Numerical Analysis of the Effect of Carbon Monoxide Addition on Soot Formation in an Acetylene/Air Premixed Flame
2012-11-06JIANGYongQIURong
JIANG Yong QIU Rong
(State Key Laboratory of Fire Science,University of Science and Technology of China,Hefei 230026,P.R.China)
Numerical Analysis of the Effect of Carbon Monoxide Addition on Soot Formation in an Acetylene/Air Premixed Flame
JIANG Yong*QIU Rong
(State Key Laboratory of Fire Science,University of Science and Technology of China,Hefei 230026,P.R.China)
The effect of carbon monoxide addition on soot formation in an acetylene/air premixed flame was investigated by detailed numerical simulation.This work focused on both the temperature effect and chemical effect of carbon monoxide addition on soot formation by comparing the results of flames with different CO contents.We find that the addition of carbon monoxide consistently reduces the formation of soot.The soot volume fraction and nucleation rate increase until a threshold temperature is reached and then decrease as the temperature increases.Considering that soot formation took place at the active site by H-abstraction mechanism,the addition of CO promotes the formation of soot.The concentration of H radicals increases and the concentration of OH radicals decreases because of the increased forward rate of the reaction OH+CO=CO2+H.For soot formation to occur by the C-addition mechanism,the degradation rates of C2H2tends to decrease and this promotes the formation of soot along with CO addition.On the other hand,the addition of CO may greatly reduce the volume fraction of C2H2in fuel resulting in a lower surface growth rate.
Soot;Acetylene;Carbon monoxide;Fuel enrichment;Modeling
Acetylene is one of the most abundant intermediates in hydrocarbon combustion as well as in the combustion of halogenated hydrocarbons such as CH3Br and CH2Cl2.Acetylene is also known to play a very important role in the formation of soot[1-2].Soot emissions resulting from combustion have long been recognized as a significant problem.Soot itself is intrinsically toxic and soot particles are strongly associated with detrimental health effects.In the earth atmosphere soot contributes to the entrapment of the solar radiation that is believed to lead to global warming.Besides,soot is present in hydrocarbon/air flames,where it affects flame reaction mechanisms and structure[3-5].Fuel enrichment combustion is a promising concept for substantial reduction in fuel consumption and pollutant emissions into the atmosphere.Guo et al.[6]have shown that hydrogen enrichment can extend the flammability limit and reduce NO formation incounterflow CH4/air premixed flames by allowing a combustor to operate at leaner condition.Ren et al.[7]studied strain-rate effects on hydrogen-enhanced lean premixed combustion by experimental and numerical approaches,and verified that it can increasetheleancombustionstabilitythrough addition ofH2.Coppens et al.[8]focused on the effects of composition on burning velocity and nitric oxide formation in non-stretched laminar premixed flames of CH4+H2+O2+N2.By reforming hydrocarbon fuels,reformate gas contains not only H2but also CO.CO is a primary component of a reformate gas or a syngas which is an effective and practical enrichment additive[9].Assuming the reformate gas is the product of partial oxidation of methane by air which consists of H2,CO,and N2,Guo et al.[9]studied the effect of reformate gas enrichment on extinction limits and NOxformation in counterflow CH4/air premixed flames.It is found that the addition of the reformate gas extends the lean flammability limit and greatly reduces the formation of NO at leaner condition without any effect on flammable range.Wu et al.[10]investigated the effects of CO addition on the characteristics of premixed CH4/air opposed-jet flames experimentally and numerically.The results show that the maximum burning velocity occurs at the rich side of stoichiometry for a fixed fuel composition.The maximum burning velocity increases with increasing CO concentration, and it reaches its highest value and then decreases along with the increase in CO concentration.
For the effect of additives on soot formation,previous studies have focused on addition of H2or inert gases.Gülder et al.[11]studied the influence of soot of hydrogen addition to the fuel in diffusion flames.They found the influence was negligible for soot formation by addition of hydrogen to the fuel side for an ethylene diffusion flame,and hydrogen addition did not show any influence on soot formation apart from the dilution effect for propane and butane flames.Glassman[12]examined the effect of inerts additives on sooting laminar coaxial diffusion flames,and it showed that temperature effect caused a logarithmic variation in soot volume fraction.Guo et al.[13]investigated numerically the influence of transport properties of inert additives on soot formation in a coflow axisymmetric ethylene/air diffusion flame. They took into account the interactions between the soot and gas-phase chemistry and used a simplified two-equation soot model.The effects of adding argon and helium to either fuel or oxidant were determined,and the results showed that the effects of the additives on soot formation process were different because of the difference in their transport properties.Guo et al.[14]also studied the influence of hydrogen addition to the fuel of an atmosphere pressure coflow laminar ethylene/air diffusion flame on soot formation by numerical simulation,and it indicated that the addition of hydrogen to the fuel in an ethylene/air diffusion flame suppressed soot formation through the effects of dilution and chemistry.Pandey et al.[15]investigated experimentally the effect of hydrogen addition on soot formation and soot morphology for axisymmetric coflowing acetylene/air laminar diffusion flames under different flow arrangements.Surprisingly,there is not much information available in the literature about the effect of CO addition on soot formation,especially the chemical effect of CO addition.Until recently,Guo et al.[16]investigated the effect of CO addition on soot formation in an ethylene/air diffusion flame.They believed that although the addition of carbon monoxide monotonically reduced the formation of soot,the chemical effect promoted the formation of soot in an ethylene/air diffusion flame.CO is a non-sooting fuel,but it contains carbon atom.CO addition has a dilution effect in terms of soot formation on one side,but participates in reactions that may influence soot formation on the other side.Consequently,the addition of CO may have complex effect on soot formation.Measurements were made by Du et al.[17].They observed that CO addition caused a linear decrease in soot formation in an ethylene diffusion flame,and a complicated behavior in a propane diffusion flame.They explained that the addition of CO could promote soot inception chemistry,but did not provide any discussion on the sooting chemistry.For diffusion flames,Guo et al.[16]argued that there existed two main reasons for CO addition causing some change in soot formation.One is that the chemical effect of CO addition may be caused by the modifications of flame temperature,the other is that the addition of CO increases the surface growth reaction rates through the reaction CO+OH= CO2+H.In previous studies,there is almost no discussion on important sub-process of soot formation.Therefore,further investigation on the detailed mechanism of the effect of CO addition on soot formation in premixed flames is needed.
Soot formation is an extremely complex physical and chemical process which involves gas and surface phase chemistries as well as particle dynamics.Although it has been studied for several decades,models for soot formation are still at the formative stage[18].It is generally acknowledged that polycyclic hydrocarbons(PAHs)are the precursors to soot particles.Appel et al.[19]have developed detailed PAH based soot models for laminar premixed flames of C2hydrocarbons which describe the formation and growth of PAHs.The H-abstraction C2H2-addition mechanism(HACA mechanism)along with the condensation of PAHs to soot surface are usually employed to describe the heterogeneous soot surface reactions.On the treatment of soot particles dynamics,there are several approaches:the method of moment[19-22],the stochastic method[23],and the sectional model[24-26]. The method of moments is less expensive compared to sectional methods and can be solved simultaneously with the gas-phase chemistry.Additionally,information about the method of moments neglects the exact distribution of particle size,mean quantities of interest are easily determined,and it can yield encouraging results for laminar flames.As a result,the method of moments has become the focus of research for modeling soot for-mation[18,27-28].
This paper investigates the effect of CO addition on soot formation in an acetylene/air premixed flame by numerical simulation.Specifically,we are interested in the chemical and temperature effect of CO addition on soot formation.The moment method was used to calculate soot formation,in which kinetic modeling with detailed chemistry and physics was considered. The model combines recent developments in gas-phase reactions,aromatic chemistry,soot particle coagulation,soot particle aggregation,and soot surface growth.
1 Model summary
The details ofthe model have been reported previously[19-22,27-29]. The formation and growth for PAHs is modeled by ABF mechanism[19].The ABF mechanism can be split into four main components:(1)GRI-Mech.2.11 for H2/O2[30],C1and C2chemistry;(2)a C3-up section leading to the formation of benzene and phenyl, designated by A1and A1-,respectively;(3)a PAHs growth section up to pyrene,designated by A4;(4)an oxidation section for A1,A1-and PAH.The further growth by cyclopentadienyl addition from Marinov et al.[31],and combinative growth of aromatics by Skjøth-Rasmussen et al.[32].The evolution of the statistical moments Mr(r=0,1,2,3,…)of the particle size distribution function(PSDF)can be derived from Smoluchowski′s equation,

where the index N indicates nucleation(inception);A represents agglomeration;G is mass growth;O indicates oxidation;and t is the reaction time;

Mris the rth concentration and size PSDF moments;miand Niare the mass and number density of particles of size class i,respectively.
Nucleation is the formation of the first soot particles from PAH molecules.Nucleated soot particles are assumed to form from the collision of two dimers which are formed from the collision of two PAH molecules,and the nucleation model used here is put forward by Pitsch and his coworkers[27].Particle growth is considered in the free,transition and continuum regimes. Aggregates obey a fractal relationship with a dimension Dfof ca 1.8[33],and the regimes are determined by the value of the Knudsen number.In the free molecular regime agglomeration is expressed as

where Kfand f(x,y)lare the free molecular regime coagulation constant and the grid function,respectively,which was determined in Ref.[20].Expressions for agglomeration in the continuum regime can be derived as
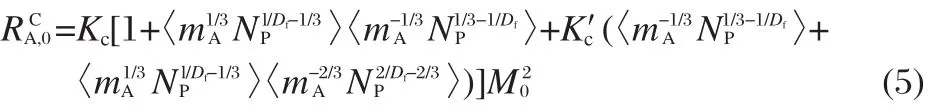
where Kc=2kBT/η,kBis the Boltzmann constant,NPis the number density,T is the temperature,η is gas viscosity,K′c=Kc/2.There is a lack of physical models describing the transition from coalescent growth to aggregation.Kazakov and Frenklach[20]suggested approximating the coagulation rate in the transition regime with the harmonic mean of the free molecular and continuum terms.
Appel et al.[19]proposed the following soot growth and oxidation mechanism,based on the corresponding gaseous reactions of aromatic species:

where CSOOTH and CSOOT●represent the arm-chair site and the radical site on the soot particle surface,respectively.The H abstraction reaction could also occur via reactions with methyl(CH3), ethynyl(C2H),and propargyl(C3H3)radicals:
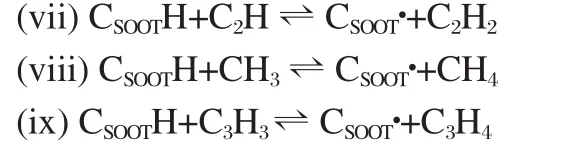
2 Model validation
In this section,the above presented soot model is validated in simulations of high temperature premixed ethylene and acetylene as well as benzene flames with the C/O molar ratio varied between 0.6 and 1.3.All simulations presented in this work were performed with our developed code based on PREMIX program[34-35],and the code has soot subroutines which contain the above particle dynamics.Wang and Frenklach[36]studied the formation and growth of PAHs in laminar premixed acetylene and ethylene flames,and suggested a detailed reaction mechanism (WF mechanism)consisting of 99 chemical species and 531 reactions.Appel et al.[19]augmented WF mechanism and developed ABF mechanism for PAHs mass growth and oxidation.In the current article,ABF mechanism is used to describe gasphase chemistry for soot formation.
The flames examined numerically were those investigated experimentally in five different laboratories,thus providing a diversity of different techniques and individual characteristics.The properties of these flames are summarized in Table 1[5,19,37-39]. The above present soot model can reproduce the existing experiment results in the literature with good accuracy.Most of the flames reached a maximum flame temperature from 1700 to 2100 K,with an exception of the ethane flame,CS1_0.748,with the reported maximum flame temperature of 1270 K.Theseflames contain the information required to test various parts of the numerical model,and collectively the data taken together can test many of the critical aspects of the soot formation model: the major combustion products and intermediates,single-ring aromatics and their precursors,polycyclic aromatics,soot volume fraction,particle size,and particle aggregation.
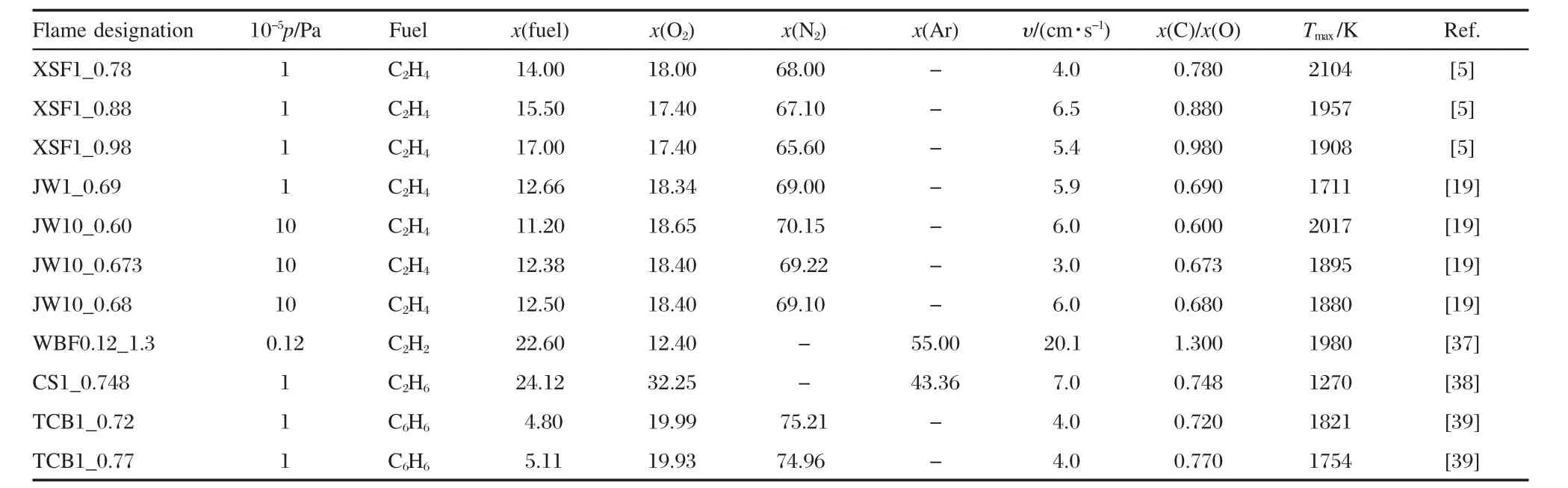
Table 1 Summary of flame conditions
As shown in Fig.1,the prediction results for the soot volume fraction are excellent,and the soot density,expressed as the total mass of soot particles per unit volume,is reasonably well predicted for both premixed benzene flames shown in Fig.2.In the experiments,soot particles were collected in the flames and then extracted in dichloromethane(DCM)to isolate them from condensed species(CS).We turned to flame CS1_0.748 which reported experiment data for both small hydrocarbons and one-to multi-ring PAHs,thus allows the examination of the transition from the small-hydrocarbon chemistry to PAH formation.As demonstrated in Fig.3,most of the measured small species (where a-C3H4is allene,A1C2H3represents styrene,A1C2H is the species in which a hydrogen atom from the benzene ring has been substituted by an ethynyl group C2H,P2is biphenyl)were predicted well,and the aromatic species(A1-A4)were also predicted reasonably close.


Eleven of the flames considered here report soot measurements,and all of these data are reproduced by the model within a factor of 2.5 (defined in Ref.[19]).This level of agreement is very encouraging,considering the difficulties associated with experimental measurements for polyaromatics and soot,and the current uncertainties in the thermodynamics and kinetics of the aromatics chemistry as well as soot particle nucleation and surface reactions.It implies that the above soot formation model can be used to predict soot formation in laminar premixed flames for research purpose in this paper.
3 Results and discussion
In the present,some parameters used are defined as follows: ratio of carbon monoxide addition a,a=xCO/(xCO+xC2H2);dilution factor D,D=xO2/(xO2+xN2)(D is taken to be 0.21 as constant in this work);fuel-equivalence ratio φ,

where xkrepresents mole fraction of the species k.The problem is modelled to solve burner-stabilized flame,and temperature profile was obtained by the coupled energy-species equations. Multi-component diffusion and thermal diffusion options were taken into account.Due to the complexity of numerical model about soot formation,the CNTN option was used in order to converge more easily or speed up iteration,which made the adaptive grid parameters GRAD and CURV vary from 1.0 and 1.0 to 0.1 and 0.5,respectively.Relative and absolute error crite-ria were RTOL=1×10-5and ATOL=1×10-10,respectively,and total number of grid points was typically 100-200.

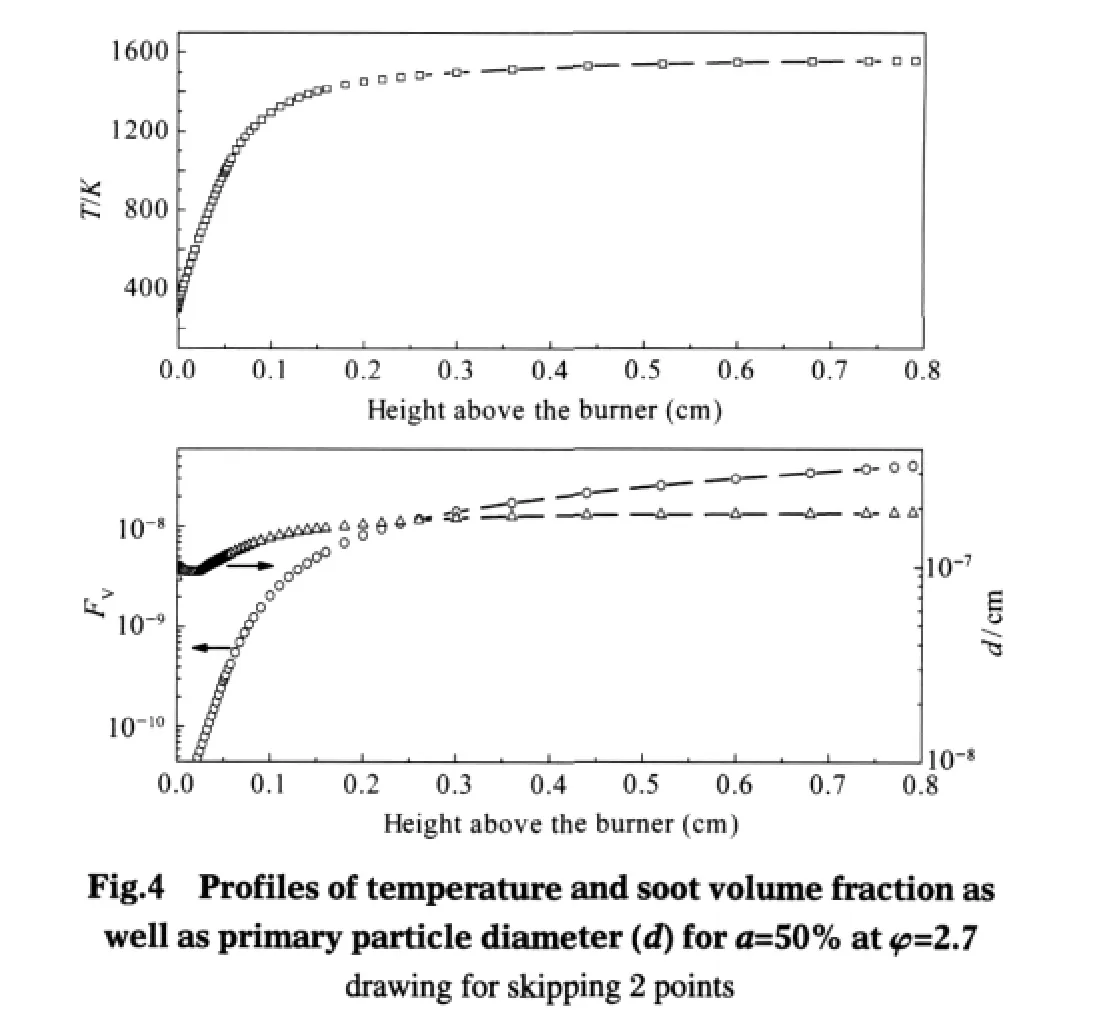
Firstly,we give a glimpse at profiles of temperature and soot volume fraction as well as primary particle diameter for CO addition in acetylene/air premixed flame shown in Fig.4.The parameters of this flame condition are:CO addition a=50%;fuelequivalence ratio φ=2.7;mass flow rate through the burner FLRT= 0.005 g·cm-2·s-1,the maximum temperature will rise along with the increase of FLRT while other parameters are the same.As shown in Fig.4,the maximum temperature in flame stability zone reaches 1556 K,the temperature rises rapidly during the height of 0.01-0.1 cm and temperature already reachs 1295 K in the height of 0.1 cm.Soot volume fraction FVand primary particle diameter d increase as the temperature rises,and the trend is similar to the temperature curve,but d declines during the initial phase of rapid temperature rising.FVand d read 2.06×10-9and 1.40×10-7cm respectively in the height of 0.1 cm above the burner.
Fig.5 shows the profiles of temperature,soot volume fraction and primary particle diameter as well as nucleation rate for different CO additions at fuel-equivalence ratio φ=2.3.It seems that the addition of CO inhibits soot formation.The temperature in flame stability zone reaches above 1900 K,for example 1909 K in the height of 0.3 cm above the burner.The temperature decreases by 22 and 63 K,respectively,for 25%and 50%CO addition at 0.3 cm above the burner.In this location,soot volume fractions fall to 5.57×10-8and 2.18×10-8respectively for 25% and 50%CO addition from original 8.17×10-8,and with the increase of CO addition primary particle diameters drop to 2.635× 10-7and 2.349×10-7cm respectively from 2.856×10-7cm.It can be found that a rapid increase in nucleation rate during ignition stage,then the nucleation rate declines in the stability zone of flame.The addition of CO can greatly reduce the nucleation rate as shown in Fig.5.It needs to point out that Guo et al.[16]suggested CO addition promotes the formation of soot in the ethylene/ air diffusion flame,but this conclusion was drawn from the comparison of CO and N2diluted flames,that is,CO addition promotes more soot formation than that of N2dilution.In fact,the experiments by Guo et al.show that the addition of both CO and N2monotonically reduces the formation of soot,and addition ofN2is more effective than that of CO in suppressing soot formation.



As mentioned above,the addition of CO decreases the temperature in the flame,but it is still unknown whether the decreases of soot volume fraction and primary particle diameter are due to temperature drop,the chemical effects by CO addition also influence soot formation.Guo et al.[16]suggested that the chemical effect of CO addition might be caused by the modifications of the flame temperature,soot surface growth,and oxidation reactions.They argued that flame temperature increases relative to a nitrogen diluted flame,which results in a higher surface growth rate,when CO is added.So it is very important to analyze the effects of temperature on the formation of soot.Fig.6 and Fig.7 show profiles of soot volume fraction,primary particle diameter,nucleation and coagulation rates with temperature in height of 0.6 cm above the burner in flame stability zone in different conditions.Each legend represents a set of numerical results,therefore,Fig.6 and Fig.7 are got through calculations for 72 rounds.Keeping fuel-equivalence ratio φ and CO addition constant,it makes temperature at the height varies by changing the mass flow rate through the burner,the purpose of doing that is decoupling the influence of C/O ratio on soot formation.Soot volume fraction and nucleation rate increase until a threshold temperature and then decrease as the temperature further increases.Primary particle diameter increases along with the temperature rising,and there is an approximate mirror symmetry relation between the nucleation rates and the coagulation rates. Along with increase ofCO addition,soot volume fraction and nucleation rate fall rapidly,while primary particle diameter has a slight decline in the low or medium temperature area.Under the condition of the same CO addition a,soot volume fraction and nucleation rate rise greatly with the fuel-equivalence ratio φ increases,while primary particle diameter keeps almost unchanged in the low or medium temperature area.

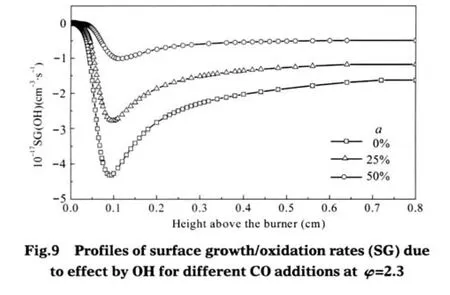
Fig.8 shows mole fraction changes of H and OH and Fig.9 illustrates profiles of surface growth/oxidation rates due to effect by OH for different CO additions at φ=2.3.The addition of CO increases the concentration of H radical in the lower flame region owing to the intensified forward rate of the reaction CO+OH=CO2+H and therefore increases the soot surface growth rates.The addition of CO also slows the oxidation rate of soot because the same reaction CO+OH=CO2+H results in a lower concentration of OH and a higher concentration of H,then it causes the higher active site formation rate of the key reaction CSOOTH+H⇌CSOOT●+H2(soot formation of active site by H-abstraction).This implies that the addition of CO chemically promotes the formation of soot,which is different from the chemical effect of CO2or H2addition.


Another key reaction for soot formation is the C-addition reaction CSOOT●+C2H2→CSOOTH+H.One of the primary destruction reactions of C2H2in which there exists carbon monoxide species is C2H2+O=CO+CH2.The reverse rate of this reaction is intensified by the addition of CO,resulting in the lower C2H2destruction rate and thus higher concentration of C2H2,and it seems that CO addition promotes the formation of soot.Guo et al.[16]found that in the upper flame region,the difference in C2H2of the different flames in the peak surface growth region is negligible in a laminar ethylene/air coflow diffusion flame,so they believedthat C2H2may not be a factor that results in the higher surface growth rate due to the chemical effect of CO addition.Profiles of production rates of C2H2for different CO additions at φ=2.3 can be seen in Fig.10,and Fig.11 shows the profiles of surface growth/oxidation rates due to C2H2for different CO additions at φ=2.3.The addition of CO weakens production rates for C2H2, but the addition of CO greatly reduces the volume fraction of C2H2in fuel and then reduces the soot surface growth due to C2H2.One of the key production reactions of C2H2is C2H3+O2= C2H2+HO2.In addition,it is important about PAH chemistry in soot formation.Two main routes for the formation of benzene and phenyl are combination of propargyl C3H3+C3H3→A1and reaction of n-C4H3+C2H2=A1-(phenyl).Fig.12 shows rates of progress for these reactions for different CO additions at φ=2.3,it can be found that the rates of progress for these reactions decrease with the increase of CO addition.

4 Concluding remarks
A detailed numerical study has been conducted on the effect of CO addition on soot formation in an acetylene/air premixed flame,and it shows that the addition of CO monotonically reduces the formation of soot.The formation of soot particles depends on a wide range of parameters,in which temperature and chemical effects play particularly important roles.There is a strong coupling between the chemical effect and thermal effect in soot formation.The changed rates of the reactions due to variations in temperature can cause different heat releases,and feed back to modify the temperature.The effect of temperature is isolated from the chemical effect by comparing the results of CO diluted flames at fixed height above the burner,and the fuelequivalence ratio φ and CO addition a remain constant,it makes temperature at the fixed height vary by changing the mass flow rate through the burner.The soot volume fraction and nucleation rate increase until a threshold temperature and then decrease as the temperature further increases.Primary particle diameter increases along with the increase of temperature,and there is an approximate mirror symmetry relation between the nucleation rates and the coagulation rates.The analysis of the details from numerical simulation suggests that there are mainly three factors for chemical effect of CO addition on the formation of soot.(1)The concentration of H radical is increased due to the intensified forward rate of the reaction OH+CO=CO2+H,which results in the higher surface growth rate for soot formation known as formation of active site by H-abstraction.Meanwhile, the addition of CO reduces the concentration of OH radical due to the same reaction and consequently slows the oxidation rates of soot.(2)The rates of progress for the primary destruction and production reactions of C2H2(C2H2+O=CO+CH2and C2H3+O2= C2H2+HO2)tend to decrease with CO addition,while the addition of CO can greatly reduce the volume fraction of C2H2in fuel,resulting in lower concentration of C2H2.So we draw a different conclusion with Guo et al.that C2H2may be a factor that results in the lower surface growth rate due to the CO addition.(3) In PAH chemistry,two main routes for the formation of benzene and phenyl(C3H3+C3H3→A1and n-C4H3+C2H2=A1-)were also analyzed,it shows that the formation of soot is suppressed by C O addition.
1 Yamamoto,M.;Duan,S.;Senkan,S.Combust.Flame,2007,151: 532
2 Kunioshi,N.;Komori,S.;Fukutani,S.Combust.Flame,2006, 147:1
3 Joo,H.I.;Gülder,Ö.L.Proc.Combust.Inst.,2009,32:769
4 Kim,Y.;Hatsushika,H.;Muskett,R.R.;Yamazaki,K.Atmos. Environ.,2005,39:3513
5 Xu,F.;Sunderland,P.B.;Faeth,G.M.Combust.Flame,1997, 108:471
6 Guo,H.;Smallwood,G.J.;Liu,F.;Ju,Y.;Gülder,Ö.L.Proc. Combust.Inst.,2005,30:303
7 Ren,J.Y.;Qin,W.;Egolfopoulos,F.N.;Tsotsis,T.T.Combust. Flame,2001,124:717
8 Coppens,F.H.V.;Ruyck,J.D.;Konnov,A.A.Combust.Flame, 2007,149:409
9 Guo,H.;Smallwood,G.J.;Gülder,Ö.L.Proc.Combust.Inst., 2007,31:1197
10 Wu,C.Y.;Chao,Y.C.;Cheng,T.S.;Chen,C.P.;Ho,C.T. Combust.Flame,2009,156:362
11 Gülder,Ö.L.;Snelling,D.R.;Sawchuk,R.A.Proc.Combust. Inst.,1996,26:2351
12 Glassman,I.Proc.Combust.Inst.,1998,27:1589
13 Guo,H.;Liu,F.;Smallwood,G.J.;Gülder,Ö.L.Proc.Combust. Inst.,2002,29:2359
14 Guo,H.;Liu,F.;Smallwood,G.J.;Gülder,Ö.L.Combust.Flame, 2006,145:324
15 Pandey,P.;Pundir,B.P.;Panigrahi,P.K.Combust.Flame,2007, 148:249
16 Guo,H.;Thomson,K.A.;Smallwood,G.J.Combust.Flame, 2009,156:1135
17 Du,D.X.;Axelbaum,R.L.;Law,C.K.Combust.Flame,1995, 102:11
18 Bhatt,J.S.;Lindstedt,R.P.Proc.Combust.Inst.,2009,32:713
19 Appel,J.;Bockhorn,H.;Frenklach,M.Combust.Flame,2000, 121:122
20 Kazakov,A.;Frenklach,M.Combust.Flame,1998,114:484
21 Frenklach,M.Chem.Eng.Sci.,2002,57:2229
22 Lindstedt,R.P.;Louloudi,S.A.Proc.Combust.Inst.,2005,30: 775
23 Zhao,B.;Yang,Z.;Johnston,M.V.;Wang,H.;Wexler,A.S.; Balthasar,M.;Kraft,M.Combust.Flame,2003,133:173
24 Smooke,M.D.;McEnally,C.S.;Pfefferle,L.D.;Hall,R.J.; Colket,M.B.Combust.Flame,1999,117:117
25 Park,S.H.;Rogak,S.N.;Bushe,W.K.;Wen,J.Z.;Thomson,M. J.Combust.Theor.Model.,2005,9:499
26 Zhang,Q.;Guo,H.;Liu,F.;Smallwood,G.J.;Thomson,M.J. Proc.Combust.Inst.,2009,32:761
27 Mueller,M.E.;Blanquart,G.;Pitsch,H.Proc.Combust.Inst., 2009,32:785
28 Blanquart,G.;Pitsch,H.Combust.Flame,2009,156:1614
29 Jiang,Y.;Qiu,R.;Fan,W.C.Journal of Combustion Science and Technology,2005,11:218 [蒋 勇,邱 榕,范维澄.燃烧科学与技术,2005,11:218]
30 Bowman,C.T.;Hanson,R.K.;Davidson,D.F.;Gardiner,W.C.; Lissianski,V.;Smith,G.P.;Golden,D.M.;Frenklach,M.; Goldenberg,M.GRI 2.11 detailed mechanism.http://www.me. berkeley.edu/gri_mech/,Berkley CA,USA
31 Marinov,N.M.;Pitz,W.J.;Westbrook,C.K.;Vincitore,A.M.; Castaldi,M.J.;Senkan,S.M.;Melius,C.F.Combust.Flame, 1998,114:192
32 Skjøth-Rasmussen,M.;Glarborg,P.;Østberg,M.;Johannessen,J.; Livbjerg,H.;Jensen,A.;Christensen,T.Combust.Flame,2004, 136:91
33 Köylü,Ü.Ö.;Faeth,G.M.;Farias,T.L.;Carvalho,M.G. Combust.Flame,1995,100:621
34 Kee,R.J.;Rupley,F.M.;Miller,J.A.Chemkin-II:a Fortran chemical kinetics package for the analysis of gas-phase chemical kinetics.Report SAND89-8009,Sandia,1989
35 Kee,R.J.;Grcar,J.F.;Smooke,M.D.;Miller,J.A.PREMIX:a Fortran program for modeling steady laminar one-dimensional premixed flames.Report SAND85-8240,Sandia,1985
36 Wang,H.;Frenklach,M.Combust.Flame,1997,110:173
37 Wieschnowsky,U.;Bockhorn,H.;Fetting,F.Proc.Combust.Inst., 1989,22:343
38 Castaldi,M.J.;Senkaw,S.M.Combust.Sci.Technol.,1996,116: 167
39 Tregrossi,A.;Ciajolo,A.;Barbella,R.Combust.Flame,1999, 117:553
乙炔/空气预混火焰中CO添加对炭黑生成影响的数值分析
蒋 勇*邱 榕
(中国科学技术大学火灾科学国家重点实验室,合肥 230026)
针对乙炔/空气预混火焰中CO添加对炭黑生成的影响进行了详细的数值模拟.通过对含有不同CO添加量的火焰的模拟结果比较,研究CO添加的温度和化学作用对于炭黑形成的影响.计算结果显示CO添加使炭黑的生成单调下降.炭黑体积分数和成核速率随着温度的升高而增加,到达一个阈值后,再随温度的升高而减少.从炭黑生成的H-萃取角度来看,由于反应OH+CO=CO2+H的向右速率增加,H原子增加以及OH自由基减少,CO添加会激发炭黑生成.从炭黑生成的C-添加机理来看,CO添加减小了C2H2的消耗速率,CO添加也有助于炭黑生成,但CO添加降低了燃料中C2H2的体积分数,使得炭黑表面增长速率降低.
炭黑;乙炔;一氧化碳;燃料改进;模化
O643
Received:February 3,2010;Revised:April 8,2010;Published on Web:June 25,2010.
*Corresponding author.Email:yjjiang@ustc.edu.cn;Tel:+86-551-3607827.
The project was supported by the National Natural Science Foundation of China(50876097)and New Century Excellent Talents in University of China(NCET-06-0546).
国家自然科学基金(50876097)和教育部新世纪优秀人才支持计划(NCET-06-0546)资助项目
ⒸEditorial office of Acta Physico-Chimica Sinica
猜你喜欢
杂志排行
物理化学学报的其它文章
- Adsorption Mechanism of Nonylphenol Polyethoxylate onto Hypercrosslinked Resins
- Coexistence of Oligonucleotide/Single-Chained Cationic Surfactant Vesicles with Precipitates
- Influence of Calcination Temperature on the Performance of Cu-Al-Ba Catalyst for Hydrogenation of Esters to Alcohols
- Novel Synthesis of Mesoporous Nanocrystalline Zirconia
- Fluorescence Behavior of Biphenyl Containing Side-Chain Liquid Crystalline Polyacetylene with Various Lengths of Spacers
- Synthesis,Crystal Structure,Thermal Behavior and Sensitivity of[Mn(AZT)2(H2O)4](HTNR)2·4H2O
