Expression of HBx protein in hepatitis B virusinfected intrahepatic cholangiocarcinoma
2012-06-11
Shanghai,China
Introduction
Intrahepatic cholangiocarcinoma (ICC) is a devastating malignancy originating from the biliary epithelium,and ranks as the second most common primary liver cancer after hepatocellular carcinoma (HCC).[1]There are several documented risk factors for ICC,including primary sclerosing cholangitis,liver fluke infection (Clonorchis sinensis or Opisthorchis viverrini),fibropolycystic liver disease,hepatolithiasis,and thorotrast exposure.[2-4]Recent studies[5-7]have shown that hepatitis B virus (HBV) infection,a major risk factor for the development of HCC,also increases the risk of ICC,but the pathogenic mechanisms remain unclear.
The small,3.2-kb DNA genome of HBV contains four known open reading frames,called S,C,P and X.The X gene-encoded protein (HBx),consisting of 154 amino acid residues with a molecular weight of 17 kD,is expressed in many HCCs.It acts as a transactivator on various cellular genes and plays a crucial role in HCC carcinogenesis.[8]HBx immunochemical staining in bile duct cells of cancerous and surrounding hepatic tissues also has been shown in some HBV-infected ICC specimens,[9]but the significance of these findings has not been fully determined.
p53 is one of the most commonly inactivated tumor suppressor genes associated with the development of human cancer.[10]It has multiple functions in several central cellular processes,including gene transcription,DNA repair,cell cycling,genomic stability,chromosomal segregation,senescence,and apoptosis.Inactivation of the p53 gene abrogates its normal function,leading to genomic instability and loss of growth control.[11]Whether the oncogenic effect of HBx in fluences p53 alterations in HCC has been studied extensively.[12]In contrast,to date,no data are available regarding the relationship of HBx to p53 gene alterations in ICC.
In the present study,we investigated the clinicopathological significance of HBx expression in HBV-infected ICC and determined whether HBx expression correlates with that of p53 protein.
Methods
Patients and tissue specimens
Surgical resection specimens were collected from 54 patients with HBV infection who underwent surgery with curative intent between December 2008 and April 2009 at the Eastern Hepatobiliary Surgery Hospital of the Second Military Medical University (Shanghai,China).HBV infection was confirmed by serological detection of HBV antigens.None of the patients had received preoperative radiotherapy or chemotherapy.All patients had histologically confirmed ICC,and patients with combined HCC and cholangiocarcinoma were excluded from this analysis.There were 36 men and 18 women,with a mean age of 49 years (range 23-73).Tumors arising in small intrahepatic bile ducts were considered to be peripheral,whereas those arising in major intrahepatic ducts were considered to be hilar.[13]Tumors <3 cm in diameter were classified as small ICC.
Immunohistochemistry
Paraffin-embedded sections of ICC were deparaffinized in xylene and rehydrated in graded ethanol.Endogenous peroxidase activity was blocked for 30 minutes by absolute methanol containing 0.3% hydrogen peroxidase.The samples were pretreated with citrate buffer (pH 6.0) for 20 minutes at 100 ℃ in a microwave oven.The sections were washed with phosphate buffered saline (PBS) three times for 5 minutes each.After treatment with blocking serum for 30 minutes,the sections were incubated at 4 ℃ overnight with the monoclonal anti-p53 antibody (1:50 dilution;NeoMarkers,Fremont,CA,USA) and the monoclonal anti-HBx antibody (1:50 dilution; Abcam,Cambridge,MA,USA).Samples were then incubated with biotinylated antibody for 20 minutes at room temperature.After incubation with avidin-biotin complex (NeoMarkers)for further 20 minutes,samples were developed with 3,3'-diaminobenzidine tetrahydrochloride.Finally,the samples were counterstained with hematoxylin and mounted.Twentyfields were randomly selected in each stained section,and the percentage of stained cells in eachfield was counted.Specimens were considered positive when >10% of cells had staining.[14]
All specimens stained for HBx and p53 were evaluated by two independent investigators (Dong H and Xian ZH) who were blinded to the patient groups and treatments.
This study was conducted in accordance with the Helsinki Declaration and approved by the Ethics Committees at our institutions.Informed consent was obtained from all subjects.
Statistical analysis
Statistical analysis was performed using SPSS for Windows version 9.0 (SPSS,Chicago,IL,USA).Categorical data were compared using the Chi-square test or Fisher's exact test.A P value <0.05 was considered statistically significant.
Results
HBx expression was found in 70.4% (38/54) of the cases.HBx immunoreactivity was observed mainly in the cytoplasm of tumor cells of ICC and the hepatic parenchymal cells of surrounding non-tumor tissue (Fig.A).
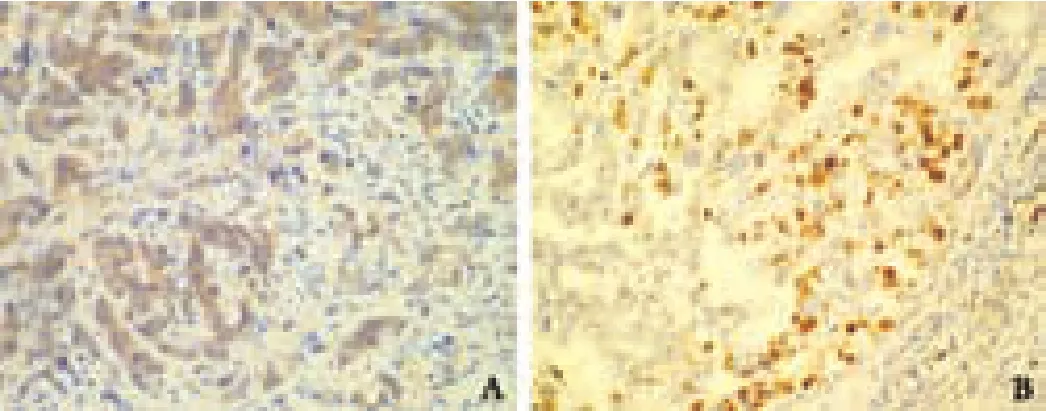
Fig.Representative immunohistochemical staining of HBx (A,original magnification ×400) and p53 (B,original magnification×200) in ICC.

Table.Clinicopathological correlation of HBx expression in HBV-infected ICC
The Table summarizes the relationship of immunohistochemical HBx expression with various clinicopathological parameters.HBx expression was seen in 79.5% (35/44) of the peripheral type,which was notably higher than the incidence in the hilar type(30%,3/10,P=0.002).All three well-differentiated ICCs expressed HBx,whereas 76.9% (30/39) of moderatelydifferentiated and 41.7% (5/12) of poorly-differentiated ICCs showed HBx expression (P=0.033).Patients with HBx expression had a higher rate of elevated serum alpha-fetoprotein (AFP) (P=0.033).There was no significant correlation between HBx expression and age,gender,serum carbohydrate antigen 19-9 level,tumor size,cirrhosis,lymph node metastasis or tumor stage.
p53 staining was found in 33.3% (18/54) of the cases.Staining was confined to the nuclei of tumor cells,and no surrounding non-tumor tissue was positive for p53(Fig.B).There was no correlation between HBx and p53 expression.In 36 of 54 (66.7%) cases,the staining pattern was either positive for p53 and negative for HBx,or negative for p53 and positive for HBx.In the other 18(33.3%) cases,p53 and HBx were either both positive or both negative.
Discussion
In our study,HBx was found in both tumorous and surrounding non-tumorous cells in >70% of HBV-infected ICC specimens,which supports the study by Wang et al,[9]who used antibodies against the X-gene product to show that the X-gene was expressed in >80%of ICCs.Taken together,these results suggest that HBx may contribute to the pathogenesis of ICC.
ICC is classified into hilar or peripheral type by the location of the tumor; they have different clinical and biological features,and have different surgical outcomes.[13,15,16]These differences may re flect a major difference in their pathogenesis.A particularly interesting point was our finding that the expression of HBx was significantly more frequent in peripheral than in hilar ICC.Hilar ICC originates from the liver hilum or in close vicinity of the bifurcation of the right and left hepatic ducts.[13]It commonly develops in relation to chronic biliary diseases such as hepatolithiasis or primary sclerosing cholangitis.[17]In contrast,peripheral ICC is thought to arise from the second or more distal branches of the biliary tree where hepatic progenitor cells (HPCs) are located.These HPCs are capable of differentiating into hepatocytes and cholangiocytes,and are considered to be a target population for carcinogenesis.[18]It has been demonstrated that HPCs can be infected with HBV,and proliferation of large numbers of HPCs is seen in HBV-associated chronic liver diseases and cirrhosis.[19-21]HBx is a transactivating protein that alters gene expression by binding to nuclear transcription factors,and by stimulating cytoplasmic signaling pathways that promote cell growth and survival.[12]We speculate that HBV infection may induce the activation of HPCs,and this process may be accompanied by abnormal genetic alteration activated by HBx,and thus contribute to the malignant transformation of HPCs.
AFP is a normal fetal serum glycoprotein that is synthesized and secreted by fetal hepatocytes,gastrointestinal cells,and yolk sac cells.A recent study[19]showed that HPCs express AFP mRNA and produce AFP during differentiation.In our study,patients with HBx expression had a significantly higher rate of elevated serum AFP; this finding is compatible with the HPC origin of tumors with HBx expression.
In our study,a close correlation was found between HBx immunoreactivity and the differentiation status of ICC specimens,but there was no significant correlation between HBx expression and tumor stage.These results suggest that HBx acts in concert with genotoxic substances at an early stage in malignant transformation in chronically HBV-infected cells.In accord with a previous study on HBx expression in HCC,in some patients with ICC,HBx was also expressed only in the surrounding non-tumor tissue,and not in the tumor itself,indicating that continuous expression of the HBx gene may not be required for the persistence of ICC; other factors may also contribute to malignant transformation.[22]
Wild-type p53 protein has a half-life of about 20 minutes and is not regularly detectable.However,mutated p53 has an increased half-life (up to 4 hours) and the protein is stabilized in the nucleus,thus making it readily detectable by immunohistochemistry.[10]Therefore,immunohistochemical detection of p53 is thought to re flect mutations of the p53 gene.[23]In our study,patients with and without HBx expression showed no difference in p53 overexpression,suggesting that HBx did not alter the p53 gene in ICC.However,to clarify their relationship,p53 gene status should be determined,because although p53 protein alterations detected by immunostaining have been reported to be fairly consistent with alterations at the genetic level,the underlying type or location of the mutations in this gene may not always be detectable with immunohistochemistry.[14]
In conclusion,these data indicate that HBx may contribute to the pathogenesis of ICC,particularly the peripheral type.p53 abnormality may not play a significant role in HBx-mediated oncogenicity during ICC carcinogenesis.Further elucidation of HBx function in cholangiocarcinogenesis may afford an important opportunity to define a novel molecular target for ICC prevention and treatment.
Acknowledgements:The authors thank Drs.Hui Dong and Zhi-Hong Xian for interpreting the pathological data.
Contributors:YZF proposed the study.ZYM wrote the first draft and analyzed the data.All authors contributed to the design and interpretation of the study and to further drafts.YZF is the guarantor.
Funding:None.
Ethical approval:Not needed.
Competing interest:The authors do not choose to declare any con flict of interest related directly or indirectly to the subject of this article.
1 Poultsides GA,Zhu AX,Choti MA,Pawlik TM.Intrahepatic cholangiocarcinoma.Surg Clin North Am 2010;90:817-837.
2 Parkin DM,Srivatanakul P,Khlat M,Chenvidhya D,Chotiwan P,Insiripong S,et al.Liver cancer in Thailand.I.A case-control study of cholangiocarcinoma.Int J Cancer 1991;48:323-328.
3 Kanemitsu E,Esaki M.A case of intrahepatic cholangiocarcinoma associated with primary sclerosing cholangitis.Jpn J Clin Oncol 2010;40:600.
4 Hur H,Park IY,Sung GY,Lee DS,Kim W,Won JM.Intrahepatic cholangiocarcinoma associated with intrahepatic duct stones.Asian J Surg 2009;32:7-12.
5 Zhou YM,Yin ZF,Yang JM,Li B,Shao WY,Xu F,et al.Risk factors for intrahepatic cholangiocarcinoma:a case-control study in China.World J Gastroenterol 2008;14:632-635.
6 Tanaka M,Tanaka H,Tsukuma H,Ioka A,Oshima A,Nakahara T.Risk factors for intrahepatic cholangiocarcinoma:a possible role of hepatitis B virus.J Viral Hepat 2010;17:742-748.
7 Fwu CW,Chien YC,You SL,Nelson KE,Kirk GD,Kuo HS,et al.Hepatitis B virus infection and risk of intrahepatic cholangiocarcinoma and non-Hodgkin lymphoma:a cohort study of parous women in Taiwan.Hepatology 2011;53:1217-1225.
8 Wu CG,Salvay DM,Forgues M,Valerie K,Farnsworth J,Markin RS,et al.Distinctive gene expression profiles associated with Hepatitis B virus x protein.Oncogene 2001;20:3674-3682.
9 Wang WL,London WT,Feitelson MA.Hepatitis B x antigen in hepatitis B virus carrier patients with liver cancer.Cancer Res 1991;51:4971-4977.
10 Khan SA,Thomas HC,Toledano MB,Cox IJ,Taylor-Robinson SD.p53 Mutations in human cholangiocarcinoma:a review.Liver Int 2005;25:704-716.
11 Harris CC.Structure and function of the p53 tumor suppressor gene:clues for rational cancer therapeutic strategies.J Natl Cancer Inst 1996;88:1442-1455.
12 Cougot D,Neuveut C,Buendia MA.HBV induced carcinogenesis.J Clin Virol 2005;34:S75-78.
13 Okuda K,Kubo Y,Okazaki N,Arishima T,Hashimoto M.Clinical aspects of intrahepatic bile duct carcinoma including hilar carcinoma:a study of 57 autopsy-proven cases.Cancer 1977;39:232-246.
14 Zhao B,Kimura W,Futakawa N,Muto T,Kubota K,Harihara Y,et al.p53 and p21/Waf1 protein expression and K-ras codon 12 mutation in carcinoma of the papilla of Vater.Am J Gastroenterol 1999;94:2128-2134.
15 Madariaga JR,Iwatsuki S,Todo S,Lee RG,Irish W,Starzl TE.Liver resection for hilar and peripheral cholangiocarcinomas:a study of 62 cases.Ann Surg 1998;227:70-79.
16 Aishima S,Kuroda Y,Nishihara Y,Iguchi T,Taguchi K,Taketomi A,et al.Proposal of progression model for intrahepatic cholangiocarcinoma:clinicopathologic differences between hilar type and peripheral type.Am J Surg Pathol 2007;31:1059-1067.
17 Fujii T,Zen Y,Nakanuma Y.Perihilar cholangiocarcinoma arising in hepatitis C virus-related liver cirrhosis with hepatocellular carcinoma.J Gastroenterol 2007;42:698-702.
18 Alison MR.Liver stem cells:implications for hepatocarcinogenesis.Stem Cell Rev 2005;1:253-260.
19 Ishikawa K,Sasaki A,Haraguchi N,Yoshikawa Y,Mori M.A case of an alpha-fetoprotein-producing intrahepatic cholangiocarcinoma suggests probable cancer stem cell origin.Oncologist 2007;12:320-324.
20 Lowes KN,Brennan BA,Yeoh GC,Olynyk JK.Oval cell numbers in human chronic liver diseases are directly related to disease severity.Am J Pathol 1999;154:537-541.
21 Xiao JC,Jin XL,Ruck P,Adam A,Kaiserling E.Hepatic progenitor cells in human liver cirrhosis:immunohistochemical,electron microscopic and immuno fluorenscence confocal microscopic findings.World J Gastroenterol 2004;10:1208-1211.
22 Diamantis ID,McGandy CE,Chen TJ,Liaw YF,Gudat F,Bianchi L.Hepatitis B X-gene expression in hepatocellular carcinoma.J Hepatol 1992;15:400-403.
23 Batheja N,Suriawinata A,Saxena R,Ionescu G,Schwartz M,Thung SN.Expression of p53 and PCNA in cholangiocarcinoma and primary sclerosing cholangitis.Mod Pathol 2000;13:1265-1268.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Disease spectrum and use of cholecystolithotomy in gallstone ileus
- Xanthogranulomatous cholecystitis mimicking gallbladder cancer and causing obstructive cholestasis
- Liver transplantation in Crigler-Najjar syndrome type I disease
- High-intensity focused ultrasound ablation as a bridging therapy for hepatocellular carcinoma patients awaiting liver transplantation
- Laparoscopic distal pancreatectomy with or without splenectomy:spleen-preservation does not increase morbidity
- Effect of endogenous hypergastrinemia on gallbladder volume and ejection fraction in patients with autoimmune gastritis
