Removal of Organic Matter and Ammonia Nitrogen inAzodicarbonamide Wastewater by a Combination of Power Ultrasound Radiation and Hydrogen Peroxide*
2012-03-22LIWenjun李文军WUDi吴笛SHIXin石鑫WENLixiong文利雄andSHAOLei邵磊StateKeyLaboratoryofOrganicInorganicCompositesBeijingUniversityofChemicalTechnologyBeijing00029China
LI Wenjun (李文军), WU Di (吴笛), SHI Xin (石鑫), WEN Lixiong (文利雄),** and SHAO Lei (邵磊) State Key Laboratory of Organic-Inorganic Composites, Beijing University of Chemical Technology, Beijing 00029, China
2 Chemical Industry Productivity Promotion Center of China, Beijing 100723, China
1 INTRODUCTION
The last twenty years has witnessed a swiftly deteriorating water environment as the result of rapid development of industry and extravagant use of complex organic compounds, in which the eutrophication problem caused by the discharge of ammonia-containing water, especially in lakes and touring areas, is one of the major concerns. The removal of hazardous substances from industrial waste streams containing ammonia nitrogen becomes a big challenge to the related industries, and effective and economical treatment technologies to remove ammonia-nitrogen are highly demanded. Biological processes, air stripping and breakpoint chlorination methods are well developed and have been applied to remove ammonia from some industrial or domestic wastewaters [1]. In the azodicarbonamide (ADC) industry, air/steam stripping technology is widely used for treating the wastewater that contains ammonia nitrogen at remarkably high concentration. However, it is only effective for removing the inorganic ammonia nitrogen, while not sufficiently workable on decomposing the organic wastes including hydrazine, urea and COD introduced into the wastewater in the production process. To remove such organic components, a pretreatment step is usually applied before air/steam stripping. Different oxidation methods, including supercritical water oxidation and catalytic wet oxidation, and some biological processes are normally used for treating organic hazards [1-3].However, new pretreatment methods are needed for treating ADC ammonia nitrogen wastewater, due to the complicated organic species and inorganic ammonia nitrogen at remarkably high concentration.
Ultrasound has been proven to be a very useful tool in enhancing the reaction rates in a variety of reaction systems and is widely used to degrade a number of water pollutants [4-6]. Ultrasound is one of advanced oxidation processes (AOPs), which are defined as those technologies that utilize the hydroxyl radical(·OH) for oxidation with extremely low selectivity of contaminants. In an aqueous liquid, the chemical effects of ultrasound result from the almost adiabatic collapse of cavitation bubbles. Cavitation bubble collapse produces extremely high local temperatures, as high as 4000 K and pressures over 101.325 MPa, resulting in high-energy chemical reactions [7, 8]. This phenomenon is capable of initiating and promoting chemical reactivity through thermolysis, supercritical water oxidation, and free radical oxidation [9]. Thermolysis can take place in the bubble cavity and interfacial layer surrounding the cavity and is the core mechanism for generating free radicals. Furthermore, sonication improves mass transfer and chemical reaction and is expected to reduce or eliminate chemical usage, resulting in minimal sludge and disposal problems [10].
A number of studies have been published for the sonochemical degradation of individual organic compounds. However, there have been few reports about the sonochemical treatment on heavily contaminated wastewater such as ADC wastewater and the removal of dual organic matters as well as inorganic ammonia simultaneously by ultrasound irradiation.
The objective of this paper is to investigate the removal efficiency of urea, hydrazine and ammonia nitrogen by ultrasound radiation, and to enhance the oxidation effects by combining ultrasound with hydrogen peroxide. The influence of initial pH, ultrasound format and peripheral water level on the oxidation processes is examined and the oxidation mechanism is explored.
2 EXPERIMENTAL
2.1 Materials and chemicals
The effluent of ADC industry wastewater typically contains inorganic ammonia nitrogen at high concentration and two organic ammonia nitrogen contaminants,urea and hydrazine, which were chosen as model organic ammonia pollutants in our study. Urea, hydrazine(volume fraction, 80%), hydrogen peroxide (volume fraction, 30%), potassium iodide, sodium sulfite, ammonium sulfate, sodium hydroxide, sulfuric acid, biurea, andp-dimethylaminobenzaldehyde (PDAB) were of analytical grade reagents and purchased from commercial source. All solutions were prepared with deionized water.
2.2 Experimental set-up
The schematic diagram of the ultrasound reactor is shown in Fig. 1. The ultrasonic source is a KQ-100B ultrasound generator (KUNSHAN Ultrasonic Equipment Co., Ltd.) with two planar transducers under the rectangle water tank of 4 L capacity. The ultrasound reactor can generate a maximum output power of 100 W at the frequency of 40 kHz. The temperature of ultrasound bath can be maintained from room temperature to 80 °C and controlled by circulating cooling water. The inner shell of the ultrasound reactor is covered by stainless steel in order to resist corrosion.
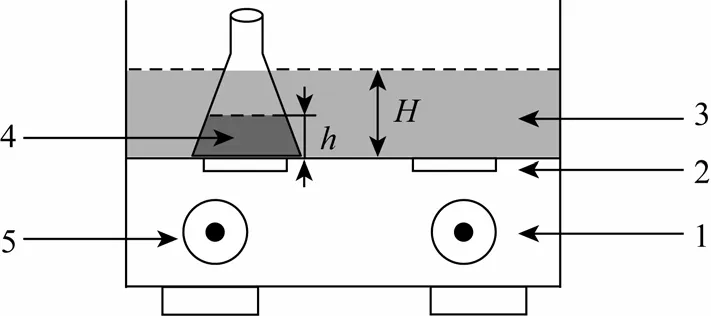
Figure 1 Scheme of ultrasound experimental setup1—radiation time control; 2—ultrasonic transducer; 3—water tank; 4—ADC wastewater; 5—temperature control
2.3 Wastewater treating procedures and concentration measurement
All experiments were carried out in a 100 ml glass conical flask containing 50 ml wastewater with flat bottom and glass plug, positioned right upon one ultrasound transducer of the water tank and partially emerged in the water tank, as illustrated in Fig. 1. The height of the inner wastewater in the flask washand the height of the surrounding water in the water tank wasH. The temperature for all experiments was 60 °C.The conical flask was filled with simulated ammonia nitrogen wastewater containing urea and hydrazine at specific initial concentrations, which are close to the industrial wastewater from ADC plant. The urea and hydrazine solution was adjusted to the required pH with 2 mol·L-1HCl or NaOH solution. COD and ammonia nitrogen concentrations of the sonicated sample were measured by a 5B-3(B) analyzer (Lian-Hua Tech Co., Ltd.) based on the K2Cr2O7and Nessler’s reagent method, respectively. Urea and hydrazine concentrations were measured by a UV-2550 UV-vis spectrophotometer (Shimadzu Co., Ltd. Japan) at the maximum wavelength (λmax). pH was measured by a pHS-3C pH meter (Shanghai Leici Instrument Factory, China).
3 RESULTS AND DISCUSSION
3.1 Determination of actual ultrasonic energy dissipated into wastewater
When a liquid is exposed to an acoustic field, the ultrasonic energy (high frequency sound waves) produces an alternating adiabatic compression and rarefaction of the liquid medium being irradiated [11]. The chemical effects of sonochemistry of ultrasound are due to the phenomenon of acoustic cavitation, which involves the formation and subsequent collapse of micro-bubbles from the acoustical wave induced compression/rarefaction. The ultrasonic power or intensity has been considered as an important factor. It is generally believed that ultrasound intensity of 0.3-10 W·cm-2could generate noticeable cavitation bubbles in liquid medium [12]. In many papers, only the input power was given as a measure of the ultrasonic power.However, the input power is not always instructive to be a sign of ultrasonic power for sonochemistry, because the energy conversion of ultrasound is primarily transducer-dependent. In addition, when an ultrasound wave travels through the liquid medium, wave energy is absorbed by the medium and converted into the form of heat, which causes the energy loss between the actual power input (Pin) and energy output quoted by the manufacture (Pout). Measurement of sound pressure with a hydrophone is useful to determine the distribution of ultrasonic intensity in a reaction vessel.However, it is not suitable to specify the mean ultrasonic power dissipated into the reaction system, because the sound field is not distributed uniformly. One of the most prevailing methods of measuringPinis based on calorimetry and assumed that the energy entering the water tank is dissipated into solutions as heat [13, 14].The ultrasonic power dissipated into the treated wastewater is calculated by the following equation:
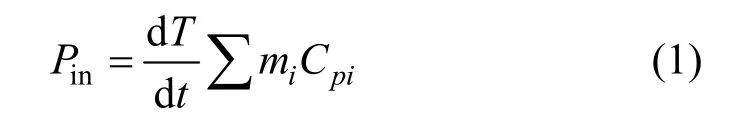
wheremiandCpiare the mass and heat capacity of the materials present in the ammonia-nitrogen wastewater,respectively, and dT/dtis the initial slope of the graph of temperatureversustime in the ultrasonic bath, as shown in Fig. 2. In this work, the sample volume of the wastewater was 50 cm3. The temperature rise was measured at room temperature by using a thermocouple, which was immersed and held at the half height of the solution. The actual ultrasonic power dissipated in the wastewater was determined by adopting the procedure reported by Hagenson and Doraiswamy [15].On basis of Eq. (1), the energy actually input to the water bath was about 68.7 W, though the maximum available ultrasonic output power quoted from the manufacturer was 100 W. The ultrasound intensity, based on the actual energy input, reached 0.359 W·cm-2in our wastewater solution, which was high enough to cause cavitation effect in the solution [12]. Due to the limit of the ultrasound generator, the effects of ultrasound intensity on the waste removal were not examined in this work, but the influence of treating time was explored.

Figure 2 Temperature rise in ultrasonic bath with ultrasound assistance (H=1.5 h; ultrasonic: 40 kHz, 100 W)
3.2 Effects of adding hydrogen peroxide on the sonolysis reduction of organic waste
Since intense oxidation-reduction reactions occur between hydrazine and hydrogen peroxide, the synergetic effect of ultrasonic and hydrogen peroxide on the removal rate of hydrazine hydrate for ADC wastewater was investigated first in this work, as shown in Table 1. It demonstrates that the introduction of hydrogen peroxide facilitates sonolysis reduction of hydrazine hydrate, especially when the initial pH is high,which proves the combinative effect of ultrasonic and hydrogen peroxide. The addition of hydrogen peroxide can generate oxygenviaits own decomposition reaction, particularly in basic environments. If the solution is saturated with oxygen, the dissolved oxygen may act as nucleation sites for acoustic cavitation.Moreover, peroxyl and more hydroxyl radicals may be created in the gas phase (upon the decomposition of molecular oxygen), and the recombination of the former at the cooler sites (interface or the solution bulk)produces additional hydrogen peroxide, as shown in Eqs. (2)-(5) [16, 17]:


Table 1 Synergetic effects of ultrasonic and hydrogen peroxide on the removal of hydrazine hydrate (H=1.5 h;ultrasonic: 60 °C, 40 kHz, 100 W; initial N2H4·H2O concentration:20 mg·L-1; treating time: 90 min; amount of 30% H2O2: 1 ml)

In the presence of oxygen acting as a scavenger of hydrogen atom (and thus suppressing the recombination of ·H and ·OH), the hydroperoxyl radical (·HO2)is formed additionally, which is an oxidizing agent.Therefore, the addition of hydrogen peroxide not only improves the oxidation ability of the overall homogenous ultrasonic system, but also favors the cavitation event to maximize chemical reaction yields and/or reaction rates by providing excessive nuclei in order to lower the cavitation threshold and speed up the initialization of cavity formation [18], hence strengthening the sonolysis reduction.
3.3 Effects of initial pH on the removal of organic waste
The effects of initial pH in the wastewater on the sonolysis reduction of urea and hydrazine with the help of H2O2are shown in Figs. 3 and 4. The initial pH has different influences on the removal efficiency in urea and hydrazine solutions. The hydrazine reduction efficiency is enhanced significantly at a basic environment as compared to acidic or neutral environment, while the effects of initial pH on urea decomposition are much less appreciable. This large difference is due to the different properties between hydrazine and urea. After 90 min ultrasound irradiation, hydrazine removal efficiency achieves 60.3% at initial pH=11.0,while only 35.2% at initial pH=3.0. The chemical property of hydrazine is quite close to that of ammonia of high volatility and its solution is basic, and the fraction in the molecular state of hydrazine is larger at higher pH. Under neutral and acidic conditions, the hydrazine ions can not vaporize into the cavitation bubbles, so the major degradation is mainly achieved by ·OH and ·H free radicals in the interfacial sheath between the gaseous bubble, surrounding liquid and bulk of solution. However, in the molecular state,gaseous hydrazine can vaporize and migrate into cavitation bubbles and degrade inside by pyrolytic reactions and radical reactions simultaneously. According to the sonochemistry theory, the efficiency of thermal cleavage is normally much superior to radical reaction.Therefore, the sonolysis reduction of hydrazine is more effective at higher pH. However, the reduction efficiency of urea solution is all around 25% with relatively minute variation at all three initial pH values,as shown in Fig. 4. This may be because that urea remains molecular state at these initial pH values and its solution is non-volatile. During ultrasonic irradiation,it tends to accumulate within the aqueous phase and cavitation bubble membrane, where it is degraded predominatelyviahydroxyl radical reactions. Thus,the initial pH has a minor influence on the sonolysis rate of urea as compared to that of hydrazine.

Figure 3 Effects of initial pH on the decompositionof hydrazine within 90 min (H=1.5 h; ultrasonic: 60 °C, 40 kHz, 100 W; initial N2H4·H2O concentration: 20 mg·L-1;amount of 30% H2O2: 1 ml)■ pH=3; ● pH=7; ▲ pH=11

Figure 4 Effects of initial pH on the decompositionof urea within 90 min (H=1.5 h; ultrasonic: 60 °C, 40 kHz,100 W; initial urea concentration: 5 mg·ml-1; amount of 30%H2O2: 1 ml)■ pH=3; ● pH=7; ▲ pH=11
The COD reduction efficiency by the combined treatment of ultrasonic and hydrogen peroxide at different initial pH is shown in Fig. 5. The wastewater samples contain urea, hydrazine and minute quantity of biurea. The COD reduction efficiency increases with increasing initial pH. The highest COD removal rate is at initial pH=11. After 90 min ultrasonic radiation, it reaches a COD removal rate of 68.45%.
3.4 Effects of ultrasonic format on the removal of organic waste
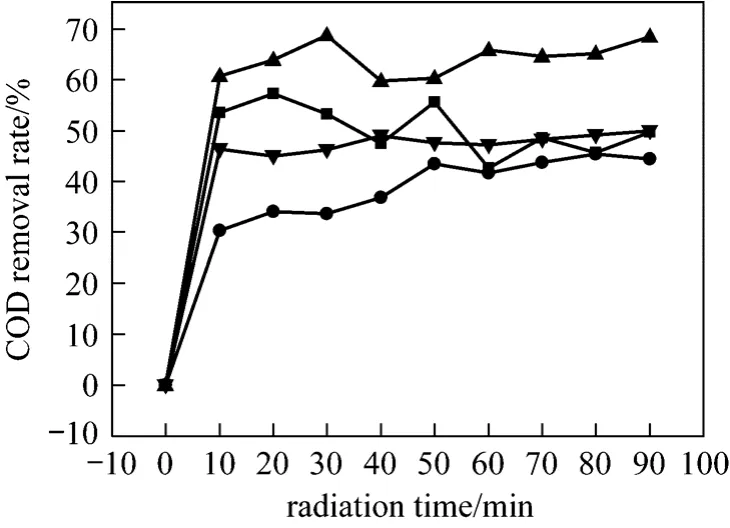
Figure 5 COD reduction efficiency under the combined treatment of ultrasonic and hydrogen peroxide (H=1.5 h;ultrasonic: 60 °C, 40 kHz, 100 W; initial COD: 1500 mg·L-1;amount of 30% H2O2: 1 ml)■ pH=3; ● pH=7; ▲ pH=11; ▼ pH=11, no H2O2
During sonolysis process using ultrasonic, some of the input ultrasonic energy is dissipated as heat to decrease the ultrasonic efficiency. At the same time the noises of ultrasonic would have negative effects on operation surroundings. To improve the cavitation efficiency and reduce the ultrasonic time, an operation technique of intermittent ultrasonic was investigated in this work. The ultrasonic generator worked at 5/5,3/3 or 1/1 min of on/off alternative mode separately,for a total treatment time of 90 min. Table 2 demonstrates the NH4-N removal rate difference between continuous and intermittent ultrasonic radiation after 90 min. It is interesting to find that the 1/1 min intermittent ultrasonic operation achieves higher ammonianitrogen removal rate than continuous mode, though the other intermittent modes with longer on/off switch time does not show the same result. Therefore, a proper intermittent ultrasonic operating format can not only enhance the sonolysis reduction of the waste, but also be more energy efficient and environment benign.

Table 2 Comparison of NH4-N removal rate in continuous and intermittent ultrasonic radiation (H=1.5 h; ultrasonic:60 °C, 40 kHz, 100 W; initial NH4-N concentration:2250 mg·L-1; amount of 30% H2O2: 1 ml)
The main and possible reason may involve the change of the collapse of micro-bubbles in cavitation and the enhancement of cavitation efficiency by intermittent ultrasound. The effective region of sonochemical reaction is spatially restricted when using continuous ultrasound, because many degassing bubbles are formed under such conditions of sonication[19]. These degassing bubbles are ineffective for sonochemical reaction, due to their small volumetric oscillation, and they restrict the propagation of sound by absorption and spreading of the sound, leading to a decrease in the sound pressure amplitude and limited sonochemical reaction. An appropriate pulse-off time that is specific to the ultrasound pulsing operation suppresses the generation of degassing bubbles by the coalescence of bubbles, promoting high amplitude of sound propagation that is responsible for the expansion and contraction of the bubbles. Therefore, the number of bubbles that are effective for sonochemical reaction is increased [20]. This suggests that ammonianitrogen removal process in a proper intermittent ultrasound field would make more efficient use of ultrasound energy due to the higher cavitation efficiency of intermittent ultrasound.
3.5 Effects of peripheral water level on the removal of ammonia-nitrogen
Ultrasonic bath systems are widely used in sonochemical research because they are readily available and relatively inexpensive. As shown in Fig. 1,the glass conical flask is typically immersed in the coupling fluid contained in the bath, which forms the indirect sonication system. Kawaseet al. [21] reported that there is a strong relationship between the inner liquid height and the ammonia removal rate. However,there has been little reported about the effect of the peripheral water level on the sonochemical efficiency.To explore these effects on ammonia-nitrogen degradation efficiency, the sonochemical experiments were conducted in an ultrasonic bath with different peripheral water levels, as shown in Fig. 1. The inner water heighthwas fixed by adding 50 ml (NH4)2SO4solution and 1 ml 30% H2O2to the flask. Fig. 6 depicts the removal percentage of ammonia-nitrogen with three different peripheral water levels at initial pH=7.3.The best ammonia-nitrogen removal rate was obtained when the peripheral water level was 1.5 times of inner water height. Further increase of the peripheral water level would lower the removal efficiency, while the least removal efficiency occurred when the peripheral water level was below the inner water height. A possible reason is that a relatively higher peripheral water height would be beneficial to uniform energy distributions within both liquids in the flask and in the bath,resulting in more efficient utilization of the input ultrasound energy by the inner ammonia-nitrogen sonolysis reaction system. However, too much excessive water in the ultrasonic bath would absorb more ultrasound input energy, while insufficient water in the bath might not be able to transfer the input ultrasound energy to the liquid in the flask which was above the peripheral water level, both reducing energy utilization of the input ultrasound energy by the sonolysis reaction system within the flask. Therefore, an appropriate height difference between the surrounding water level and the inner water level of the flask is also an important factor for optimizing the indirect sonication system.
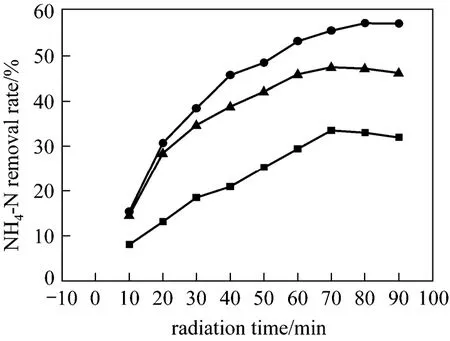
Figure 6 Effects of peripheral water level on the removal rate of ammonia-nitrogen (ultrasonic: 60°C, 40 kHz, 100 W;initial NH4-N concentration: 2250 mg·L-1; amount of 30%H2O2: 1 ml)■ H=0.5 h; ● H=1.5 h; ▲ H=2 h
4 CONCLUSIONS
The removal of organic matter and ammonia nitrogen in ADC wastewater by a combination of power ultrasound radiation and hydrogen peroxide was investigated and the effects of reaction time, initial pH,ultrasonic format and peripheral water level on the COD reduction and ammonia removal efficiency were explored. It is found that the initial solution pH has a significant influence on the degradation of water contaminants. Higher initial pH is favorable to the sonolysis of hydrazine, whereas this effect on the degradation of urea is relatively small. Adding hydrogen peroxide to the sonolysis reaction can enhance the sonolysis reduction of organic waste as well, especially at high pH. In addition, a proper intermittent ultrasonic operating format and/or an appropriate height difference between the surrounding water level and the inner water level of the flask can further improve the utilization efficiency of the input ultrasound energy by the inner ammonia-nitrogen sonolysis reaction system within the flask, and therefore, boosting the sonolysis reduction of the waste in ADC wastewater by ultrasonic treatment.
1 Tchobanoglous, G., Burton, F.L., Wastewater Engineering: Treatment,Disposal and Reuse, 2nd Edition, McGraw-Hill, New York (1991).
2 Lee, Y.W., Ong, S.K., Sato, C., “Effects of heavy metals on nitrifying bacteria”,WaterSci.Technol., 36 (12), 69-74 (1997).
3 Garrido, J.M., Guerrero, L., Mendez, R., Lema, J.M., “Nitrification of waste waters from fish-meal factories”,Water SA, 24 (3), 245-249(1998).
4 Cooper, W.J., Cramer, C.J., Martin N.H., Mezyk, S.P., O’Shea, K.E.,Sonntag, C., “Free radical mechanisms for the treatment of methyltert-butyl ether (MTBE)viaadvanced oxidation/reductive processes in aqueous solutions”,Chem.Rev., 109 (3), 1302-1345 (2009).
5 Yang, L., Rathman, J.F., Weavers, L.K., “Degradation of alkylbenzene sulfonate surfactants by pulsed ultrasound”,J.Phys.Chem.B,109 (33), 16203-16209 (2005).
6 Beckett, M.A., Hua, I., “Impact of ultrasonic frequency on aqueous sonoluminescence and sonochemistry”, J. Phys. Chem. A, 105 (15),3796-3802 (2001).
7 Suslick, K.S., Ultrasound: Its Chemical, Physical and Biological Effects, VCH, New York (1988).
8 Suslick, K.S., “The chemical effects of ultrasound”, Sci. Am., 260(2), 80-86 (1989).
9 Hoffmann, M.R, Hua, I, Hochemer, R., “Application of ultrasonic irradiation for the degradation of chemical contaminants in water”,Ultrason. Sonochem., 3 (3), S163-S172 (1996).
10 Adewuyi, Y.G., “Sonochemistry: environmental science and engineering applications”, Ind. Eng. Chem. Res., 40 (22), 4681-4715 (2001).
11 Mason, T.J., Chemistry with Ultrasound: Critical Reports on Applied Chemistry 28, Society for Chemical Industry, Elsevier, London(1990).
12 Feng, N., Li, H., Sonochemistry and Its Application, 2nd ed., Anhui Science and Technology Press, Hefei (1992). (in Chinese)
13 Contamine, F.R., Willhelm, A.M., Berlan, J., Delmas, H., “Power measurement in sonochemistry”, Ultrason. Sonochem., 2 (1), S43-S47(1995).
14 Kimura, T., Sakamoto, T., Leveque, J.M., Somiya, H., Fujita, M., Ikeda,S., Ando, T., “Standardization of ultrasonic power for sonochemical reaction”, Ultrason. Sonochem., 3 (3), S157-S161 (1996).
15 Hagenson, L.C., Doraiswamy, L.K., “Comparison of the effects of ultrasound and mechanical agitation on a reacting solid-liquid system”, Chem. Eng. Sci., 53 (1), 131-148 (1998).
16 Makkino, K., Mossoba, M.M., Riesz, P., “Chemical effects of ultrasound on aqueous solution. Evidence for hydroxyl and hydrogen free radicals (·OH and ·H) by spin trapping”, J. Am. Chem. Soc., 104(12), 3537-3539 (1982).
17 Petrier, C., Lamy, M.F., Francony, A., Benahcene, A., David, B.,Renaudin, V., Gondrexon, N., “Sonochemical degradation of phenol in dilute aqueous solutions: comparison of the reaction rates at 20 and 487 kHz”, J. Phys. Chem., 98 (41), 10514-10520 (1994).
18 Mason, T.J., Cordemans, E.M., “Practical Considerations for Process Optimization”, in Synthetic Organic Sonochemistry, Luche, J.L., Ed.,p. 301, Plenum Press, New York (1998).
19 Leighton, T.G., The Acoustic Bubble, Academic, London (1996).
20 Tuziuti, T., Yasui, K., Lee, H., Kozuka, T., Towata, A., Iida, Y.,“Mechanism of enhancement of sonochemical-reaction efficiency by pulsed ultrasound”, J. Phys. Chem. A, 112 (22), 4875-4878 (2008).
21 Kawase, Y., Masuya, T., Yasuda, K., Nakamura, M., “Effects of operation conditions on performance of concentrator using ultrasonic atomization”, J. Chem. Eng. JPN, 39 (3), 334-339 (2006).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- HPLC Analysis of Egg Yolk Phosphatidylcholine by Evaporative Light Scattering Detector
- A Geometric Approach to Support Vector Regression and Its Application to Fermentation Process Fast Modeling*
- Three Dimensional Numerical Simulation of Convection-Condensation of Vapor with High Concentration Air in Tube with Inserts*
- Nanoparticle Migration in a Fully Developed Turbulent Pipe Flow Considering the Particle Coagulation*
- Regeneration of Spent Activated Carbon by Yeast and Chemical Method*
- Optimization of Parameters for Melt Crystallization of p-Cresol*
