Adsorption Mechanism of Nonylphenol Polyethoxylate onto Hypercrosslinked Resins
2012-11-06YANGWeiBenRENLi
YANG Wei-Ben REN Li
(College of Chemistry and Environment Science,Nanjing Normal University,Nanjing 210097,P.R.China)
Adsorption Mechanism of Nonylphenol Polyethoxylate onto Hypercrosslinked Resins
YANG Wei-Ben*REN Li
(College of Chemistry and Environment Science,Nanjing Normal University,Nanjing 210097,P.R.China)
The aim of this study was to determine the behaviors and mechanism of three hypercrosslinked polymers during the adsorption of nonylphenol ethoxylated decylether(NPEO-10)from aqueous solutions.The polymers were characterized to determine their specific surface areas,pore sizes,and elemental contents.The adsorption isotherms of NPEO-10 on the three polymers fit the Langmuir and double Langmuir models better than the Freundlich model,and the isotherm curves had similar shapes on a lg-lg scale.The amount of adsorbed NPEO-10 depends on the specific surface area,the pore size of the polymer,and the temperature of the solution.Thermodynamic analysis indicated that the adsorption process was characterized by an interaction of the hydrophobic part of the surfactant molecule with the surface of the polymer and by the formation of micelle-like aggregates on the surface of the polymer.A twodimensional mixture consists of singly dispersed surfactant molecules and monolayered or bi-layered aggregates on the surface of the polymers.Adsorption dynamics confirmed that the adsorption process involved two plateaus which were related to the formation of a monolayer and a bi-layer.Finally,the elution processes were investigated to further establish the appropriate adsorption conditions for the purification of water containing NPEO-10.
Elution;Nonylphenol polyethoxylate;Isotherm;Resin;Temperature
Nonylphenol polyethoxylates(NPEOs)are the most widely used non-ionic surfactants.Over 300000 tons of NPEOs are produced and used annually throughout the world in many industries,such as those of pulp and paper,textile manufacturing,plastics,petroleum production,cleaning products,pesticides,metal processing,paint and protective coating,etc.However,NPEOs are endocrine disrupting chemicals,and their biodegradation intermediates,nonylphenol mono-ethoxylate,diethoxylate,and nonylphenol,are more toxic and persistent than their parent substances in the environment[1].Moreover,as surfactants, NPEOs can remarkably enhance the solubility of hydrophobic organic contaminants,and accordingly worsen water quality and increase the difficulty and cost of water treatment.The use of NPEOs has been banned in Europe for several industrial uses because they are common water pollutants discharged into wastewater treatment facilities or directly into the aquatic environment, whereas an exception is made for industries possessing wastewater treatment technologies which are able to completely remove theorganicfractionofthewaste(EUregulationNo.1816,2004)[2].
Several methods,such as membrane[3],oxidation[4],and adsorption[5-8],are available for the removal of NPEOs from contaminated water.Comparatively,though adsorption of NPEOs has achieved considerable attention,the study on adsorption of NPEOs by polymeric adsorbents is still limited.Specially,the importance of porous structure on the adsorption of NPEOs in aqueous solution is not well understood and needs further study.Moreover,the design and optimization of an adsorption process on an industrial scale relies on a thorough understanding of the fundamental mechanisms involved in the various adsorption interactions.
Adsorption process of NPEOs at a solid/water interface is affected by coulombic interactions,the hydrophobicity and polarity of the solid surface,the length of the hydrophobic chain and hydrophilic group of NPEOs[9].As a consequence,systematic studies are needed to explore different variables of the systems and to establish their influence on NPEOs adsorption.Non-functionalized macroporous hypercrosslinked polymer is particularly suitable for the efficient sorption of high molecular weight organic molecules with lipophilic properties.This polymeric material can be regenerated more easily by using organic solvents such as ethanol,methanol,or acetone[10-12].So,in the present work,adsorption behaviors and mechanisms of NPEO-10(representative of NPEOs)onto hypercrosslinked polymer NU-100 prepared in our laboratory were investigated.For comparison,commercially available hypercrosslinked polymers,NDA-150 and MN-200, were also adopted for study of NPEO-10 adsorption.
1 Materials and methods
1.1 Materials
NPEO-10(molecular weight 660)was obtained from Aldrich Chemical Company.The water used for the NPEO-10 adsorption was purified by distillation.Nitrobenzene,acetone,hydrochloric acid,sulfuric acid,ethanol,and benzene were all analytically pure grades and were obtained from Shanghai Chemical Reagent Plant(Shanghai,China).
1.2 Polymers
Hypercrosslinked polymer NU-100 was prepared in our laboratory with the method described in literature[13-14].Commercially hypercrosslinked polymers,MN-200 and NDA-150,were used in this work.MN-200 was supplied by Shanghai Office,Purolite International Co.,Ltd.,and NDA-150 was donated by Jiangsu Nandagede Environmental Science&Technology Co.,Ltd.in Nanjing,China.The polymers were conditioned in methanolhydrochloric acid mixtures and finally in water before used in the adsorption experiments.Nitrogen adsorption and desorption experiments were carried out at temperature 77 K to determine the textural properties of the polymeric resins.The BET surface area was calculated from the desorption isotherms using the standard Brunauer-Emmert-Teller equation,and the mesoporous pore size distribution was determined from desorption isotherms through the Barrett-Joyner-Halenda(BJH)method.All these calculations were performed by an Accelerated Surface Area and Porosimeter system(ASAP 2010,Micromeritics,USA)automatically.The elemental analysis of the polymers was peformed using a Perkin-Elmer 240C Elemental Analytical Instrument (Wellesley,MA,USA).
1.3 Adsorption assay
The equilibrium adsorption experiments of polymers were carried out at 288,303,and 318 K.Firstly,0.0500 g polymer was introduced into a series of 150 mL conical flasks and 100 mL aqueous solution of NPEO-10 with known concentration was added into each flask.The initial concentrations of the solutions were 200,400,600,800,and 1000 mg·L-1.The flasks were then completely sealed and placed in a model G25 incubator shaker at a pre-set temperature with shaking speed of 130 r· min-1.The adsorption tests were run continuously for 72 h to ensure the equilibrium.
Finally the residual concentrations of NPEO-10 in solutions were determined with spectrophotometric measurements on a UV3100-PC ultraviolet and visible spectrometer(Mapada,China) at 230 nm.The adsorption capacity was calculated using Eq.(1):

where C0is the initial concentration(mg·L-1),Ceis the residual concentration(mg·L-1)at equilibrium;qeis the adsorption capacity(mg·g-1)of NPEO-10 on the polymer at equilibrium system;V is the volume(L)of solution;and m is the mass(g)of dry polymer.
1.4 Kinetics and elution
To obtain the kinetic data and determine the time required for equilibrium for the adsorption of NPEO-10 onto the polymer, 100 mL NPEO-10 solution were introduced into a series of 150 mL conical flasks.The NPEO-10 solutions with an initial concentration of 1000 mg·L-1were shaken with 0.0500 g polymer at 303 K and sampled at different time intervals.The adsorption capacity was calculated using Eq.(1).And then,0.0500 g of the polymer that adsorbed NPEO-10 was placed in 100 mL aqueous solutions with various ethanol contents and shaken for 24 h at 303 K.The amounts of NPEO-10 eluted were estimated by measuring the absorbance of solutions at 230 nm.
2 Results and discussion
2.1 Characterization of polymers
It is well known that pore diameter and specific surface area of polymer affect its adsorption capacity[15].In order to investigate the adsorption behaviors and mechanisms of NPEO-10 onto the three hypercrosslinked polymers,the physicochemical properties of the polymers were characterized and listed in Table 1.
The data for MN-200 determined in this study are in accordance with those given by Purolite Corporation[16-17].The contents of oxygen,carbon,and hydrogen of the NU-100 resin prepared in our laboratory are similar to those of the MN-200 and NDA-150 resins.Both surface area and micropore area of the MN-200 resin are larger than those of the NU-100 and NDA-150 resins; and the average pore diameters of the polymers exhibit an opposite trend.
2.2 Adsorption isotherm
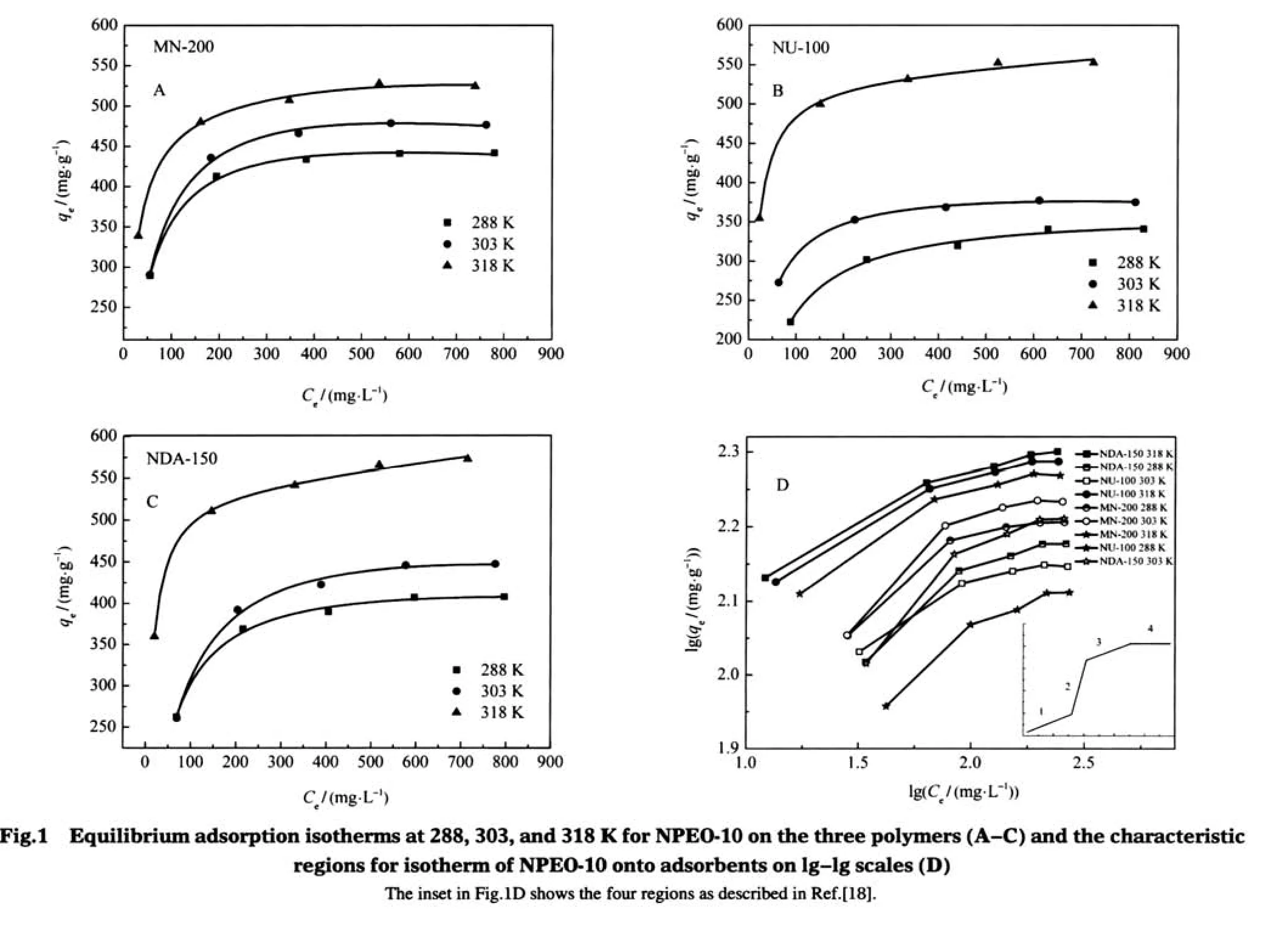
The amounts of NPEO-10 adsorbed per gram of the polymers (qe)versus the equilibrium concentration(Ce)are shown in Fig.1.From these plots it can be seen that the effect of temperature on the adsorption capacity is different for the three polymers.At 318 K with the largest concentration,the adsorption capacities of the polymers are in the following order:NDA-150>NU-100>MN-200,which agrees well with the order of their average pore diameters.In addition,for all the studied temperatures the adsorption capacities of NDA-150 are always greater than those of NU-100 at the highest concentration.Surprisingly,the adsorption capacities of the three polymers at 288 and 303 K did not increase as their specific surface area or average pore diameter increased,indicating that the adsorption capacity of the polymers was possibly affected simultaneously by several factors,including the specific area,pore structures of adsorbents,and temperature of the solution.
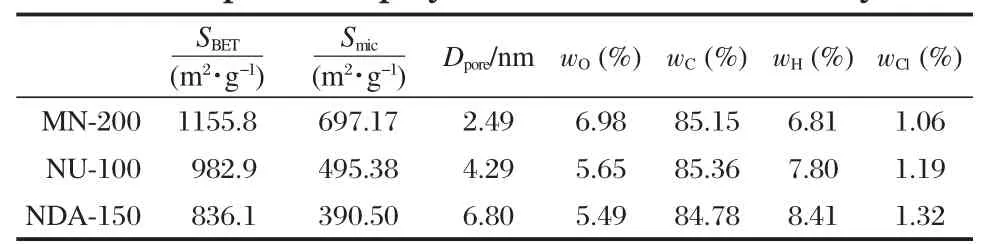
Table 1 Properties of polymers measured in our study
Generally,adsorption of surfactants onto solid-liquid interface is controlled by many factors,such as the nature of the solid surfaces,the type of head group and tail part of the surfactant molecule.In the case of NPEO-10 adsorption onto hydrophobic surfaces of the three hypercrosslinked polymers,the hydrocarbon moiety of the NPEO-10 is in contact with the surface of the polymers,and the oxyethylenic part of the molecule protrudes into the aqueous solution.From the profiles of the adsorption isotherm,the arrangement of surfactant molecules on the adsorbent surface can be modeled as a single layer of surfactant molecules at the initial stage of adsorption,a close-packed ad-sorbate layer in the further course and a multi-layer aggregate at the last stage[19-20].The following two isotherm models were employed to fit the above data.
Linear Langmuir model:

where KLis a direct measure of the intensity(L·mg-1)of the adsorption process;M is a constant relating to the surface area occupied by a monolayer of adsorbate,reflecting the adsorption capacity(mg·g-1);Kfis a system constant related to the bonding energy;Kfis defined as an adsorption or distribution coefficient that represents the general capacity of the adsorbate adsorbed onto the adsorbent for a unit equilibrium concentration;and the slope n is a measure of the adsorption intensity or surface heterogeneity.
Table 2 lists the fitting results with the Langmuir and Freundlich models and the correlation coefficients R2values. Clearly,the experimental data fit better with the Langmuir model (R2≥0.999)than that with the Freundlich one(R2ranges from 0.866 to 0.961).
Many researchers reported that the adsorption isotherms of surfactant could not be well fitted with the Freundlich equation. Moreover,when the Freundlich model is plotted on a lg-lg scale, four regions can be distinguished in the adsorption isotherms[21-22]. In the present work,it is shown in Fig.1D that the adsorptionisotherms of NPEO-10 on a lg-lg scale nearly have the same shape and consist of three main regions rather than four regions as described in the literature(plotted as an insert in Fig.1D).This is mainly due to the ignorance of the low concentration range (<100 mg·L-1)in our study.
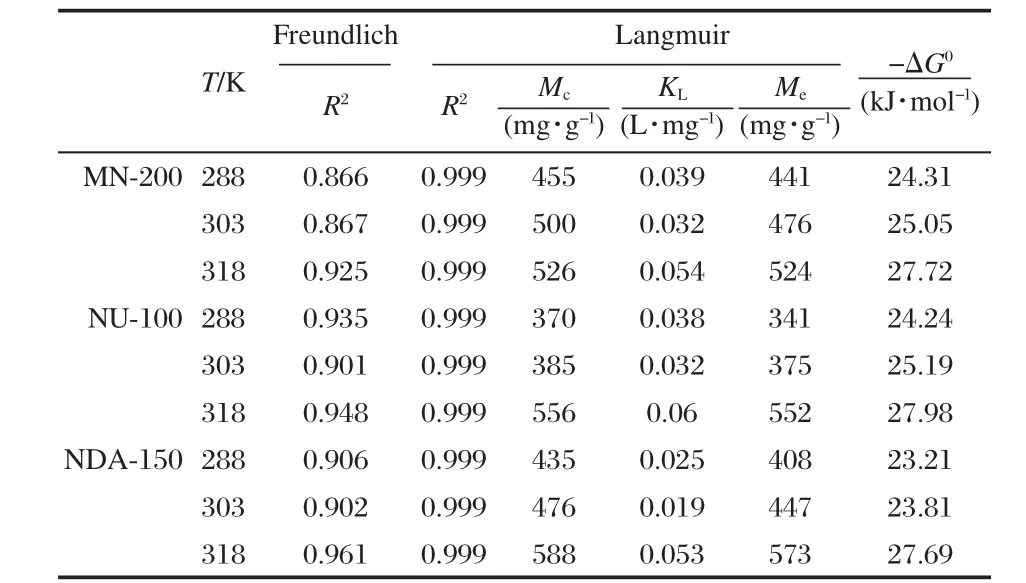
Table 2 Fitted adsorption parameters under different temperatures with the Langmuir model and the Freundlich model
Although the isotherms can be perfectly fitted with the Langmuir equation,it is difficult to interpret the arrangement of surfactant molecules on the adsorbent surface with the corresponding parameters because the basic assumption of Langmuir equation is monlayer adsorption.Double Langmuir model provides a semi-empirical method to analyze the experimental results of adsorption processes.This model,extrapolated to the solid/liquid interface,allows the arrangement of adsorbed molecules to be studied in more detail.González-Gárc and coworkers[23]analyzed the surfactant adsorption behavior by applying the Double Langmuir equation and interpreted the experimental data successfully.Double Langmuir equation is given as follows:

where Mtis the total amount of adsorbate adsorbed at the equilibrium concentration Ce;M1,M2and KL1,KL2are parameters corresponding to each individual Langmuir equation.
Similarly,the results of R2values listed in Table 3 show that the adsorption isotherms can be fitted well with the Double Langmuir equation.Mtis the total amount absorbed on the resins,which is the sum of M1and M2.The values of M1are almost the same as those of M2on the three polymers at 288 K, whereas the values of M1and M2are totally different at 318 K. Furthermore,the values of KL1and KL2are identical at 288 and 303 K,whereas the values of KL1are much smaller than those of KL2at 318 K.These observations indicate that the arrangement of NPEO-10 molecules on the polymers′surface is fundamentally affected by the temperature of the studied system.
According to the fitting results of the Langmuir,Freundlich, and Double Langmuir equations,the NPEO-10 molecules appeared to be united as a“monolayer”on the surface of the polymers,and this“monlayer”resulted from the merger of two or more layers.At very low surface concentrations,the adsorption proceeds via attachment of single molecule,and a certain number of the segments of the hydrophobic tail conformably attaches to the surface of the adsorbent.The sudden steep rise in region 2in the inset of Fig.1D indicates a dramatic change in the adsorption mechanism which is due to the formation of small cluster of NPEO-10 molecules onto the surface until the surface is saturatedwiththehemimicelles[24-26].In region 3,formation of new surface cluster slows down;hence there is a decrease in the slope of isotherm.At higher concentrations,the slope of isotherm decreases,and a plateau forms(region 4),where the adsorption amounts remain constant.NPEO-10 may also adsorb onto the surfaces containing oxygen by electrostatic interaction between the oxyethylenic groups and the surfaces.The adsorbed phase is assumed to be a two-dimensional mixture consisting of singly dispersed surfactant molecules,monolayered and bilayered aggregates of various sizes,and empty sites[27-28].The explanations for the nature of adsorption curve in the three regions are depicted in Fig.2.

Table 3 Regression data of isotherms by double Langmuir equation
2.3 Factors affecting the adsorption process
The factors affecting the adsorption process include the length of the hydrophobic and hydrophilic chains in the molecule and the relative size and cross-sectional area of the solvent and solute molecules[29].The interaction between adsorbate and adsorbent is reflected by the experimental adsorption isotherm and,if some theoretical models are assumed,by the predictions of the free energy of adsorption evaluated from the models.In this study, Eq.(5)is employed to determine the standard Gibbs energy change(ΔG0)[30].

where KLis a thermodynamic constant determined from Langmuir equation;T is the absolute temperature in K;and R is the gas constant with a value of 8.314 J·mol-1·K-1.The values of ΔG0were calculated and listed in Table 2.These values are very similar for the three polymers at the same temperature.Further, to a certain adsorbent,the absolute values of ΔG0increases as the temperature increases.ΔG0indicates the degree of spontaneity of an adsorption process,and a higher absolute value reflects a more energetically favorable adsorption.This observation is consistent with the adsorption behavior of ethoxylated nonionic surfactants,that is,at higher temperature,the larger the pore size,the more the amount adsorbed on the polymer surface.
Typically,the polar chain of non-ionic surfactants has a size well above the dimension of alkyl chain and forms an extended polar corona.The NEPO-10 molecule has a length of 4.9 nm, with a hydrophobic part of 1.8 nm and a hydrophilic part of 3.1 nm[31-32].Therefore,during the transfer of the adsorbate molecule onto the surface of the polymers,steric repulsion and stretching deformation should be considered.Essentially,the critical micelle concentration(CMC)of NEPO-10 was affected by the temperature.At different adsorption temperatures the NEPO-10 molecules possessed different conformations,and thus different adsorption behaviors were observed[33].As the surface concentration increased,the NEPO-10 molecules tended to compete with each other to adsorb on the available adsorption sites,the amounts of which depend on the pore size and the free adsorbent surface area.As a result,the adsorption on the wall of the pores became a rate-limiting step.A small pore size made the formation of double layer aggregates of surfactant unfavorable[34]. As shown in Table 1 and Fig.1,in comparison to MN-200 resin, NDA-150,which has the largest pore size,exhibited the highest adsorption capacity toward NPEO-10 at 318 K,even though its surface area is the least among the three polymers.
As described above,the specific surface area and pore size are important factors affecting the adsorption amount.Therefore,the process of NPEO-10 adsorption on the surface of the polymer can be characterized by the interaction of the hydrophobic part of the surfactant molecule with the surface of the polymer and the formation of micelle-like aggregates on the surface of the polymer.The former interaction is exothermic in nature,whereas the latter is related to the endothermic interactions[35-36].The experimental results suggest that the adsorption mechanisms are quite similar for the studied polymers,and the adsorption capacity mainly depends on the surface area and average pore diameter of the adsorbents.High temperature is favorable to the formation of micelle-like structures,since the intermicellar interactions undergoes a change from repulsive to attractive as the temperature increases.Therefore,an increase of temperature will result in an increase of the adsorption amounts[37-38].


2.4 Adsorption kinetics and elution
In order to develop NU-100 as a polymeric adsorbent for the removal and recovery of nonylphenol polyethoxylates from industrial wastewater,adsorption kinetics and elution of NPEO-10 from 1000 mg·L-1aqueous solution onto NU-100 at 303 K and desorption with various contents of ethanol were tested.
Fig.3 shows that the amount of NPEO-10 adsorbed on NU-100 adsorbent from aqueous solution increased with time.A plateau with an adsorption amount of about 250 mg·g-1was observed from 10 to 24 h.After 24 h,the adsorption amount started to increase again until 50 h.A second plateau was observed after 50 h,with the adsorption amount of about 350 mg·g-1.The above adsorption kinetics is interpreted as follows.At a low surface coverage the adsorption takes place with the hydrocarbon chain lying flat.The first plateau region corresponds to a close-packed monolayer of flat-lying surfactant.With a further increase in the adsorbed NPEO-10 molecules,the amount of the molecules is enough to form a partial bi-layer until the kinetic curve extends to the second plateau region[39].According to the above explanation of the adsorption process,we can speculate that the actual aggregate structure on the polymers tested may be intermediate between the monolayer structure and the partial bi-layer structure(Fig.2),such as 1.3 layers,1.5 layers,or 1.7 layers[40].
In general,the high adsorption potential and the high affinity of the polymer matrix for the surfactant might render surfactant elution from the polymers very difficult.It is worthwhile to investigate the elution behavior of NPEO-10 from the polymer and the possibility of recycled use of the polymer.Fig.3 shows the influence of ethanol on the elution rate of NPEO-10 from NU-100 resin.The elution rate increased as the ethanol content in solution increased.The elution rate of solute on NU-100 polymer is greater than 98%with an ethanol concentration of 40% (volume fraction,φ).The observed high elution rate may be related to the fact that the non-ionic surfactants are mainly adsorbed physically,therefore can be desorbed easily with organic solvents.By additional distilling,both NPEO-10 and ethanol can be easily recovered.
3 Conclusions
The presence of non-ionic surfactants in aqueous environment has considerably increased,and there is a need to search for a suitable method to remove them from wastewater.The adsorption characteristics of nonylphenol polyethoxylates in aqueous solution on the three hypercrosslinked polymers have been examined.The following conclusions can be drawn from the present study:
(1)The three polymers have similar structure and their contents of oxygen,carbon,and hydrogen are almost identical.Both surface area and micropore area of the MN-200 resin are bigger than those of the NU-100 resin and NDA-150 resin,whereas the average pore diameters of the polymers exhibit an opposite trend.
(2)The adsorption isotherm can fit the Langmuir model and double Langmuir model better than the Freundlich model;the adsorption isotherms on a lg-lg scale nearly have the same shape and consist of three main regions,which can be interpreted by the molecule arrangement on the adsorbent surface.The specific surface area,pore size,and temperature are fundamental factors affecting adsorption amount.The temperature dependence of intermicellar interactions results in the increase of the adsorption amounts at elevated temperatures.
(3)The investigation on adsorption dynamic indicates that the adsorption process involves two plateaus which are related to the formation of the monolayer and bi-layer.The elution process shows that the non-ionic surfactants are mainly adsorbed physically and can be desorbed easily with organic solvent.
1 Hou,S.G.;Sun,H.W.;Gao,Y.Chemosphere,2006,63:31
2 Gioiaa,D.D.;Sciubbaa,L.;Bertina,L.Water Res.,2009,43: 2977
3 Hu,J.Y.;Chen,X.;Tao,G.Environ.Sci.Technol.,2007,41: 4097
4 Ike,M.;Asano,M.;Belkada,F.D.Water Sci.Technol.,2002,46: 127
5 John,D.M.;House,W.A.;White,G.F.Environ.Toxicol.Chem., 2000,19:293
6 Misra,K.P.;Dash,U.;Somasundaran,P.Ind.Eng.Chem.Res., 2009,48:3403
7 Caruso,F.;Serizawa,T.;Furlong,D.N.Langmuir,1995,11:1546
8 Espantaleón,A.G.;Nieto,J.A.;Fernández,M.Appl.Clay Sci., 2003,24:105
9 Nevskaia,D.M.;Sepulveda-Escribano,A.;Guerrero-Ruiz,A. Phys.Chem.Chem.Phys.,2001,3:463
10 Streat,M.;Sweetland,L.A.React.Funct.Polym.,1997,35:99
11 Penner,N.A.;Nesterenko,P.N.J.Chromatogr.A,2000,884:41
12 Valderrama,C.;Cortina,J.L.;Farran,A.J.Colloid Interface Sci., 2007,310:35
13 Tsyurupa,M.P.;Davankov,V.A.React.Funct.Polym.,2002,53: 193
14 Ahn,J.H.;Jang,J.E.;Oh,C.G.Macromolecules,2006,39:627
15 Yang,W.B.;Li,A.M.;Fan,J.Chemosphere,2006,64:984
16 Valderrama,C.;Gamisans,X.;de las Heras,F.X.React.Funct. Polym.,2007,67:1515
17 Streat,M.;Sweetland,L.A.Trans IChemE B,1998,76:115
18 Per,W.;Bengt,J.Langmuir,1994,10:3268
19 Misra,P.K.;Mishra,B.K.;Somasunduran,P.J.Colloid Interface Sci.,2003,265:1
20 Shalaby,M.N.Polym.Adv.Technol.,2004,15:533
21 Marcel,R.B.;Luuk,K.K.Langmuir,1992,8:2649
22 Li,B.Q.;Eli,R.Langmuir,1996,12:5052
23 González-García,C.M.;González-Martín,M.L.;Gómez-Serrano, V.;Bruque,J.M.;Labajos-Broncano,L.Langmuir,2000,16: 3950
24 Drach,M.;Narkiewicz-Michałek,J.;Rudziński,W.Phys.Chem. Chem.Phys.,2002,4:2307
25 Calvoa,E.;Bravoa,R.;Amigoa,A.Fluid Phase Equilib.,2009, 282:14
26 Zhang,R.;Somasundaran,P.Langmuir,2004,20:8552
27 Edwards,D.A.;Adeel,Z.;Luthy,R.G.Environ.Sci.Technol., 1994,28:1550
28 Adeel,Z.;Luthy,R.G.Environ.Sci.Technol.,1995,29:1032
29 Paria,S.;Yuet,P.K.Ind.Eng.Chem.Res.,2007,46:108
30 Liu,Y.J.Chem.Eng.Data,2009,54:1981
31 Urbina-Villalba,G.;Reif,I.;Márquez,M.L.Colloids Surf.A, 1995,99:207
32 Levitz,P.E.C.R.Geoscience,2002,334:665
33 Muller,N.Langmuir,1993,9:96
34 Kibbey,T.C.G.;Hayes,K.Environ.Sci.Technol.,1997,31:1171
35 Wesemeyer,H.;Muller,B.W.;Muller,R.H.Int.J.Pharm.,1993, 89:33
36 Ghiaci,M.;Kalbasi,R.J.;Abbaspour,A.Colloids Surf.A,2007, 297:105
37 Lindheimer,M.;Keh,E.;Zaini,S.;Partyka,S.J.Colloid Interface Sci.,1990,138:83
38 Winnik,M.A.;Bystryak,S.M.;Odrobina,E.Langmuir,2000,16: 6118
39 Mishra,S.K.;Kanungo,S.B.;Rajeev.J.Colloid Interface Sci., 2003,267:42
40 Gallardo-Moreno,A.M.;González-García,C.M.;González-Martín,M.L.;Bruque,J.M.Colloids Surf.A,2004,249:57
超高交联树脂对壬基酚聚氧乙烯醚的吸附机理
杨维本*任 丽
(南京师范大学化学与环境科学学院,南京 210097)
研究了壬基酚聚氧乙烯醚(NPEO-10)在3种具有不同比表面积和孔径大小的超高交联树脂上的吸附行为与机理.3种超高交联树脂对壬基酚聚氧乙烯醚的吸附量受它们的比表面积和孔径大小以及溶液温度的影响.壬基酚聚氧乙烯醚在3种超高交联树脂上的吸附等温线可以用Langmuir和双Langmuir模型很好地拟合,而用Freundlich模型拟合则效果不好,但这些拟合曲线都具有相似的形状.热力学分析表明吸附过程主要表现为吸附质分子的疏水部分和吸附剂表面的作用以及吸附质分子在其表面形成胶束状的聚集体,即分散的、单层及双层聚集体的混合分布.吸附动力学曲线中的两个平台也证明了吸附过程存在单层和双层聚集体.脱附研究为实现超高交联树脂吸附分离水溶液中的壬基酚聚氧乙烯醚提供了合适的操作条件.
脱附;壬基酚聚氧乙烯醚;等温线;树脂;温度
O642
Received:February 22,2010;Revised:April 1,2010;Published on Web:June 7,2010.
*Corresponding author.Email:yangwb007@njnu.edu.cn;Tel:+86-25-85560233;Fax:+86-25-85572627.
The project was supported by the National Natural Science Foundation of China(50978137)and Natural Science Foundation of Jiangsu Province,China(BK2008436).
国家自然科学基金(50978137)和江苏省自然科学基金(BK2008436)资助项目
ⒸEditorial office of Acta Physico-Chimica Sinica
猜你喜欢
杂志排行
物理化学学报的其它文章
- Coexistence of Oligonucleotide/Single-Chained Cationic Surfactant Vesicles with Precipitates
- Influence of Calcination Temperature on the Performance of Cu-Al-Ba Catalyst for Hydrogenation of Esters to Alcohols
- Novel Synthesis of Mesoporous Nanocrystalline Zirconia
- Fluorescence Behavior of Biphenyl Containing Side-Chain Liquid Crystalline Polyacetylene with Various Lengths of Spacers
- Numerical Analysis of the Effect of Carbon Monoxide Addition on Soot Formation in an Acetylene/Air Premixed Flame
- Synthesis,Crystal Structure,Thermal Behavior and Sensitivity of[Mn(AZT)2(H2O)4](HTNR)2·4H2O
