Synthesis,Crystal Structure,Thermal Behavior and Sensitivity of[Mn(AZT)2(H2O)4](HTNR)2·4H2O
2012-11-06FENGJinLingZHANGJianGuoZHANGTongLaiCUIYan
FENG Jin-Ling ZHANG Jian-Guo,* ZHANG Tong-Lai,* CUI Yan
(1State Key Laboratory of Explosion Science and Technology,Beijing Institute of Technology,Beijing 100081,P.R.China;2The 6th Department,Research Institute of Chemical Defense(PLA),Beijing 102205,P.R.China)
Synthesis,Crystal Structure,Thermal Behavior and Sensitivity of[Mn(AZT)2(H2O)4](HTNR)2·4H2O
FENG Jin-Ling1ZHANG Jian-Guo1,*ZHANG Tong-Lai1,*CUI Yan2
(1State Key Laboratory of Explosion Science and Technology,Beijing Institute of Technology,Beijing 100081,P.R.China;2The 6th Department,Research Institute of Chemical Defense(PLA),Beijing 102205,P.R.China)
A novel energetic coordination complex of[Mn(AZT)2(H2O)4](HTNR)2·4H2O(AZT=3-azido-1,2,4-triazole,HTNR=2,4,6-trinitro resorcinol)was prepared by the reaction between the acidic manganese(II)salt of 2,4,6-trinitro resorcinol and AZT in an aqueous solution.The complex was characterized by elemental analysis and FTIR spectroscopy.The molecular structure was determined by X-ray single crystal diffraction.The crystal belongs to the triclinic system with a P1 space group.The central manganese(II)cation has a slightly distorted octahedron feature.Three-dimension networks were formed and the layers are linked by extensive hydrogen bonding.The thermal decompositionmechanismof[Mn(AZT)2(H2O)4](HTNR)2·4H2O was predicted based on differential scanning calorimetry(DSC)and thermogravimetry-derivative thermogravimetry(TG-DTG)analyses.One endothermic peak and three exothermic peaks are present during the thermal decomposition process with the final residue at 600℃being MnO and MnO2.The kinetic parameters of the exothermic process for the complex were studied using Kissinger′s and Ozawa-Doyle′s methods.Furthermore,impact sensitivity,flame sensitivity,and friction sensitivity tests reveal that the title complex is sensitive and selective towards external stimulants.
Crystal structure;3-Azido-1,2,4-triazole;Manganese(II)complex;Sensitivity;Thermal behavior
Nitrogen-rich compounds have attracted significant attention due to their potential applications as high-energy-density materials(HEDMs)in propulsion and explosive fields[1-7].They derive most of their energies either from the oxidation of the carbon backbone,or from their high positive heats of formation[8].Good HEDMs possess high density,have a fast velocity of detonation, and are energetically unstable with respect to their reaction products.Five-member nitrogen-rich heterocyclic compounds, such as triazole series[9-15],are one of the representative candidates of HEDMs.The high positive formation enthalpy and high nitrogen content of triazole have made its complexes interesting for being used as energetic materials.The addition of azide group strongly immproves its energetic properties[16].The nitrogen content of 3-azido-1,2,4-triazole(AZT)is 76.36%,and its standard heat of formation(ΔH⊖f)is 458 kJ·mol-1[17].
Hydroxyl O atoms and triazolyl ring N atoms have good coordination capacities.Therefore,1,2,4-triazole has potential bridging fashions(μ1,2,μ2,4,and μ1,2,4)and strong coordination capabilities to bridge transition metal ions[18].To the best of our knowledge in published studies on 1,2,4-triazole-based energetic complexes,the ligands have been limited to 1,2,4-triazol-5-one[19-20],3-nitro-1,2,4-triazol-5-one[21-24],and 4-amino-1,2,4-triazol-5-one[25]. Thusfar,theligandsofothertriazolederivatives,suchas3-amino-1H-1,2,4-triazole[26]and 3,4,5-triamino-1,2,4-triazole[27],have been briefly studied.For the compound of AZT,main attention has been focused on its energetic ionic salts[28-32],except for our early research of using AZT as ligands for zinc(II)and cadmium(II) cations,where the structure and thermal properties were investigated[33-34].
Enlightened by previous studies,we have selected other transition metals to construct novel AZT-based coordination compounds with new structures.Herein,we report a new manganese coordination compound[Mn(AZT)2(H2O)4](HTNR)2·4H2O,with AZT and H2O molecules as ligands,acidic anion of 2,4,6-trinitro resorcinol(HTNR)as outer anion.Thermal decomposition mechanism was studied with differential scanning calorimetry(DSC), thermogravimetry-derivative thermogravimetry(TG-DTG)analyses,and Fourier transform infrared(FTIR)spectroscopy.Furthermore,the investigations of sensitivity properties revealed the potential application of the title compound as an energetic material in ammunitions.
1 Experimental
General caution:AZT and its coordination compound are energetic materials and tend to explode under certain conditions. Appropriate safety precautions,such as safety glasses,face shields,leather coat,and ear plugs,should be taken during the synthesis,test,and measurement processes,especially when these compounds are prepared on a large scale and in dry states.
1.1 Materials and instruments
All chemicals from commercial sources were of analytical pure and used without further purification.AZT was prepared according to the method literature[35]reported.
FTIR spectrum was recorded as KBr pellets on a Bruker Equinox 55 infrared spectrometer(Germany)in the range of 4000-400 cm-1with the resolution of 4 cm-1.Elemental analysis was carried out by a Flash EA 1112 full-automatic trace element analyzer(USA).
DSC studies were performed on Perkin-Elmer Pyris-1 Differential Scanning Calorimeter(USA)with heating rates of 2,5,10, 15,and 20℃·min-1,respectively.TG analysis was conducted on Perkin-Elmer Pyris-1 Thermo-gravimetric Analyzer(USA) with a heating rate of 10℃·min-1in a flow of dry oxygen-free nitrogen at 20 mL·min-1.The sample of 0.5 mg was sealed in aluminum pans for DSC and held in platinum pans for TG-DTG.
1.2 Preparation
MnCO3(2.30 g,20 mmol)was added to a suspending solution of 2,4,6-trinitro resorcinol(9.80 g,40 mmol)in 20 mL distilled water at 50℃with vigorous stirring.Mn(HTNR)2solution was obtained after filtering the mixture.AZT(4.40 g,40 mmol)was dissolved in 40 mL distilled water,and the solution was added dropwise when the temperature reached 60℃.The mixture was stirred for an additional 1 h to complete the reaction.The jacinth precipitate was collected by filtration,washed with ethanol and dried in vacuum,with a yield of 84%(based on AZT).Element analysis,Calcd.(%)for[Mn(AZT)2(H2O)4](HTNR)2·4H2O:C, 21.16;H,2.64;N,27.77;Found(%):C,21.11;H,2.62;N,27.95. IR data(KBr pellet,ν/cm-1):3394(s),2150(s),1631(s),1572(s), 1531(s),1465(s),1333(s),1272(m),1180(m),1085(s),971(w), 927(m),873(w),735(m),692(m),626(w).
1.3 X-ray crystallography
Single crystals of[Mn(AZT)2(H2O)4](HTNR)2·4H2O were obtained through slow evaporation of a saturated water solution at 15℃for 25 d(distilled water,white rods,0.22 mm×0.20 mm× 0.18 mm).A Bruker Smart 1000 CCD diffractometer(Germany) with graphite monochromatic Mo Kαradiation(λ=0.07107 nm) was used for data collection at 21℃.Intensity measurements were performed using φ and ω scan modes.A total of 2793 reflections were used to determine the lattice parameters and orientation matrix in the range of 2.38°≤θ≤26.34°.The structure was solved by direct methods using SHELXS-97[36]and refined by full-matrix least squares techniques based on F2with the SHELXL-97 program[37].All non-hydrogen atoms were obtained from the difference Fourier map and refined anisotropically.All hydrogen atoms were generated geometrically or from the difference Fourier map,and treated by a constrained refinement. Crystallographic and refinement data are listed in Table 1.
2 Results and discussion
2.1 Structure description
[Mn(AZT)2(H2O)4](HTNR)2·4H2O crystallizes with a triclinic unit cell in the space group P1.The coordination environment of themanganesecationandthemolecularunitof[Mn(AZT)2(H2O)4] (HTNR)2·4H2O,with atom labeling and the intra-molecular hydrogen bonds,are shown in Fig.1.Packing diagram of [Mn(AZT)2(H2O)4](HTNR)2·4H2O viewed along a-axis is shownin Fig.2.Selected bond data of[Mn(AZT)2(H2O)4](HTNR)2· 4H2O,compared with those in the molecular crystal of AZT[34], are listed in Table 2.The hydrogen bond lengths and bond angles are listed in Table 3.
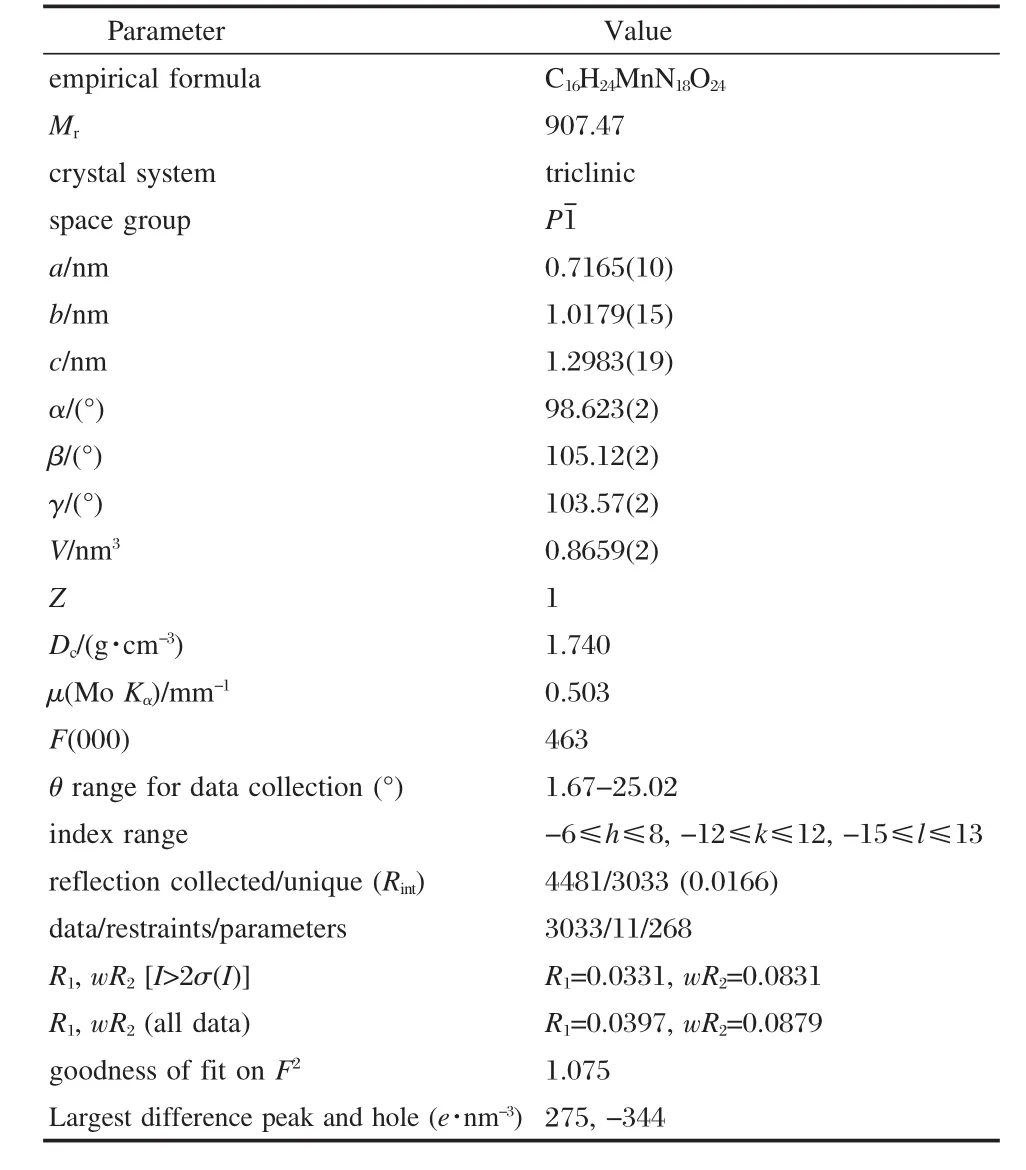
Table 1 Crystal data and structure refinement for [Mn(AZT)2(H2O)4](HTNR)2·4H2O
As we can see from Fig.1,in the molecular unit of[Mn(AZT)2(H2O)4](HTNR)2·4H2O,each central manganese(II)ion is hexacoordinated with two N(4)atoms from two AZT molecules and four O atoms from four H2O molecules to form a centrosymmetric slightly distorted octahedron.There are three parallelogram planes in the structure,N(4)—O(9)—Mn(1)—N(4)#1—O(9)#1 (plane A),O(10)—N(4)—Mn(1)—O(10)#1—N(4)#1(plane B), and O(10)—O(9)—Mn(1)—O(10)#1—O(9)#1(plane C).The angles between plane A and plane B,plane B and plane C,plane C and plane A,all slightly derivate from 90°,are 86.0°,91.4°, and 91.2°,respectively.The bond lengths of manganese(II)centertothesixcoordinationatomscanbeobservedtobe0.2140(13) nm for Mn(1)—O(10)and Mn(1)—O(10)#1,0.2212(14)nm for Mn(1)—O(9)and Mn(1)—O(9)#1,0.2277(16)nm for Mn(1)—N(4)and Mn(1)—N(4)#1.The bond angles for O(10)—Mn(1)—O(10)#1,O(9)—Mn(1)—O(9)#1 and N(4)#1—Mn(1)—N(4)are almost the same(180°),while those around the central Mn2+derivate from 90°by the value of 1.28°,1.06°,and 3.97°,for the reason of the steric hindrance effect by the bulky AZT molecules and H2O molecules.
In the crystal structure of[Mn(AZT)2(H2O)4](HTNR)2·4H2O, thecoordinationsiteofAZTisalsoN(5)atom,whichhasthemost negative charge(-0.49387e)as calculated at the B3LYP/6-311+ G**level using the Gaussian 98 program package[34].Most of the bond lengths and bond angles for the AZT ligand differ slightly from the corresponding ones of AZT molecule(Table 2),except for the azido-group with relatively obvious changes.The bond lengths of N(6)—C(8),N(7)—C(8)and N(8)—N(9)are shortened by 0.0007,0.0012 and 0.0007 nm,respectively,the elongation of the N(4)—C(8)bond is 0.0008 nm,otherwise.The rea-son for this can be concluded as the existence of the small steric hindrance effect of four H2O molecules.Besides,the coordination of Mn—N(4)destroys the natural p-π conjugation and π-π conjugation among the crystal structure of AZT in which the triazole ring is aromatic and contains six delocalized π-electrons. Corresponding to the length changes,the bond angels of N(8)—N(7)—C(8)and N(6)—N(8)—N(7)are increased by ca 3°,while that of N(9)—N(8)—N(7)is decreased by ca 3°.This kind of arrangement can facilitate the coordinated small-sized H2O molecules and the bulky AZT molecules with central manganese(II)cation to complete the octahedral sphere figure.The bond lengths and bond angles of HTNR are usual for coordination compounds with HTNR as outer anions.

Table 2 Selected bond lengths and bond angles for[Mn(AZT)2(H2O)4](HTNR)2·4H2O and AZT
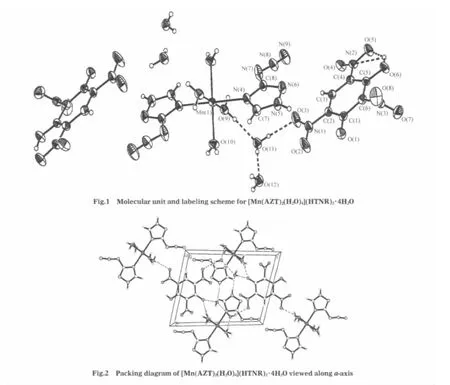
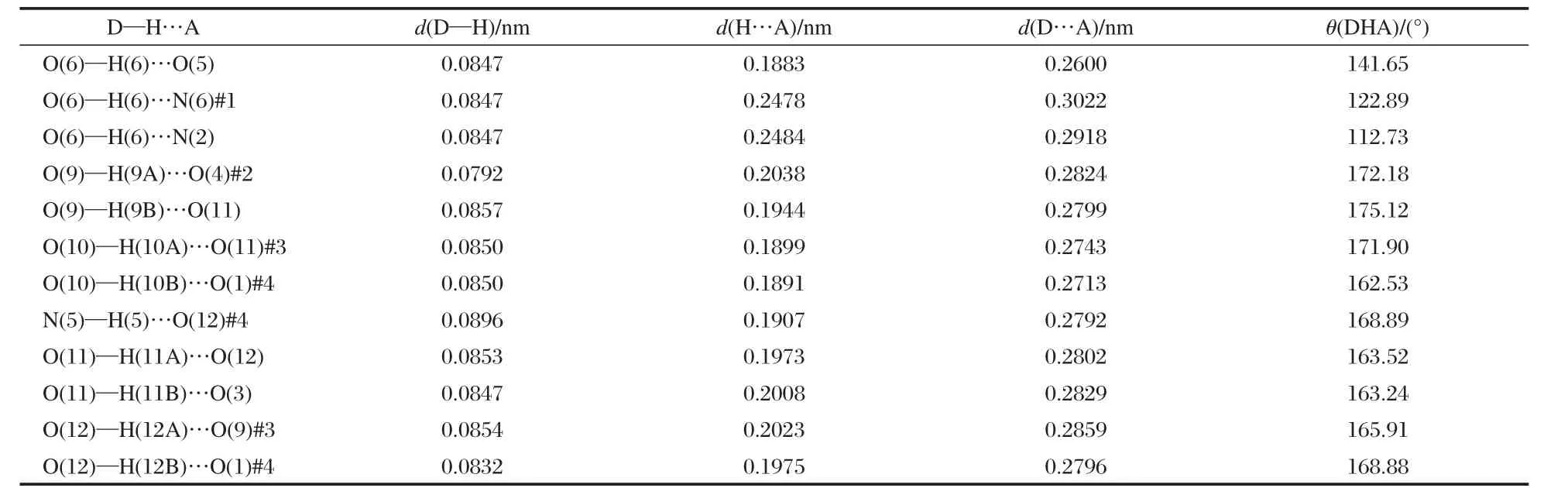
Table 3 Hydrogen bond lengths and bond angles for[Mn(AZT)2(H2O)4](HTNR)2·4H2O
There are many kinds of hydrogen bonds in the crystal structure of[Mn(AZT)2(H2O)4](HTNR)2·4H2O.Lattice and coordination H2O molecules play vital roles in the formation of the structure.Three types of intra-molecular hydrogen bonds can be observed from Fig.1.The first type is formed between the hydroxylgroup and nitro-group of the HTNR anion,O(6)—H(6)…O(5) and O(6)—H(6)…N(2).The second type occurs between the lattice H2O molecules and the adjacent nitro-group of the HTNR anion,O(11)—H(11A)…O(3).The third type takes place among the coordinated and lattice H2O molecules of O(9),O(11),and O (12).we can see from Fig.2 that all the molecular units are linked together into 3D structures by the intermolecular hydrogen bonds,which are formed among different molecular units and can be divided into two types.One type takes place between the lattice H2O molecules and coordinated H2O molecules or AZT molecule,O(12)—H(12A)…O(9)#3,O(10)—H(10A)…O (11)#2,N(5)—H(5)…O(12)#4.The similar mode occurs between the hydroxyl-group or deprotonated hydroxylgroup of the outer HTNR anion and the coordinated H2O molecules or AZT molecule,O(10)—H(10B)…O(1)#4,O(9)—H(9A)…O(4)#2, and O(6)—H(6)…N(6)#1,O(12)—H(12B)…O(1)#4.These extensive hydrogen bonds also make an important contribution totheformationandstabilityofthecrystalstructureof[Mn(AZT)2(H2O)4](HTNR)2·4H2O.
2.2 Thermal decompositions
Under the linear heating rate of 10℃·min-1,DSC and TGDTG experiments were carried out in order to investigate the thermal behaviors of[Mn(AZT)2(H2O)4](HTNR)2·4H2O.The DSC and TG-DTG curves of[Mn(AZT)2(H2O)4](HTNR)2·4H2O are illustrated in Fig.3 and Fig.4.
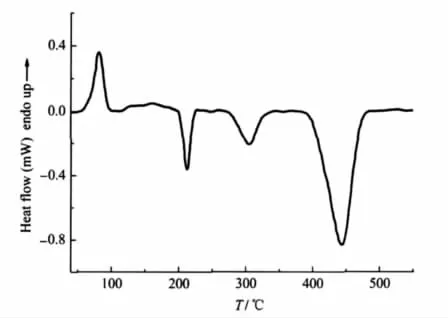
Fig.3 DSC curve of[Mn(AZT)2(H2O)4](HTNR)2·4H2O at a heating rate of 10℃·min-1
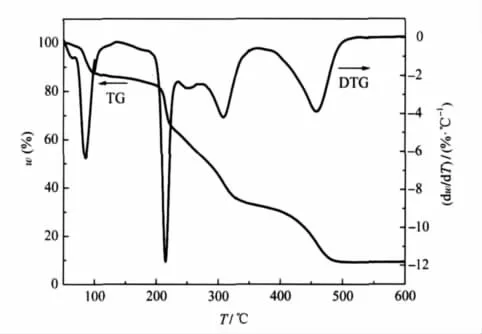
Fig.4 TG-DTG curves of[Mn(AZT)2(H2O)4](HTNR)2·4H2O at a heating rate of 10℃·min-1
There are one endothermic process and three exothermic peaks in the range of 45.3-600℃.A mass loss of 15.8%can be seen in the TG-DTG curves during the first stage of 45.3-176.2℃,which is in coincident with the theoretical value of losing four lattice H2O molecules and four coordination H2O molecules. The structure of the compound is unstable and begins decomposing with three steps after lossing all the H2O molecules.The first-step is a sharp exothermic decomposition occurred during the temperature range of 176.2-229.8℃,with the peak temperature at 210.9℃and the exothermic enthalpy change of 185.9 kJ·mol-1.There is a mass loss of 20.2%in the TG-DTG curve. The compound undergoes further decomposition in the range of 229.8-339.2℃,with a peak temperature at 303.9℃and a mass loss of 29.7%.The exothermic enthalpy of this process is 187.1 kJ·mol-1,the value of which is similar to the first exothermic process.The last exothermic process occurs with the onset temperature at 388.2℃and the peak temperature at 444.1℃,the mass loss corresponding to this stage in TG-DTG curves is 35.0%.The exothermic enthalpy change(1038.3 kJ·mol-1)of this process is very strong.The mass fraction of the final residue is 9.3%,which is between the theoretical values of MnO and MnO2,7.8%and 9.6%.The absorption band at 600 and 650 cm-1in the FTIR spectrum of the residue at 600℃also proves that the final residue is the mixture of MnO[38]and MnO2[39].
Based on the experimental and calculated results,the thermal decomposition processes of the complex can be proposed as follows:
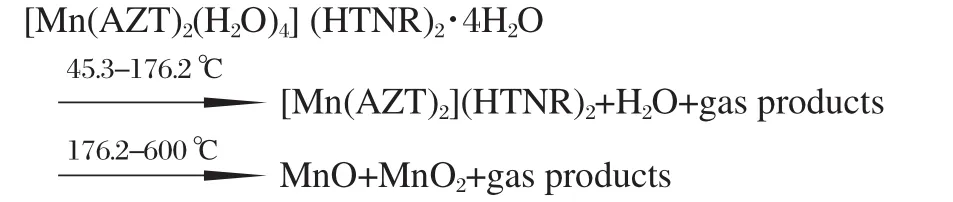
2.3 Non-isothermal kinetics analysis
We can see,from the DSC and TG-DTG curves,the first exothermic process has dominant effects on the decomposition of the complex.Hence,we studied the kinetics parameters of the first exothermic process of[Mn(AZT)2(H2O)4](HTNR)2·4H2O by using the Kissinger′s[40]and Ozawa-Doyle′s[41]methods.The Kissinger equation(1)and Ozawa-Doyle equations(2)are as follows:

where Tpis the peak temperature,℃;R is the gas constant,8.314 J·mol-1·℃-1;β is the linear heating rate,℃·min-1;C is a constant.
Based on the multiple non-isothermal DSC curves obtained atfive different heating rates of 2,5,10,15,20℃·min-1,the peak temperatures Tpcan be observed as 196.5,203.3,211.1,214.8, 215.7℃.The values of apparent activation energy Ekand Eo, which are calculated by the Kissinger′s method and Ozawa-Doyle′s method,are 206.3 and 203.7 kJ·mol-1,respectively.The corresponding linear coefficients rkand roare 0.9934 and 0.9939. The pre-exponential factor Akdetermined with the Kissinger′s method is 20.55 s-1.The calculated results using both methods are similar and within the normal range of kinetic parameters of such thermal decomposition reaction[42].
The Arrhenius equation can be expressed with Ea(the average of Ekand Eo)and lnAkas follows:lnk=20.55-206.3×103/(RT). This equation can be used to estimate the rate constants of the initialthermaldecompositionprocessof[Mn(AZT)2(H2O)4](HTNR)2· 4H2O.
2.4 Sensitivitypropertiesofcomplex[Mn(AZT)2(H2O)4] (HTNR)2·4H2O
Inordertostudythestabilityandthehazardof[Mn(AZT)2(H2O)4] (HTNR)2·4H2O,we tested its sensitivity properties.The sample of 20 mg was compacted in a copper cap with a pressure of 39.2 MPa to test the impact sensitivity and flame sensitivity.The impact sensitivity was determined with a Bruceton method[43]on the standard fall hammer apparatus,and the compacted sample was hit with 0.8 kg drop hammer on the apparatus.The results of impact sensitivity showed that[Mn(AZT)2(H2O)4](HTNR)2·4H2O did not fire at the highest apparatus limitation of 53 cm.
The flame sensitivity was determined on a designed flame sensitivity apparatus,and the compacted sample was ignited by a standard black powder pellet right above the sample.The friction sensitivity was determined by using a standard pendulum apparatus.According to the standard method[44],flame sensitivity was evaluated with the height for 50%probability of explosion (h50%)of the sample.h50%of[Mn(AZT)2(H2O)4](HTNR)2·4H2O is calculated as 15.42 cm,which reveals that the compound has a moderate flame sensitivity.
The sample was compressed firmly between two steel poles with mirror surfaces at the pressure of 1.96 MPa,then it was hit horizontally with a 1 kg hammer dropping from 90°angle.The statistical firing rate about friction sensitivity of[Mn(AZT)2(H2O)4](HTNR)2·4H2O is 80%,which shows that it is relatively sensitive to friction at the testing conditions.The sensitivity testing results are related with the molecular structure and thermal stability of the compound.
3 Conclusions
A novel energetic coordination compound[Mn(AZT)2(H2O)4] (HTNR)2·4H2O was synthesized and structurally characterized. Thermal analyses show that there are one endothermic process and three exothermic decomposition stages in the temperature range of 45.3-600℃,with the final residue at 600℃being the mixture of MnO and MnO2.The sensitivity test results indicate that[Mn(AZT)2(H2O)4](HTNR)2·4H2O has relatively strong sensitivity to friction,moderate sensitivity to flame and weak sensitivity to vertically hit,which means the compound possesses sensitivity and selectivity,which is of significance interest for ammunition application.
Supporting information: CCDC No.746340 contains the supplementary crystallographic data for this article.These data can be obtained free of charge at http://www.ccdc.cam.ac.uk[or from the Cambridge Crystallographic Data Centre(CCDC),12 Union Road,Cambridge CB2 1EZ,UK;Fax:+44(0)1223-336033;Email:deposit@ccdc.cam.ac.uk].
1 Mondal,T.;Saritha,B.;Ghanta,S.;Roy,T.K.;Mahapatra,S.; Durga,M.P.J.Mol.Struct.-Theochem.,2009,897:42
2 Kokan,T.S.;Olds,J.R.;Seitzman,J.M.;Ludovice,P.J.Acta Astro.,2009,65:967
3 Badgujar,D.M.;Talawar,M.B.;Asthana,S.N.;Mahulikar,P.P. J.Hazard.Mater.,2008,151:289
4 Cottrell,R.;McAdory,D.;Jones,J.;Gilchrist,A.;Shields,D.; Strout,D.L.J.Phys.Chem.A,2006,110:13889
5 Sikder,A.K.;Sikder,N.J.Hazard.Mater.,2004,112:1
6 Pagoria,P.F.;Lee,G.S.;Mitchell,A.R.;Schmidt,R.D. Thermochim.Acta,2002,384:187
7 Striebich,R.C.;Lawrence,J.J.Anal.Appl.Pyrolysis.,2003,70: 339
8 Turker,L.;Atalar,T.;Guemues,S.;Camur,Y.J.Hazard.Mater., 2009,167:440
9 Angelo,N.G.;Arora,P.S.J.Am.Chem.Soc.,2005,127:17134
10 Lamanna,M.E.;Horra,E.;Jacobo,S.;Accorso,N.B.React. Funct.Polym.,2009,69:759
11 Senchyk,G.A.;Lysenko,A.B.;Rusanov,E.B.;Chernega,A.N.; Krautscheid,H.;Domasevitch,K.V.Inorg.Chim.Acta,2009, 362:4439
12 Isloor,A.M.;Kalluray,B.;Shetty,P.Eur.J.Med.Chem.,2009, 44:3784
13 Mullen,K.M.;Mercurio,J.;Serpell,C.J.;Beer,P.D.Angew. Chem.Int.Edit.,2009,48:4781
14 Sherif,S.M.;Erasmus,R.M.;Comins,J.D.J.Colloid Interface Sci.,2007,311:144
15 Kuroiwa,K.;Shibata,T.;Takada,A.;Nemoto,N.;Kimizuka,N. J.Am.Chem.Soc.,2004,126:2016
16 Li,Q.S.;Duan,H.X.J.Phys.Chem.A,2005,109:9089
17 Xiao,Y.D.;Wei,L.H.;Wang,J.T.;Zhang,J.B.;Lin,S.F.;Zhou, Z.H.Chemom.Intell.Lab.Syst.,1999,45:277
18 Zhai,Q.G.;Wu,X.Y.;Chen,S.M.;Lu,C.Z.;Yang,W.B.Cryst. Growth Des.,2006,6:2126
19 Zhang,J.G.;Zhang,T.L.;Lu,Z.;Yu,K.B.Acta Chim.Sin., 1999,57:1233 [张建国,张同来,陆 政,郁开北.化学学报, 1999,57:1233]
20 Zhang,J.G.;Zhang,T.L.;Yang,L.;Mao,L.Q.;Yu,K.B.Chin.J. Inorg.Chem.,2002,18:284 [张建国,张同来,杨 利,毛利秋,郁开北.无机化学学报,2002,18:284]
21 Zhang,T.L.;Lü,C.H.;Zhang,J.G.;Zhang,Z.G.;Yu,K.B. Chin.J.Inorg.Chem.,2002,18:138 [张同来,吕春华,张建国,张志刚,郁开北.无机化学学报,2002,18:138]
22 Singh,G.;Kapoor,I.P.S.;Felix,S.P.;Agrawal,J.P.Propellants Explos.Pyrotech.,2002,27:16
23 Yun,S.S.;Kim,J.K.;Kim,C.H.J.Alloy.Compd.,2006,408: 945
24 Song,J.R.;Ma,H.X.;Huang,J.;Hu,R.Z.Thermochim.Acta, 2004,416:43
25 Zhang,J.G.;Zhang,T.L.Acta Chim.Sin.,2000,58:1563 [张建国,张同来.化学学报,2000,58:1563]
26 Liu,B.;Chen,Y.H.;Zhang,X.C.Inorg.Chem.Commun.,2008, 11:965
27 Bichay,M.;Fronabarger,J.W.;Gilardi,R.;Butcher,R.J.; Sanborn,W.B.;Sitzmanna,M.E.;Williams,M.D.Tetrahedron Lett.,2006,47:6663
28 Zhang,J.P.;Lin,Y.Y.;Zhang,W.X.;Chen,X.M.J.Am.Chem. Soc.,2005,127:14162
29 Xue,H.;Gao,Y.;Twamley,B.;Shreeve,J.M.Inorg.Chem., 2005,44:5068
30 Huang,Y.G.;Gao,H.X.;Twamley,B.;Shreeve,J.M.Eur.J. Inorg.Chem.,2008,16:2560
31 Moderhack,D.;Daoud,A.J.Heterocycl.Chem.,2003,40:625
32 Drake,G.;Hawkins,T.;Brand,A.;Hall,L.;Mckay,M. Propellants Explos.Pyrotech.,2003,28:174
33 Cui,Y.;Zhang,T.L.;Zhang,J.G.;Yang,L.Chin.J.Chem.,2008, 26:2021
34 Cui,Y.;Zhang,T.L.;Zhang,J.G.;Yang,L.;Zhang,J.;Hu,X.C. Struct.Chem.,2008,19:269
35 Kofman,T.P.;Krasnov,K.N.Russ.J.Org.Chem.,2004,40: 1651
36 Sheldrick,G.M.SHELXL-97,program for the solution of crystal structure.Gottingen,Germany:University of Gottingen,1997
37 Sheldrick,G.M.SHELXS-97,program for the refining of crystal structure.Gottingen,Germany:University of Gottingen,1997
38 Nyquist,R.A.;Kagel,R.O.Infrared spectra of inorganic compounds.New York:Academic Press,1971:218
39 Fernandes,J.B.;Desai,B.;Dalal,V.N.K.Electrochim.Acta, 1983,28:309
40 Kissinger,H.E.Anal.Chem.,1957,29:1702
41 Ozawa,T.Bull.Chem.Soc.Jpn.,1965,38:1881
42 Hu,R.Z.;Yang,Z.Q.;Liang,Y.J.Thermochim.Acta,1988, 123:135
43 Dixon,W.J.;Mood,A.M.J.Am.Stat.Assoc.,1948,43:109
44 Liu,Z.T.;Lao,Y.L.Initiating explosive experimental.Beijing: Beijing Institute of Technology,1995:238-250 [刘自汤,劳允亮.起爆药实验.北京:北京理工大学出版社,1995:238-250]
[Mn(AZT)2(H2O)4](HTNR)2·4H2O的合成、晶体结构、热行为及感度性质
冯金玲1张建国1,*张同来1,*崔 燕2
(1北京理工大学爆炸科学与技术国家重点实验室,北京 100081;2防化研究院第六研究所,北京 102205)
通过酸性2,4,6-三硝基间苯二酚(HTNR)的锰盐与3-叠氮-1,2,4-三唑(AZT)在水溶液中反应,制备得到一种新颖的锰配合物[Mn(AZT)2(H2O)4](HTNR)2·4H2O.通过元素分析和红外光谱对配合物进行了表征,用X射线单晶衍射分析确定其晶体结构.该配合物为三斜晶系,空间群为P1,中心锰(II)离子为六配位的畸变的八面体结构,分子内和分子间强烈的氢键作用构成了有序的三维(3D)网状结构.采用差示扫描量热(DSC)和热重-微分热重 (TG-DTG)分析技术研究了配合物的热分解特性,并预测了它的热分解反应机理.利用Kissinger方法和Ozawa-Doyle方法研究了其第一放热分解峰的分解动力学过程.其分解过程包括一个吸热峰和三个放热峰,在600℃的分解产物为MnO和MnO2的混合物.同时.对这个配合物进行了感度(撞击感度、火焰感度、摩擦感度)性能分析,结果表明,它对外界刺激具有很强的响应性和选择性.
晶体结构;3-叠氮-1,2,4-三唑;锰(II)配合物;感度;热行为
O641;O642;O741
Received:April 1,2010;Revised:May 23,2010;Published on Web:July 15,2010.
*Corresponding authors.ZHANG Jian-Guo,Email:zhangjianguobit@yahoo.com.cn.ZHANG Tong-Lai,Email:ztlbit@bit.edu.cn;
Tel/Fax:+86-10-68913818.
The project was supported by the National Natural Science Foundation of China(20471008)and Program for New Century Excellent Talents in Universities of the Ministry of Education of China(NCET-09-0051).
国家自然科学基金(20471008)和教育部新世纪优秀人才支持计划(NCET-09-0051)资助项目
ⒸEditorial office of Acta Physico-Chimica Sinica
