Ischemic preconditioning enhances hepatocyte proliferation in the early phase after ischemia under hemi-hepatectomy in rats
2012-06-11
Hangzhou,China
Introduction
Liver transplantation is one of the most effective treatments for end-stage liver disease.It is wellknown that ischemia/reperfusion (I/R) injury is a major barrier to liver surgery and transplantation,and is responsible for the initial poor function and consequent failure of many grafts[1,2]by impairing remnant liver/reduced-size-graft regeneration.[3-7]In the I/R injury process,hepatocyte proliferation is considered to be a key protective factor to overcome reperfusion injury after surgery,including liver operation,living donor liver transplantation,and reduced-size liver transplantation.Liver regeneration following I/R injury and partial hepatectomy involves a series of pathobiological processes.[8-10]Previous studies[11-14]showed that ischemic preconditioning (IPC) significantly enhances the regenerative capacity of hepatocytes,especially in the late phase after I/R injury,and these studies have touched upon many molecular mechanisms.As a potent protective strategy,it is important to know whether IPC also enhances the regenerative capacity of hepatocytes in the early hepatic I/R phase.To our knowledge,little information is available about the relationship between IPC and hepatocyte proliferation.Based on our previous studies and related articles,we hypothesized that IPC promotes liver regeneration in the early phase after reperfusion.To test our hypothesis,we mimicked the features of recovery of living donor livers using a warm ischemia model,and focused on cell proliferation and apoptosis <12 hours after reperfusion.Then,to assess the proliferation index and the degree of injury,we measured serum interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) concentrations and investigated the possible mechanisms involved in liver cell proliferation and apoptosis.
Methods
Animals and experimental protocol
Adult male Wistar rats purchased from the Shanghai Animal Center were provided with regular chow and water ad libitum,and housed under a 12-hour dark-light cycle.The protocol was approved by the Animal Care Committee of Zhejiang University.
A total of 90 rats were randomized into three experimental groups:1) PHx:non-ischemic and hemihepatectomy; 2) I/R:60 minutes of ischemia plus hemihepatectomy; 3) IPC:cycle of 10 minutes alternating I/R prior to 60 minutes of ischemia plus hemi-hepatectomy.And each group was divided intofive subgroups sacrificed at 0.5,2,6,12 and 24 hours (n=6/subgroup).Under general anesthesia,surgical procedures were performed according to our previous study with some modification.[15]After surgery,the animals were allowed food and water ad libitum.Four rats died accidentally(bleeding and anesthesia).All samples were harvested at the corresponding time points.Blood samples and some tissues were stored at -80 ℃,and other tissues werefixed in 10% formaldehyde at room temperature.
Measurement of serum liver enzyme and cytokines
Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured by an Automated Chemical Analyzer (7600,Hitachi,Japan).Serum cytokine concentrations were evaluated by a commercial enzyme-linked immunosorbent assay (ELISA) kit (R&D Co.,USA).According to the manufacturer's instructions,the 96-well plates were read on an ELx800 automated microplate reader (Amersham Pharmacia Biotech Co.,USA) at 450 nm.Subsequently,the serum TNF-α and IL-6 concentrations were obtained from standard curves:the limit of detection was 8-16 pg/mL.
Immunohistochemical staining
Hepatic specimensfixed in formaldehyde were embedded in paraffin and sectioned.The staining procedures were performed according to our previous study.[15]The sections were incubated overnight at 4 ℃with proliferating cell nuclear antigen (PCNA) at 1:1000 dilution (Cell Signaling Technologies,Beverly,MA,USA).After washing with PBS,sections were incubated with secondary antibody at 1:200 for 1 hour at room temperature (Zhongshang Corp.,Beijing,China).Subsequently,slides were stained with diaminobenzidine,counterstained with hematoxylin,dehydrated,and washed with PBS.At 400× magnification,30fields were randomly selected and PCNA-positive cells were counted by a pathologist using Image-Pro Plus software,version 6.0 (Media Cybernetics Inc.,Bethesda,MD,USA).
Western blotting analysis
To evaluate the PCNA expression,liver tissue was harvested at each time point,and 0.1 g fresh liver was homogenized.The supernatant was adjusted to contain an equivalent amount of protein.Western blotting was performed according to our previous experiment.[15]In this analysis,PCNA was diluted to 1:1000,phosphorylated c-Jun N-terminal kinase (p-JNK)(Cell Signaling) to 1:1000 and caspase-3 antibody (Cell Signaling) to 1:1000 and incubated for 24 hours at 4 ℃.All data were described as density ratios relative to β-actin.
Statistical analysis
All values were expressed as mean±SD,and were analyzed using SPSS 16.0.Statistical significance was obtained by ANOVA.β-actin was used as an internal control.A P value <0.05 was considered statistically significant.
Results
Effects of IPC on serum liver enzyme and cytokines
Serum levels of ALT and AST in the I/R and IPC groups dramatically increased after hemi-hepatectomy,peaking at 2 and 6 hours,respectively.These levels after 2-24 hours of reperfusion other than 0.5 hour time point in the I/R group were significantly higher than those of the PHx and IPC groups (Fig.1).
The serum level of TNF-α in the I/R group was significantly increased after 0.5-12 hours of reperfusion compared with the levels in the PHx and IPC groups,peaking at 6 hours.A significant increase of serum IL-6 level was found in the IPC group (Fig.2).
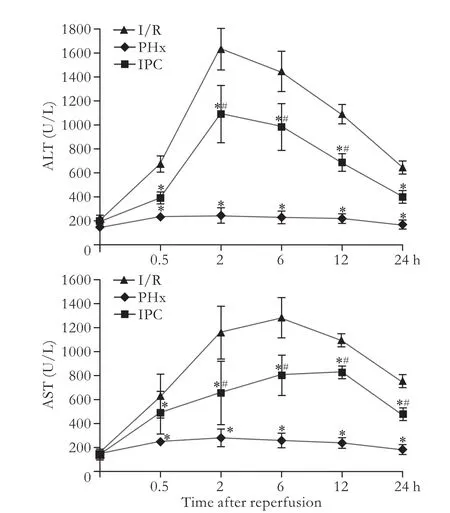
Fig.1.Changes in serum ALT and AST after hepatectomy.*:P<0.05,versus I/R; #:P<0.05,versus PHx.
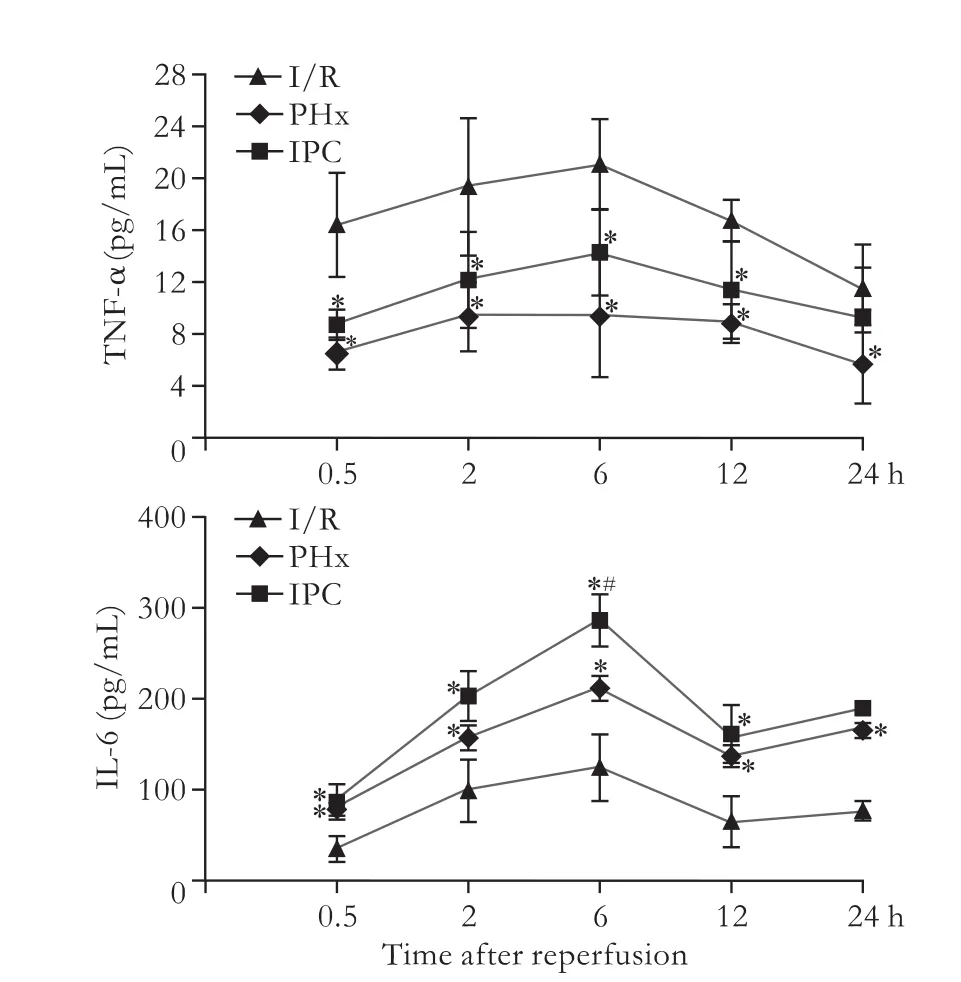
Fig.2.Kinetics of serum TNF-α and IL-6 after hepatectomy.*:P<0.05,versus I/R; #:P<0.05,versus PHx.
Effects of IPC on PCNA index after reperfusion
PCNA-positive hepatocytes were notably diffuse after reperfusion,and these positive cells were remarkably increased in the IPC group after 0.5,6 hours of reperfusion compared with the I/R group (Fig.3).
Effects of IPC on PCNA and caspase-3 proteins after reperfusion
The expression of PCNA nuclear protein re flects the state of hepatocytes in the late G1 and S phases of the mitotic cycle,while caspase-3 indicates programmed cell death.High expression of caspase-3 protein was found in the I/R group.In contrast,although IPC did not completely inhibit caspase-3 expression,it evidently strengthened the expression of PCNA protein.The changes of PCNA and caspase-3 in the I/R and IPC groups indicated a relationship between apoptosis and proliferation after reperfusion (Fig.4).
Effects of IPC on p-JNK protein after reperfusion
Hepatic p-JNK expression was higher in the IPC group than in the I/R group after reperfusion in the early phase.At the same time point,p-JNK expression was consistent with PCNA (Figs.4,5).
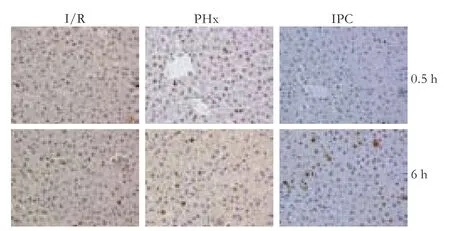
Fig.3.Numbers of PCNA-positive hepatocytes at 0.5 and 6 hours after reperfusion (original magnification ×400).
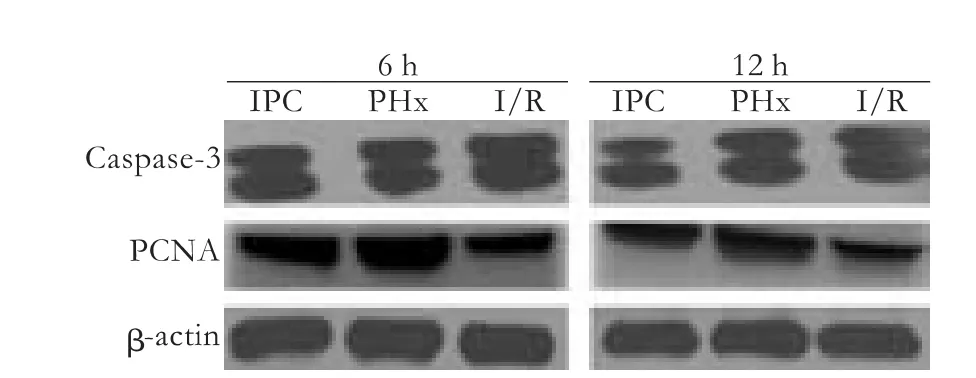
Fig.4.Representative Western blotting (upper panel) and their densitometric analyses (lower panel) showing the expression of PCNA and caspase-3 proteins in the liver.β-actin was used as an internal control.
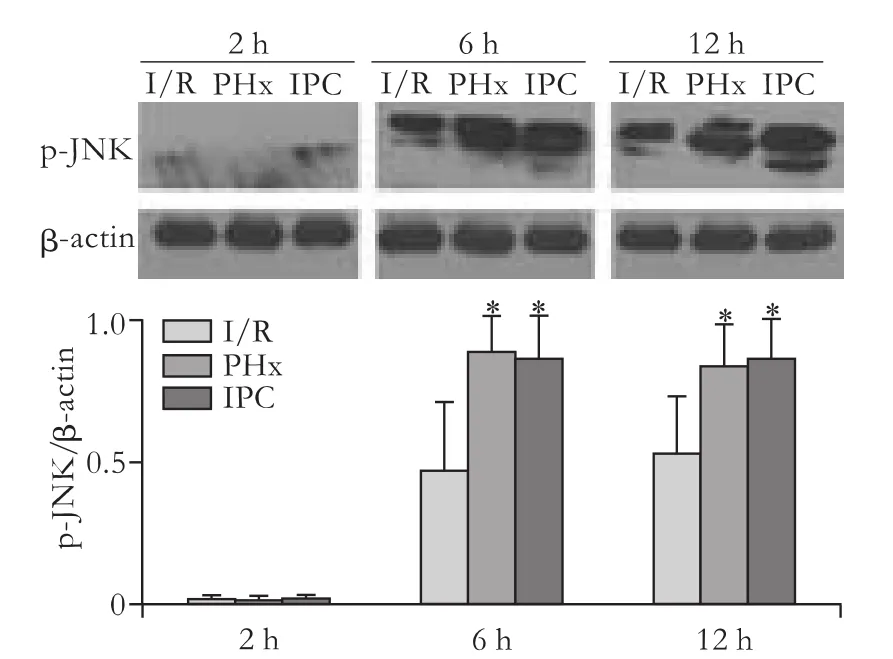
Fig.5.Representative Western blotting (upper panel) and their densitometric analyses (lower panel) showing p-JNK expression in the liver.β-actin was an internal control.*:P<0.05,versus I/R.
Discussion
Living donor liver transplantation is an effective strategy to minimize the shortage of donors.[16]However,the most important risk to the donor is bleeding after harvesting the liver.[17]Handicaps are remnant liver regeneration in the donor and small-size-graft regeneration in the recipient.[16]Whether the graft or remnant liver regenerates rapidly or not contributes to the clinical outcomes in donors and recipients.[13]Since Pringle's maneuver was gradually introduced into living donor liver transplantation,better clinical outcomes could be obtained.[17]As a modified Pringle's maneuver,IPC has a more potent protective role,not merely in decreasing bleeding and ameliorating I/R injury,but also in enhancing hepatocyte proliferation.[10,18]Theoretically,living donor liver transplantation offers a sufficient mass to average-sized patients; however,the onset of small-for-size syndrome and graft non-function is common.[19,20]The impairment of liver regeneration by I/R injury is still an inevitable problem in the liver surgery and transplantation setting,leading to smallfor-size syndrome or liver dysfunction or failure.[5,6,21]To ameliorate hepatic I/R injury and enhance the capacity for regeneration,a key factor that involves proliferation in the early phase after reperfusion is to improve the long-term function of the remnant liver or graft.In living donor liver transplantation,however,warm ischemia injury plays an important role before,during and after transplantation,especially in the early phase of reperfusion.[22]In the present study,we evaluated the effect of IPC on hepatocyte proliferation after reperfusion and investigated the relationship between cell death and proliferation by means of a rat reduced-size liver I/R injury model.We also investigated a possible mechanism for improving hepatocyte proliferation in the early phase after reperfusion.Recently,a partial liver transplantation study showed that IPC has protective effects,including the improvement regeneration of small-for-size liver grafts in the early phase by adjusting the free radical adduct signal.[7]In the present study,IPC induced smooth increases in serum ALT and AST levels in the early phase after hemi-hepatectomy compared with the I/R group,and the IPC response was associated with increased proliferative signals,IL-6,TNF-α and PCNA expression,and especially p-JNK activation.
Liver restoration after partial hepatectomy is a complicated multifactorial process,which involves a balance between cell death and proliferation.Within hours,quiescent hepatocytes are primed to leave the resting state (G0/G1),then pass through the G1/S and G2/M checkpoints,and enter DNA synthesis (S)by cyclin D1 located downstream of the proliferative pathway.In the last step,the cell-cycle nuclear protein PCNA is expressed in the late G1 and S phases.[23,24]Therefore,the process has many steps and mediators such as TNF-α and IL-6 to furnish a proliferative cycle.However,the varying outcomes of diverse studies are based on the period of ischemia,the extent of the ischemic area and whether or not the organ is excised,especially the volume of remnant liver after hepatectomy.[10,15,24,25]The paradoxical results may be due to the percentage of resected liver beyond the extended volume.[14]Clavien et al[10]demonstrated that the induction of a protective IPC effect requires a suitable remnant liver.Our results showed that the PCNA expression in the IPC group was significantly higher than in the PHx and I/R groups after 6-12 hours of reperfusion.Apart from its correlation with a suitable liver volume,increased PCNA is associated with the expression of early gene transcription,[21]which mediates a series of tissue repair processes in the early phase.In addition,we found that p-JNK levels were significantly increased by IPC,while the expression of PCNA was consistent with the p-JNK levels.p-JNK is activated by cellular stressors,and mitogens can modulate proproliferative or pro-apoptotic signals depending on cell type and context;[26]this is another plausible candidate to induce cell cycle entry during hepatocyte proliferation,because c-Jun is a component of activator protein-1 on the cyclin D1 promoter.[26]Based on the finding that the higher the expression of PCNA protein,the higher the activation of p-JNK in the early phase after reperfusion,we presumed that the activated p-JNK supported the beneficial effects of IPC.Furthermore,the related proliferative cytokines TNF-α and IL-6 also showed a similar activation of p-JNK.These results suggest that IPC not only induced higher p-JNK expression,but also reduced the degree of hepatic injury,including low caspase-3 expression and a better biochemical index.Therefore,p-JNK may play an important role in the cell proliferation cycle.
On the other hand,the early serum levels of TNF-α and IL-6 were also consistent with the PCNA expression.It is known that TNF-α and IL-6 are required for liver regeneration by NF-κB and STAT3.[26-29]After partial hepatectomy,STAT3 is activated by IL-6 within 30 minutes and peaks at 3 hours; NF-κB,an anti-apoptotic transcription factor,increases within 15 minutes.[30]Among the in flammatory mediators,TNF-α and IL-6 contribute to the initiation of the cell cycle.[26,27,31,32]A series of cells and mediators lead to the release of TNF-α and IL-6,which in turn facilitate the remnant hepatocytes to reenter the proliferation state.[4,8,33,34]We also found that the concentration of TNF-α was higher at 2-12 hours in the IPC group than in the PHx group,but lower than in the I/R group.This phenomenon is similar to previous studies showing that a medium dose of TNF-α expression may mediate IPC protection.[26,31,34]At corresponding time points,the liver PCNA level was consistent with the serum TNF-α concentration,suggesting that TNF-α does mediate cell proliferation in the early phase after reperfusion.In fact,the protective effect of TNF-α is associated with the early activation of STAT3.[26]A low dose of TNF-α is effective at reducing hepatic I/R injury,while a higher dose (25 μg/kg body weight) augments hepatotoxicity rather than reducing hepatic I/R injury.The pleiotropic effects of TNF-α may only depend on its time of appearance and the intensity of its interactions with other cytokines,rather than its concentration.[26,34]Many previous investigations have suggested that the hepatoprotective effects of IPC are simulated by low doses of TNF-α.[26,27,31,34]Therefore,we inferred that TNF-α may play a protective role in this model,which seems to re flect the diverse pathophysiological situations in the early and late phases.
Although some investigators believed that the onset of liver regeneration occurs in the late phase after hepatectomy,others confirmed that quiescent hepatocytes initiate proliferation within 24 hours.[33,35]IPC can initiate reentry into the cell cycle ahead of schedule,associated with hepatocyte proliferation and cyclin D1 expression during early ischemia,and then diminish or compensate for subsequent hepatocyte injury induced by prolonged ischemia and I/R injury.[35,36]The reduced degree of liver injury is at least associated with the protective role by which IPC enhances liver cell proliferation.
The expression of PCNA was inversely associated with the expression of caspase-3 at 6-12 hours after reperfusion,indicating a close relationship between apoptosis and proliferation.This phenomenon was also coincident with the biochemical and histological alterations and changes in the molecular profile of p-JNK expression.As a consequence,the fate of hepatocytes depends on the interaction of cytokines and signal transduction pathways in the early phase after reperfusion,showing that IPC may be an early intervention to overcome hepatic ischemia damage.
In conclusion,IPC can initiate hepatocyte proliferation action in the early phase after ischemia under hemihepatectomy by a signaling mechanism that may be associated with p-JNK,the trigger of TNF-α/IL-6 signals.
Acknowledgment:We thank Prof.Jian Wu and Drs.Xiao-Wen Feng and Hui Chen for their technical support and advice in this study.
Contributors:ZL,XHY,YS,XX and ZSS proposed the study.JLM and LYX performed research and wrote the first draft.JLM and JSF collected and analyzed the data.All authors contributed to the design and interpretation of the study and to further drafts.ZSS is the guarantor.
Funding:This study was supported by grants from the National Basic Research Program of China (973 Program) (2009CB522403),the National Key Technology R&D Program (2008BA160b02),the National S&D Major Project (2008ZX10002-026),the Projects of the Ministry of Public Health (200802006),the Special Foundation for Young Scientists of Zhejiang Province Traditional Chinese Medicine,China (2011ZQ008) and the Medical Scientific Research Foundation of Health Bureau of Zhejiang Province,China(2012KYB143).
Ethical approval:The protocol was approved by the Animal Care Committee of Zhejiang University.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Banga NR,Homer-Vanniasinkam S,Graham A,Al-Mukhtar A,White SA,Prasad KR.Ischaemic preconditioning in transplantation and major resection of the liver.Br J Surg 2005;92:528-538.
2 DeOliveira ML,Graf R,Clavien PA.Ischemic preconditioning:promises from the laboratory to patients--sustained or disillusioned? Am J Transplant 2008;8:489-491.
3 Suzuki S,Inaba K,Konno H.Ischemic preconditioning in hepatic ischemia and reperfusion.Curr Opin Organ Transplant 2008;13:142-147.
4 Selzner M,Camargo CA,Clavien PA.Ischemia impairs liver regeneration after major tissue loss in rodents:protective effects of interleukin-6.Hepatology 1999;30:469-475.
5 Franco-Gou R,Peralta C,Massip-Salcedo M,Xaus C,Serafín A,Roselló-Catafau J.Protection of reduced-size liver for transplantation.Am J Transplant 2004;4:1408-1420.
6 Watanabe M,Chijiiwa K,Kameoka N,Yamaguchi K,Kuroki S,Tanaka M.Gadolinium pretreatment decreases survival and impairs liver regeneration after partial hepatectomy under ischemia/reperfusion in rats.Surgery 2000;127:456-463.
7 Rehman H,Connor HD,Ramshesh VK,Theruvath TP,Mason RP,Wright GL,et al.Ischemic preconditioning prevents free radical production and mitochondrial depolarization in smallfor-size rat liver grafts.Transplantation 2008;85:1322-1331.
8 Cressman DE,Greenbaum LE,DeAngelis RA,Ciliberto G,Furth EE,Poli V,et al.Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice.Science 1996;274:1379-1383.
9 Taub R.Liver regeneration:from myth to mechanism.Nat Rev Mol Cell Biol 2004;5:836-847.
10 Clavien PA,Selzner M,Rüdiger HA,Graf R,Kadry Z,Rousson V,et al.A prospective randomized study in 100 consecutive patients undergoing major liver resection with versus without ischemic preconditioning.Ann Surg 2003;238:843-852.
11 Yamada F,Saito T,Abe T,Tsuchiya T,Sato Y,Kenjo A,et al.Ischemic preconditioning enhances regenerative capacity of hepatocytes in long-term ischemically damaged rat livers.J Gastroenterol Hepatol 2007;22:1971-1977.
12 Kerem M,Bedirli A,O fluoglu E,Deniz K,Turkozkan N,Pasaoglu H,et al.Ischemic preconditioning improves liver regeneration by sustaining energy metabolism after partial hepatectomy under ischemia in rats.Liver Int 2006;26:994-999.
13 Bedirli A,Kerem M,Pasaoglu H,Erdem O,O fluoglu E,Sakrak O.Effects of ischemic preconditioning on regenerative capacity of hepatocyte in the ischemically damaged rat livers.J Surg Res 2005;125:42-48.
14 Habib MM,Selden C,Hodgson H,Davidson BR.Ischemic preconditioning impairs liver regeneration in extended reduced-size livers.Ann Surg 2006;244:328-330.
15 Liu YX,Jin LM,Zhou L,Xie HY,Jiang GP,Chen H,et al.Sirolimus attenuates reduced-size liver ischemia-reperfusion injury but impairs liver regeneration in rats.Dig Dis Sci 2010;55:2255-2262.
16 White SA,Al-Mukhtar A,Lodge JP,Pollard SG.Progress in living donor liver transplantation.Transplant Proc 2004;36:2720-2726.
17 Imamura H,Kokudo N,Sugawara Y,Sano K,Kaneko J,Takayama T,et al.Pringle's maneuver and selective in flow occlusion in living donor liver hepatectomy.Liver Transpl 2004;10:771-778.
18 Heizmann O,Loehe F,Volk A,Schauer RJ.Ischemic preconditioning improves postoperative outcome after liver resections:a randomized controlled study.Eur J Med Res 2008;13:79-86.
19 Dahm F,Georgiev P,Clavien PA.Small-for-size syndrome after partial liver transplantation:definition,mechanisms of disease and clinical implications.Am J Transplant 2005;5:2605-2610.
20 Heaton N.Small-for-size liver syndrome after auxiliary and split liver transplantation:donor selection.Liver Transpl 2003;9:S26-28.
21 Ishii S,Abe T,Saito T,Tsuchiya T,Kanno H,Miyazawa M,et al.Effects of preconditioning on ischemia/reperfusion injury of hepatocytes determined by immediate early genetranscription.J Hepatobiliary Pancreat Surg 2001;8:461-468.
22 Yan S,Jin LM,Liu YX,Zhou L,Xie HY,Zheng SS.Outcomes and mechanisms of ischemic preconditioning in liver transplantation.Hepatobiliary Pancreat Dis Int 2010;9:346-354.
23 Fausto N.Liver regeneration:from laboratory to clinic.Liver Transpl 2001;7:835-844.
24 Eipel C,Glanemann M,Nuessler AK,Menger MD,Neuhaus P,Vollmar B.Ischemic preconditioning impairs liver regeneration in extended reduced-size livers.Ann Surg 2005;241:477-484.
25 Yao A,Li X,Pu L,Zhong J,Liu X,Yu Y,et al.Impaired hepatic regeneration by ischemic preconditioning in a rat model of small-for-size liver transplantation.Transpl Immunol 2007;18:37-43.
26 Teoh N,Leclercq I,Pena AD,Farrell G.Low-dose TNF-alpha protects against hepatic ischemia-reperfusion injury in mice:implications for preconditioning.Hepatology 2003;37:118-128.
27 Teoh N,Field J,Farrell G.Interleukin-6 is a key mediator of the hepatoprotective and pro-proliferative effects of ischaemic preconditioning in mice.J Hepatol 2006;45:20-27.
28 Sudo K,Yamada Y,Saito K,Shimizu S,Ohashi H,Kato T,et al.TNF-alpha and IL-6 signals from the bone marrow derived cells are necessary for normal murine liver regeneration.Biochim Biophys Acta 2008;1782:671-679.
29 Camargo CA Jr,Madden JF,Gao W,Selvan RS,Clavien PA.Interleukin-6 protects liver against warm ischemia/reperfusion injury and promotes hepatocyte proliferation in the rodent.Hepatology 1997;26:1513-1520.
30 Black D,Lyman S,Heider TR,Behrns KE.Molecular and cellular features of hepatic regeneration.J Surg Res 2004;117:306-315.
31 Teoh N,Field J,Sutton J,Farrell G.Dual role of tumor necrosis factor-alpha in hepatic ischemia-reperfusion injury:studies in tumor necrosis factor-alpha gene knockout mice.Hepatology 2004;39:412-421.
32 Matsumoto T,O'Malley K,Efron PA,Burger C,McAuliffe PF,Scumpia PO,et al.Interleukin-6 and STAT3 protect the liver from hepatic ischemia and reperfusion injury during ischemic preconditioning.Surgery 2006;140:793-802.
33 Clavien PA,Petrowsky H,DeOliveira ML,Graf R.Strategies for safer liver surgery and partial liver transplantation.N Engl J Med 2007;356:1545-1559.
34 Jin LM,Liu YX,Zhou L,Xie HY,Feng XW,Li H,et al.Ischemic preconditioning attenuates morphological and biochemical changes in hepatic ischemia/reperfusion in rats.Pathobiology 2010;77:136-146.
35 Cai FG,Xiao JS,Ye QF.Effects of ischemic preconditioning on cyclinD1 expression during early ischemic reperfusion in rats.World J Gastroenterol 2006;12:2936-2940.
36 Teoh N,Dela Pena A,Farrell G.Hepatic ischemic preconditioning in mice is associated with activation of NF-kappaB,p38 kinase,and cell cycle entry.Hepatology 2002;36:94-102.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- High-intensity focused ultrasound ablation as a bridging therapy for hepatocellular carcinoma patients awaiting liver transplantation
- Effect of endogenous hypergastrinemia on gallbladder volume and ejection fraction in patients with autoimmune gastritis
- Expression of HBx protein in hepatitis B virusinfected intrahepatic cholangiocarcinoma
- Laparoscopic distal pancreatectomy with or without splenectomy:spleen-preservation does not increase morbidity
- Xanthogranulomatous cholecystitis mimicking gallbladder cancer and causing obstructive cholestasis
- Disease spectrum and use of cholecystolithotomy in gallstone ileus
