不对称三芳基三氮唑锰配合物的合成、晶体结构和性质研究
沈国平1赵 建1陈 浪1孙 峰1沈 旋1朱敦如*,1,2刘晓勤*,1
(1南京工业大学化学化工学院,材料化学工程国家重点实验室,南京 210009) (2江苏省低维材料化学重点建设实验室,淮阴 223300)
以3-对甲氧苯基-4-对氯苯基-5-(2-吡啶基)-1,2,4-三氮唑(L)为配体,合成了1个单核锰配合物trans-[MnL2(NCS)2],对其进行了红外、电喷雾质谱、热重和单晶结构表征,该配合物属于单斜晶系,空间群为P21/c,a=1.4585(3)nm,b=0.91500(18)nm,c= 1.6163(3)nm,β=101.70(3)°,V=2.1122(7)nm3,Z=2,R1=0.0585。单晶结构表明,锰离子处于1个扭曲的八面体配位环境中,2个硫氰根离子呈反式配位,每个配体L通过三氮唑上1个氮原子和吡啶上1个氮原子参与配位。热重分析表明该配合物在260℃开始发生分解。变温磁化率显示该配合物为顺磁性(1.8~300 K)。
锰配合物;晶体结构;三氮唑;性质
O614.71+1
A
1001-4861(2012)01-0159-05
2011-07-08。收修改稿日期:2011-10-14。
国家自然科学基金(No.20771059,20976082,21171093),江苏省自然科学基金(No.BK2008371)和江苏省低维材料化学重点建设实验室开放课题(No.JSKC09057)资助项目。
*通讯联系人。E-mail:zhudr@njut.edu.cn;会员登记号:S060015982P。
猜你喜欢
杂志排行
无机化学学报的其它文章
- 碳酸盐共沉淀法制备Li[Li0.2Co0.13Ni0.13Mn0.54]O2中加料方式对产物性能的影响
- Sonochemical Synthesis of Different Morphological CaF2Microstructures
- S2-控制剂对Ag纳米产物的形貌及光学性能的影响
- Syntheses and Crystal Structures of Nickeand CopperComplexes with Schiff Base Ligand of 5-Chlorosalicylaldehyde
- Hydrothermal Synthesis,Crystal Structure and Catalytic Properties of a Polyoxovanadate Organicamine
- Tumor-Imaging Core-Shell Nano-Models for Catalase
