3-溴-9-苯基咔唑的合成
2012-10-10吕宏飞李淑辉王艳华
李 猛,吕宏飞,李淑辉,王艳华,杨 杰
(黑龙江省科学院石油化学研究院,黑龙江哈尔滨150040)
有机电致发光二极管(OLED)是近年来热门的领域。相对于液晶显示器(LCD)相比,OLED具有更高的亮度,更快速的应答时间,更高的色彩纯度、视角和对比度以及更低的电压和功耗。1990年,英国剑桥大学J.Burroughes等人开发出高分子型发光二级管(PLED)对OLED的发展起了重要的作用[1],使OLED可以制备更大的显示器。目前,小分子型OLED刚刚商业化,正在应用于手机、MP3、PDA、数码相机、数码摄像机等小型显示器中。对于大型的显示器,应用于笔记本电脑,电视中的高分子型发光二级管在技术上已有突破,正在逐渐开发研制中。而弯曲式有机电致发光二极管(FOLED)也是现在欧美、日本、韩国等国家研究的热点。今后,OLED将替代LED,成为新的显示材料,应用在各种显示器中,同时,科研人员正在开发研制出应用于照明的OLED材料,所以,OLED应用前景异常广阔。
OLED利用电子与空穴复合所产生的激子扩散到发光层而发光,磷光发光体材料可以作为主发光材料,其化学结构几乎都含有具有空穴传输的咔唑基团[2,3],最常用的就是含有苯基咔唑基团,这方面文献很多[4]。而3-溴-N-苯基咔唑就是合成这一中间体的重要底物,从它可以合成的N-苯基咔唑-3-硼酸,进而合成苯基咔唑基团。由于3-溴-N-苯基咔唑在OLED中用量很大,目前正是OLED蓬勃兴起的阶段,所以3-溴-N-苯基咔唑在OLED方面将会有很大的市场前景。
3-溴-N-苯基咔唑也成为3-溴-9-苯基咔唑。目前,其合成方法是N-苯基咔唑在溶剂下与溴化试剂反应得到。
1 3-溴-N-苯基咔唑的合成路线
3-溴-N-苯基咔唑的合成方法是在溶剂中,加入溴化试剂对N-苯基咔唑进行溴化。溴化试剂通常使用NBS,也有文献报道使用溴素或KBr-KBrO3,后者价格低一些,但反应收率也差一些。日本半导体能源实验室有限公司(Semiconductor Energy Laboratory Co.LTD)发表了多篇专利和文章,由于专利合成方法相似或相同,这里不一一列举。反应在不同溶剂、不同温度、不同时间下收率不同,为了便于比较,现分类列表如下。反应方程式如下:
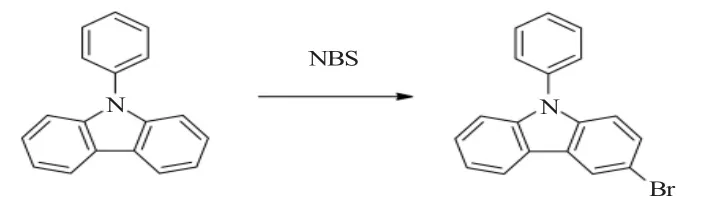
从文献上看,溴化试剂通常使用NBS,使用KBr和KBrO3也可以作为溴化试剂,但反应收率最低,而是用液溴作为溴化试剂,丙酮重结晶后收率只有57%。反应温度基本上都是室温或0℃,证明反应和容易进行。这样,就要求溴化试剂与N-苯基咔唑的摩尔比要控制在1∶1,溴化试剂过量会产生二溴代化合物。使用冰醋酸作为溶剂的反应收率低于二氯甲烷作为溶剂,而最常用的是使用DMF,而收率最高的是使用甲苯-乙酸乙酯混合溶剂。

表1 N-苯基咔唑的溴化反应Table 1 Bromation of N-benylcarbazole
2 N-苯基咔唑的合成
N-苯基咔唑是3-溴代N-苯基咔唑的原料,它的合成方法很多,下面按照原料的不同分述如下。
2.1 N-苯基咔唑的芳基化
N-苯基咔唑的芳基化是指N-苯基咔唑在过渡金属催化剂下,与卤代芳烃反应,脱去卤化氢,使芳烃连接在N-苯基咔唑的N上。这个方法是著名的人名反应——Ullmann C-N偶联反应[18]。反应通常是在碱性条件下进行,催化剂是含贵金属Pt2+,Pd2+等化合物及其配合物,也可以是Ni2+,Cu2+等化合物,甚至是Cu粉;反应一般在惰性气体保护下进行;卤代烃主要是卤代芳烃,如碘代芳烃,溴代芳烃。在反应体系中,为了从经济角度考虑,使用Cu2+作为催化剂,采用溴代芳烃作为反应底物是经济实惠的。N-苯基咔唑的芳基化反应方程式如下:
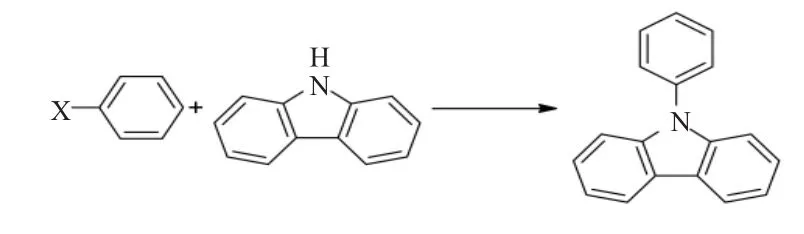
其中 X=1,Br,CI

表2 以碘苯为原料N-苯基咔唑的芳基化反应Table 2 Arylation of N-benylcarbazole from iodobenzene
使用溴苯代替碘苯,可以降低原料的消耗定额。以溴苯为原料咔唑芳基化的反应与碘苯为原料的反应相类似见表3。

表3 以溴苯为原料N-苯基咔唑的芳基化反应Table 3 Arylation of N-benyl-carbazole from bromobenzene
在铜催化下,溴苯与咔唑钾盐在180-220℃下反应,可以得到N-苯基咔唑[40]。
氯苯活性较低,目前文献中只有一条路线。以二甲苯为溶剂,在叔丁基醇钠作用下,加入cBRIDP配体和 bis(η3-allyl-μ-chloropalladium(II))的 Pd催化剂作用下,120℃反应3h,收率94%[33]。
从上述反应路线可以看出,以溴苯为底物,加入一定量的碱,使用CuI催化的Ullmann反应是较为合理的,反应过程中加入表面活性剂,以加快反应速度。避免了昂贵的Pd催化剂,以及复杂的配体。
2.2 以N-苯基甲胺与邻卤代苯合成路线

其中,X=Br,Cl
使用醋酸钯和三己基磷的配合物为Pd催化剂,以甲苯为溶剂,N-苯基甲胺与邻卤代苯在高温下反应,可以得到N-苯基咔唑。在105℃下,使用邻二溴苯、1-氯-2-溴苯,邻二氯苯为底物,得到N-苯基咔唑的收率分别为96%,88%,85%[41]。
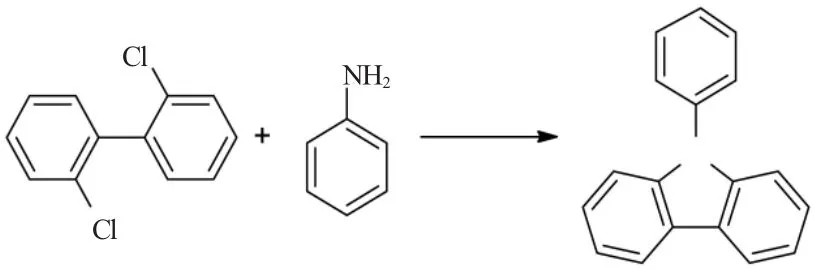
2.3 以2,2’-二取代-1,1’-联苯与苯胺为原料
当联苯的2和2’位取代有卤素,如氯、溴、碘,或是磺酸基时,2,2’-二取代联苯与苯胺在过渡金属钯的催化剂作用下,与苯胺反应,生成N-苯基咔唑[42]。
以三(二亚苄基茚丙酮)二钯(0)催化剂,甲苯为溶剂,2,8-二溴联苯与苯胺为原料,在叔丁醇钠盐作用下,加入不同的配合物,加热反应,能够得到N-苯基咔唑。


表4 苯胺与2,2’-二溴代-1,1’-联苯合成路线Table 4 Synthesis route of aniline and 2,2’-bromo-1,1’-biphenyl
类似地,2,2’-二氯联苯也可以作为原料生成N-苯基咔唑。以三(二亚苄基茚丙酮)二钯(0)催化剂,甲苯为溶剂,2,8-二氯联苯与苯胺为原料,在叔丁醇钠盐作用下,100℃反应24h,能够得到N-苯基咔唑。再加入非离子超强碱,可以提高收率,达到95%[42]。
以邻氯碘苯为原料,乙酸钯为催化剂,三环己基膦为配体,叔丁醇钠作用下,惰性气体保护下,105℃下反应18h,可以得到N-苯基咔唑,收率84%[41]。
当联苯的取代基一个为氯,一个为氟代烷基磺酸基时,与苯胺反应,在三(二亚苄基茚丙酮)二钯(0)催化下,加入配体,碱,在甲苯中100℃反应,也会产生N-苯基咔唑。不同的配体催化性能不同,使用4,5-双二苯基膦-9,9-二甲基氧杂蒽(Xantphos)时,只能得到痕量的N-苯基咔唑,使用联苯基膦(biphenylphosphine)时,收率提高,丁醇钠作为碱时,收率可达76%,使用磷酸钾时,收率可达86%[45]。


当2和2’位基团同为氟代磺酸基时,也能够合成N-苯基咔唑。
在以三氟乙酸铜为催化剂,二氯甲烷为溶剂,联苯二酚三氟甲磺酸酯与苯胺在室温下反应50h,收率84%[46]。在以三(二亚苄基丙酮)二钯氯仿配合物为催化剂,在磷酸钾作用下,加入2-(dicyclohexylphosphino)-2'-methylbiphenyl配体,以甲苯作为溶剂,联苯二酚三氟甲磺酸酯与苯胺在100℃反应18h,收率 88%[47]。
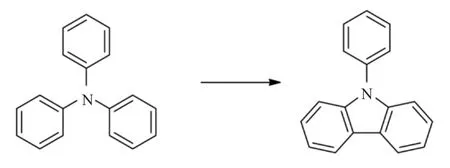
2.4 三苯胺脱氢
在水中,Pd/C为催化剂,在250℃下反应12h,三苯胺脱氢得到N-苯基咔唑,收率76%[48]。
在以乙酸为溶剂,乙酸钯为催化剂,加热反应3h,三苯胺脱氢得到N-苯基咔唑,收率45%。
在乙腈溶剂中,光照、通氧条件下,三苯胺定量地转化为N-苯基咔唑[49]。
在甲醇溶剂中,光照条件下,能够使三苯胺定量地转化为N-苯基咔唑,文献中还测定了反应动力学以及热力学数据[50]。
3 总结
3-溴-N-苯基咔唑的制备方法比较简单,就是N-苯基咔唑溴化的方法。其溴化试剂通常选用NBS,在室温或0℃下进行,收率很高。用于合成3-溴-N-苯基咔唑的底物N-苯基咔唑的合成方法较多,比较简单的是使用咔唑与卤代苯进行Ullmann的C-N偶联反应得到,其它方法都存在着底物合成困难,反应条件复杂的情况。在合成中,选用由咔唑与卤代苯反应生成N-苯基咔唑,再溴化合成3-溴-N-苯基咔唑的路线是合理的。
[1]J H BURROUGHES,D D C BRADLEY,A.BROWN,et al.Light-emitting diodes based on conjugated polymers [J].Nature,1990,348(6301)∶539~541.
[2]D M PAI,J F YANUS,M STOLKA,et al.Trap-Controlled Hopping Transport[J].Journal of Physical Chemistry.1984,88(20)∶4714~4717.
[3]X GONG,M R ROBINSON,J C OSTROWSKI,et al.High-Efficiency Polymer-Based Electrophosphorescent Devices[J].Advanced Materials,2002,14(8),∶581 ~ 585.
[4]卡姆.W.劳努斯蕾拉.朱布兰 兹比格纽.托卡斯基具有新型电荷转移化合物的用于电子照相的有机光受体CN,02148210.1[P].2003-04-06.
[5]KAORI OGITA,SACHIKO KAWAKAMI,TSUNENORI SUZUKI,et al.,Anthracene derivative and light-emitting devices,electronic devices,and lighting device using the anthracene derivative US,7960566[P].2011-06-14.
[6]FENG GUO-LIANG,JI SHUN-JUN,LAI WEN-YONG,et al.Synthesis and Optical Properties of Starburst Carbazoles Based on 9-Phenylcarbazole Core[J].Synlett 2006,2006(17)∶2841-2845
[7]LIU YU,WANG HAI-YING,CHEN GANG,et al.Synthesis and Properties of Novel'Ethyne-linked'Compounds Containing Carbazole and 1,8-naphthalimide Groups[J].Australian Journal of Chemistry,2009,62(8)∶934~940.
[8]NAKASHIMA HARUE,KUMAKI DAISUKE,KOJIMA KUMi.Carbazole derivative,and light-emitting element and light-emitting device using the carbazole derivative.WO,2006070912[P].2006-
[9]SEO SATOSHI,NOMURA RYOJI,KAWAKAMI SACHIKO.Stilbene derivatives,light-emitting element and light-emitting device.US,7476745[P].2009-01-13.
[10]LEE DAE-WOONG,HONG SUNG-KIL,PARK TAE-YOON,et al.novel compound and organic electronic device using same.EP,2343277[P].2011-09-12.
[11]ROBERTO GRISORIO,ANTONIO DELL'AQUILA,GIUSEPPE ROMANAZZI,et al.Novel bifluorene based conjugated systems∶synthesis and properties[J].Tetrahedron,2006,62(4)∶627~634.
[12]KIRILL TCHABANENKO,MARCUS G O TAYLOR,ROBERT M ADLINGTON,et al.Synthesis of substituted pyrano[3,2-c]pyridines via Diels-Alder reaction of 3-methylenepyridin-4-one[J].Tetrahedron Letters,2006,47(1),∶39~41.
[13]WAI-YEUNG WONG,CHEUK-LAM HO,ZHI-QIANG GAO,et al..Multifunctional Iridium Complexes Based on Carbazole Modulesas Highly Efficient Elect rophos phors [J].Angewandte Chemie,International Edition.2006,45(46)∶7800~7803.
[14]张玉祥,胡灵峰,张春林.取代咔唑联嘧啶类三价铱金属有机配合物及其有机电致发光器件.CN,200910218998[P].2009-01-16.
[15]FENG GUO-LIANG,JI SHUN-JUN;GENG LI-JUN;et al.Synthesis and optical properties of novel compounds containing carbazole and 1,8-naphthalimide groups[J].ournal of Chemical Research,Synopses,2008,3∶137~140.
[16]CHEUK-LAM HO,WAI-YEUNG WONG,BING YAO,et al.Synthesis,characterization,photophysics and electrophos-phorescent applications of phosphorescent platinum cyclometalated complexes with 9-arylcarbazole moieties[J].Journal of Organometallic Chemistry,2009,694(17)∶2735~2749.
[17]MARiA B PONCE,FRANCOM CABRERIZO,SERGIO M.BONESI,et al..Synthesis and Electronic Spectroscopy of Bromocarbazoles.Direct Bromination of N-and C-Substituted Carbazoles by N-Bromosuccinimide or a N-Bromosuccinimide/Silica GelSystem [J].Helvetica Chimica Acta,2006,89(6)∶1123~1139.
[18]梁云,李金恒.钯催化卤代芳烃Ullmann偶合反应[J].有机化学,2005,25(2)∶147~151.
[19]JAE KWAN KWON,JOONG HYUN CHO,YOUNG-SIL RYU,et al.N-Arylation of carbazole by microwave-assisted ligand-free catalytic CuI reaction [J]. Tetrahedron, 2011, 67(26)∶4820~4825.
[20]XI ZHENXING,LIU FENGHUI,ZHOU YONGBO,et al..CuI/L(L=pyridine-functionalized 1,3-diketones)catalyzed C N coupling reactions of aryl halides with NH-containing heterocycles[J].Tetrahedron,2008,64(19)∶4254-4259.
[21]LEE DAE-WOONG,HONG SUNG-KIL,PARK TAE-YOON,et al...novel compound and organic electronic device using same.EP,2343277[P],2011-09-12.
[22]YOUNG-KOOK KIM,SEOK-HWAN HWANG,YOON-HYUN KWAK.Carbazole-based compound and organic light-emitting device including organic layer including the carbazole-based compound.US,20080174237[P].2008-07-24.
[23]RAHMAN HOSSEINZADEH,MAHMOOD TAJBAKHSH,MOHAMMAD ALIKARAMI,et al..N-Arylation of N-H heterocycles with aryl bromides and aryl iodides using cui and kf/Al2O3[J].Journal of Heterocyclic Chemistry,2008,45(6)∶1815~1818.
[24]D V DAVYDOV,I P BELETSKAYA.Palladium-and copper-catalyzed synthesis of triarylamines in an aqueous-organic emulsion[J].Russian Chemical Bulletin,1995,44(6)∶1141.
[25]RUI ZHU,LIXIN XING,XINYAN WANG,et al.Highly Practical“Ligand-Free-Like”Copper-Catalyzed N-Arylation of A-zoles in Lower Nitrile Solvents [J].Advanced Synthesis and Catalysis,2008,350(9)∶1253~1257.
[26]ZHONG-LIN XU,HONG-XI LI,ZHI-GANG REN,et al.Cu(OAc)2·H2O-catalyzed N-arylation of nitrogen-containing heterocycles[J].Tetrahedron,2011,67(29)∶5282~5288.
[27]CARMEN AVENDA?O,MODESTA ESPADA,BLANCA OCA?A,et al..The problem of the existence of C(Ar)-H N intramolecular hydrogen bonds in a family of 9-azaphenyl-9H-carbazoles[J].Journal of the Chemical Society,Perkin Transactions 2∶Physical Organic Chemistry,1993,(8)∶1547~1555.
[28]PER-FREDRIK LARSSON,ARKAITZCORREA,MONICA CARRIL, et al. Copper-Catalyzed Cross-Couplings with Part-per-Million Catalyst Loadings[J].Angewandte Chemie,International Edition,2009,48(31)∶,5691~5693.
[29]DOUGLAS B.GROTJAHN,DEREK B.BROWN,JESSICA K.Martin,Evolution of Iridium-Based Molecular Catalysts during Water Oxidation with Ceric Ammonium Nitrate[J].Journal of the American Chemical Society,2009,131(6)∶2244~2251.
[30]Yabunouchi Nobuhiro,Kawamura Masahiro.Aromatic amine derivative and organic electroluminescent device using the same.EP,2177516[P].,2011-05-09.
[31]FATMA BAYCAN KOYUNCU,SERMET KOYUNCU,EYUP OZDEMIR.A novel donor-acceptor polymeric electrochromic material containing carbazole and 1,8-naphtalimide as subunit[J].Electrochimica Acta,2010,55(17)∶4935 ~ 4941.
[32]MIYAZAKI,TAKANORI,NISHIYAMA,MASAKAZU,MATSUMOTO,NAOKI.Catalyst for producing arylamine and process for producing arylamine by means thereof.US,20070282111[P].2007-12-06.
[33]KEN SUZUKI,YOJI HORI,TOHRU KOBAYASHI.A New Hybrid PhosphineLigand for Palladium-Catalyzed Amination ofArylHalides[J].AdvancedSynthesisandCatalysis,2008,350(5)∶652~656.
[34]KUBO SHINJI.Method for producing arylamine.US,6043370,[P].2000-03-28.
[35]KUBO SHINJI,SHINTOU TAICHI,AOKI HIDENORI.Process for producing arylamine.US,2006069287[P].2006-06-29.
[36]NIRANJAN PANDA,ASHIS KUMAR JENA,SASMITA MOHAPATRA,et al.Copper ferrite nanoparticle-mediated N-arylation of heterocycles∶a ligand-free reaction [J].Tetrahedron Letters,2011,52(16)∶1924~1927.
[37]MAKOTO WATANABE,MASAKAZU NISHIYAMA,TOSHIHIDE YAMAMOTO,et al.Palladium/P(t-Bu)3-catalyzed synthesis of N-aryl azoles and application to the synthesis of 4,4’,4’’-tris(N-azolyl)triphenylamines[J],Tetrahedron Letters,2000,41(4)∶481~484.
[38]KAI-JU WEI,JIA NI,JIAN GAO,et al..Self-Assembly of Silver(I)Coordination Polymers from AgX (X=BF4-,ClO4-,CF3COO-,and SO3CF3-)and a Rigid Bent 3,6-Dicyano-9-phenylcarbazole Ligand∶The Templating Effect of Anions[J].European Journal of Inorganic Chemistry,2007,24∶3868~3880.
[39]YABIN SONG,CHONG-AN DI,ZHONGMING WEI1.Synthesis,Characterization,and Field-Effect Transistor Properties of Carbazolenevinylene Oligomers∶From Linear to Cyclic Architectures[J].Chemistry-A European Journal,2008,14(15)∶4731~4740.
[40]LEOPOLD CASSELLA&CO.Verfahren zur Darstellung indophenolartiger Produkte aus N-alkyl-und N-aryl-carbazolen.DE,224951[P].1974-04-18.
[41]LUTZ ACKERMANN,ANDREAS ALTHAMMER,PETER MAYER.Palladium-Catalyzed Direct Arylation-Based Domino Synthesis of Annulated N-Heterocycles Using Alkenyl or(Hetero)Aryl 1,2-Dihalides[J].Synthesis,2009,20∶3493~3503.
[42]T.KITAWAKI,Y.HAYASHI,A.UENO,et al.One-step construc-tion of carbazoles by way of the palladium-catalyzed double N-arylation reaction and its application to the total synthesis of murrastifoline-A[J].Tetrahedron,2006,62(29)∶6792~6801.
[43]YIBO ZHOU,JOHN G.VERKADE.Highly Efficient Ligands for the Palladium-Assisted Double N-Arylation of Primary Amines for One-Sep Construction of Carbazoles[J].Advanced Synthesis and Catalysis,2010,352(4)∶616~620.
[44]KYOKO NOZAKI,KEITA TAKAHASHI,KOJI NAKANO,et al.The Double N-Arylation of Primary Amines∶Toward Multisubstituted Carbazoles with Unique Optical Properties[J].Angewandte Chemie,International Edition,2003,42(18)∶2051~2053.
[45]KEIKO KAWAGUCHI,KOJI NAKANO,KYOKO NOZAKI.Synthesis of ladder-type π-conjugated heteroacenes via palladium-catalyzed double N-arylation and intra-molecular O-arylation[J].Journal of Organic Chemistry,2007,72(14)∶5119~5128.
[46]DEREK H.R BARTONA,JEAN-PIERRE FINETA,JAMAL KHAMSIA.Copper catalysed phenylation of indoles by triphenylbismuthBIS-trifluoroacetate[J].Tetrahedron Letters,1988,29(10)∶1115~1118.
[47]Atsushi Kuwahara,Koji Nakano,Kyoko Nozaki.Double N-Arylation of Primary Amines∶Carbazole Synthesis from 2,2‘-Biphenyldiols[J].Journal of Organic Chemistry,2005,70(2),413~419.
[48]MITSURU YAMAMOTO,SEIJIRO MATSUBARA.Carbazole Synthesis by Platinum-catalyzed C-H Functionalizing Reaction Using Water as Reoxidizing Reagent[J].Chemistry Letters,2007,36(1)∶172~173.
[49]MARYE ANNE FOX,MARIA T.DULAY,KEVIN KROSLEY.Comparison ofOxidative and Excited-State Cyclizations of N-Benzyldiphenylamines to N-Benzylcarbazoles[J].Journal of the American Chemical Society,1994,116(24)∶10992~10999.
[50]NITIN CHATTOPADHYAY,CARLOS SERPA,LUIS G.ARNAUT,et al.Energetics of photocyclization of polyphenylamines and assignment of the intermediate∶A time-resolved photoacoustic calorimetric study [J].Physical Chemistry Chemical Physics,2001,3(17)∶3690~3695.
